The results of a confidential audit of maternal mortality due to preeclampsia and eclampsia in Russia in 2017–2018
Aim. To identify the contemporary features of the development and progression of the most severe forms of PE and its complications, specify medical errors and management flaws in obstetric care associated with maternal deaths (both in the outpatient and hospital settings), and conduct the next stage of the confidential audit of maternal mortality due to PE and eclampsia in the regions of the Russian Federation in 2017–2018.Sidorova I.S., Nikitina N.A., Guseva E.V.
Materials and methods. The audit was based on a retrospective analysis of primary medical documentation of 25 cases of maternal deaths from preeclampsia, eclampsia, and their complications in 2017–2018 in the Russian Federation. The data sources included outpatient medical records, maternity records, protocols of clinical examinations, inpatient medical records, and anatomic pathology reports. The documentation was provided by the Department of Child’s Care and Maternity Services of Minzdrav of Russia.
Results. In 2017, according to Minzdrav of Russia, 80.9% of maternal deaths were avoidable (preventable and amenable). Analysis of our findings and literature showed that main complications of PE and eclampsia accounting for the majority of maternal deaths included cerebral edema with brainstem dislocation, multiple organ failure, massive coagulopathic hemorrhage, and, most notably, increasing incidence of stroke. We also report the most common medical errors and management flaws in obstetric care during pregnancy, delivery, and postpartum associated with maternal deaths.
Conclusion. A thorough analysis of all cases of maternal deaths made it possible to formulate and substantiate several recommendations aimed at reducing maternal mortality due to PE, eclampsia, and their complications.
Keywords
In recent years in Russia, as in many countries worldwide, there has been a significant decrease in the maternal mortality. In 2017, as estimated by Minzdrav of Russia, the maternal mortality rate was 9.6 per 100,000 live births, which was 8.6% lower than in 2016 and 60.3% lower than 10 years before (Fig. 1).
At the same time, there have been little changes in the structure of the causes of maternal death. According to the latest published data from Minzdrav of Russia, the leading causes of maternal mortality include non-obstetric diseases (35%), obstetric hemorrhage (23.3%), and hypertensive disorders, including preeclampsia (PE) and eclampsia (12, 9%) [1].
The decrease in maternal mortality attributed to PE and eclampsia is extremely unstable, as shown in Fig. 2. In 2017, this indicator decreased by 10.1% compared to the previous year and amounted to 1.24 per 100,000 live births (Fig. 2) [1]. According to preliminary data from Minzdrav of Russia, in 2018, this indicator is almost 20% lower than in 2017.
Complications of PE and eclampsia that account for the majority of maternal deaths include severe liver dysfunction (acute liver failure, HELLP syndrome), kidneys dysfunction (acute renal failure), brain injury (eclampsia, acute cerebrovascular stroke) and massive coagulopathic hemorrhage. The incidence of these life-threatening complications, according to Minzdrav of Russia, is shown in Fig. 3.
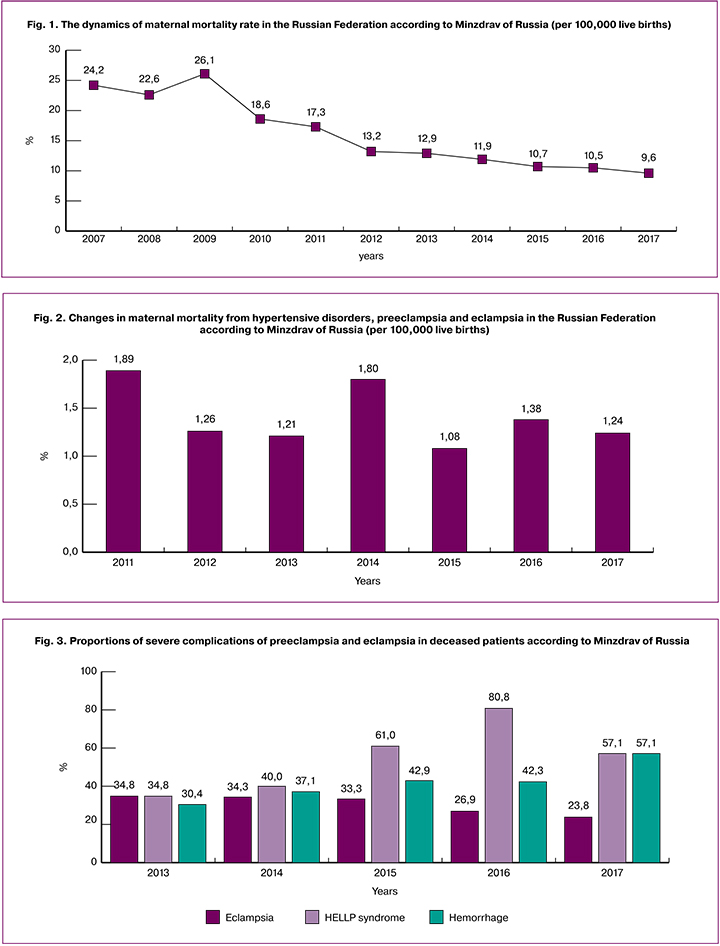
The percentage of preventable and potentially preventable deaths from PE, according to Minzdrav of Russia, increased to 80.9% in 2017 (in 2016, it was 69.2%). All patients died in secondary and tertiary hospitals.
We conducted a confidential audit of maternal mortality associated with PE and eclampsia in the regions of the Russian Federation in 2017–2018. The audit was aimed to identify the contemporary features of the development and progression of the most severe forms of PE and its complications, specify medical errors and organizational flaws in obstetric care associated with maternal deaths (both in the outpatient and hospital settings). We also performed a comparative analysis of the recent and previous audit results, which have been published in leading Russian journals and methodology letters of Minzdrav of Russia (2013–2015).
Materials and methods
The audit was based on a retrospective analysis of primary medical documentation of 25 cases of maternal deaths from preeclampsia, eclampsia, and their complications in 2017-2018 in the Russian Federation. The data sources included outpatient medical records, maternity records, protocols of clinical examinations, inpatient medical records, and anatomic pathology reports. The documentation was provided by the Department of Child’s Care and Maternity Services of Minzdrav of Russia.
Results and discussion
Eighty, 16%, and 4% of deaths due to preeclampsia occurred in women aged over 30, 20-25, and 25-30 years, respectively. Only 20% of them were primigravida, and 80% were multigravida.
Five (20%) women in the study group were not registered for antenatal care and were not examined.
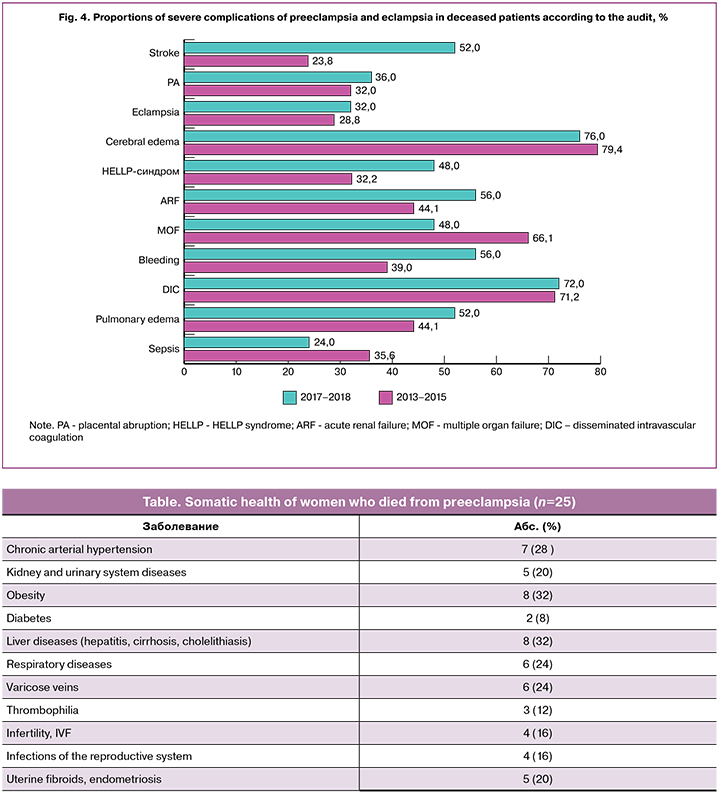
All patients (100%) had 1-2 or more somatic and gynecological diseases (Table 1). These data are consistent with the results of the previous audit and confirm the fact that multimorbid patients are more likely to die from preeclampsia. The most common comorbidities were arterial hypertension, kidney and urinary tract infections, obesity, and liver diseases (infectious and non-infectious). In 3 (12%) fairly young women (27, 30, and 36 years), postmortem pathological examination revealed early severe atherosclerosis of the aorta and coronary arteries. In another 3 (12%) women, comorbidities were not diagnosed during life and were found only postmortem (chronic hepatitis, cirrhosis, chronic glomerulonephritis). It should be noted that 3 (12%) patients were examined for congenital and acquired thrombophilia already after being in a critical condition (an eclampsia-induced coma, hemorrhagic stroke).
Twenty-three (92%) deceased women had early preeclampsia occurring before 34 weeks’ gestation. At the same time, 13 (52%) of the deceased had the most unfavorable variant of preeclampsia with clinical symptoms emerging before 30 weeks. The ratio of early and late preeclampsia in the study group was approximately 12: 1, while in the general population, this ratio is usually equal to 1: 4 or more.
Among the deceased, the most common first clinical manifestations of preeclampsia were pathological weight gain and gestational edema (13/21; 61.9%). The second most common symptom was arterial hypertension (7/21; 33.3%), and only in one woman, the first symptom was proteinuria (1/21; 4.8%). In 4 deceased, clinical manifestations at the onset of preeclampsia could not be determined (not observed).
At the same time, every third (32%) deceased had circulatory disorders of the fetal-placental unit before the onset of the classic symptoms of preeclampsia, every fifth (20%) had changes in the hemostatic system, usually activated intravascular coagulation (moreover, no pregnant women were examined for thrombophilia).
As before, we noted that contemporary patients had an atypical, non-classical clinical presentation of severe preeclampsia, which may be largely due to the therapy (antihypertensive drugs, antiplatelet agents, anticoagulants, etc.) and the development of some complications. So, severe arterial hypertension, massive proteinuria, and severe edema, anasarca were observed only in 52%, 58.8%, and 58.8% of the deceased, respectively. Some deceased women (8%) had arterial hypotension (against the background of preterm placental abruption and bleeding), which made preeclampsia difficult to diagnose.
Fifty-two percent of the deceased had severe arterial hypertension refractory to combination therapy with three or more antihypertensive drugs in an adequate dosage, thus allowing for a safe level of blood pressure to be achieved. This is one of the main causes of the rupture of cerebral vessels and intracerebral hemorrhage, which in most cases, led to death.
The main complications that led to the death were cerebral edema with brainstem dislocation, multiple organ failure, massive coagulopathic bleeding. Especially noteworthy is an increase in the incidence of brain stroke. Lethal complications reported in the last and previous audits are presented in fig. 4.
Due to the high incidence of stroke, there was a 4-fold increase in the rates of craniotomy for evacuation of intracranial hematoma (up to 12% compared to 3% in previous years). The need for this operation was seen more often, but it was impossible due to the severity of the patients’ condition. There is still a high rate of operative delivery (84%), hysterectomy (36%), regional vessel ligation (24%), and re-laparotomy (28%).
The direct cause of death in most deceased patients was intracerebral hemorrhage, cerebral edema with brainstem dislocation (48%), multiple organ failure (32%), hemorrhagic shock (8%), obstetric embolism (8%), and septic shock (4%).
In 4 (16%), 11 (44%), and 10 (40%) patients death occurred on the first day, days 2-7, and beyond seven days after delivery, respectively.
Twenty-eight children were born, including three twins. Perinatal losses decreased from 42.9% in previous years to 28.6% according to the latest audit. The proportion of preterm births rose to 90% compared with 79.4% in previous years. However, the rates of complications such as hypoxic-ischemic damage to the central nervous system, intraventricular hemorrhage, severe asphyxia, and malnutrition in newborns did not change significantly.
Considerable attention should be given to the incidence of stroke according to the latest audit, which more than doubled and was reported in 13 (52%) of the deceased. Of them, only 8% had ischemic strokes, and 92% had hemorrhagic strokes. Hemorrhagic stroke has a particularly unfavorable prognosis: according to published data, they account for 40 to 60% of eclampsia-related deaths [2].
Of all cerebrovascular accidents, 90.9% occurred during delivery or immediately postpartum, and 9.1% were observed during pregnancy. In this group of the deceased, 81.8% of women had severe arterial hypertension before the onset of a stroke. By the time of the stroke, 100% of them had thrombocytopenia. In 72.7% of them, stroke occurred concurrently with HELLP syndrome (which is also characterized by thrombocytopenia). Almost all of these patients (90.9%) underwent cesarean delivery under general endotracheal anesthesia.
According to the literature, the incidence of stroke in pregnant women is 2-3 times higher than in non-pregnant women of reproductive age [2] constituting 11 cases per 100,000 non-pregnant women aged 15-44 and 25-34 cases per 100,000 births. In pregnant women with arterial hypertension of any genesis, the risk of stroke is 6–9 times higher than in pregnant women without hypertension [3].
Recently published data [4] showed that the highest risk of stroke during gestation is associated with delivery (stroke develops in 161.1 out of 100,000) and the early postpartum period (in 47.1 out of 100 000). During pregnancy, according to the same authors, stroke develops in 10.7 cases per 100,000 births.
After analyzing all the clinical data of our study and taking into account the literature data, we identified the following main risk factors for the development of stroke:
- severe refractory to therapy hypertension persisting for a long time;
- thrombocytopenia, a hypercoagulable state that accompanies the most severe forms of preeclampsia and HELLP syndrome;
- the destructive nature of vascular endothelial lesions in women with preeclampsia, described by us earlier and repeatedly published;
- the delivery process itself (birth stress). Of significant importance are large fluctuations in blood pressure (against the background of inadequate antihypertensive therapy, at the time of intubation and extubation, inadequate pain relief, etc.);
- significant changes in hemodynamics in all organs and systems including in the brain in the early postpartum period;
- pathogenetic features of preeclampsia, which are included in the concept of PRES (posterior reversible encephalopathy syndrome). In particular, the forced vasodilation of cerebral arterioles described by many authors, excessive hyper-perfusion can lead to rupture of cerebral vessels;
- failure of cerebral blood flow autoregulation, which can occur in preeclamptic patients with significantly lower blood pressure (eclampsia with moderate hypertension) [5];
- changes in cerebral arteries’ architectonics, malformation of cerebral vessels. Such vessels have insufficient content of collagen, low elasticity, and strength of the vascular wall, impaired microcirculation, which contributes to the rupture of blood vessels.
Eclampsia was reported in 7 (28%) of the deceased. Six (85.7%) of them suffered convulsions at various stages of pregnancy (in 1 at 38 weeks, in 5 at 22-37 weeks); 1 (14.2%) developed convulsions on the first day after cesarean section at 40 weeks’ gestation. Eclampsia was preceded by severe arterial hypertension in 5 (71.4%) women; after the attack, all of them had severe, critical hypertension of more than 180/20 mm Hg. The deceased women suffered headaches as a harbinger of eclampsia (85.7%), nausea and vomiting (42.9%), epigastric and upper right abdomen pain (28.6%), 2/3 of them had hepatic and renal dysfunction/insufficiency.
HELLP syndrome was diagnosed in 12 (48%) patients. In 2/3 of them, symptoms of HELLP syndrome were observed during pregnancy (66.7%). Only in a third of them the development and progression of the HELLP syndrome was noted after delivery. It was noteworthy that some hospitals already try to diagnose other forms of thrombotic microangiopathy (TMA), which include the HELLP syndrome, as well as use specific targeted therapy for the atypical hemolytic-uremic syndrome (found in 2 deceased). In other cases, the diagnosis of HELLP syndrome was based only on clinical presentation, and additional diagnostic procedures were not performed.
Further, we present a list of medical errors and gaps in the management of pregnancy, childbirth, and the postpartum period in women who due to preeclampsia/eclampsia and their complications.
1. Many patients do not undergo continuous blood pressure monitoring, while large fluctuations of blood pressure can cause a stroke.
Clinical case:
A pregnant woman aged 40. Obstetric history: Gravida 4, para 2, abortion 2 (one spontaneous miscarriage before 12 weeks and one medical abortion). Non-obstetric comorbidities included the hypertonic type of NCD and varicose veins. The onset of hypertension of 140/90 occurred at 20 weeks’ gestation, and at 35 weeks, she developed gestational edema. She was admitted to the hospital with a full-term pregnancy. Her blood pressure was 160/100, and she had pronounced swelling of lower extremities, hands, face, and anterior abdominal wall. Proteinuria 0.48 g/l. She was administered an IV bolus of 20 ml Sol. MgSO4 25%, Relanium 2 ml, Nifedipine 2 sublingual tablets. Ten minutes later, blood pressure decreased to 120-130/80-85. Three hours later, she underwent a cesarean section. On the first postoperative day, the patient developed HELLP syndrome, hepatic-renal failure, thrombocytopenia up to 56x109, hypertension up to 190/100 refractory to therapy, and stroke (confirmed by CT 12 hours after surgery). She died on the 3rd day of the postoperative period (cerebral edema with brainstem dislocation).
It is also necessary to remember that preeclampsia is characterized by an antiangiogenic imbalance, which leads to a decrease in the density of the capillary bed in organs and tissues, and a depletion of capillary blood flow. This fact is confirmed by our immunohistochemical studies of autopsy material. In this regard, a rapid and large decrease in blood pressure (as shown in the clinical example) can lead to a decrease in organ and tissue perfusion to the point of necrotic changes (a clinical equivalent of HELLP syndrome).
2. Many pregnant women did not undergo an appropriate examination.
Clinical examples:
A patient with recurrent miscarriage (a history 5 of spontaneous miscarriages), was not examined according to the protocol; severe multigenic thrombophilia was diagnosed only after the development of stroke;
At a visit to an antenatal clinic at 28 weeks’ gestation, a patient was found to have thrombocytopenia (78x109) and was referred to a hematologist. A record in the outpatient card says: “the patient made an appointment with a hematologist on the 22d day (!). At 29 weeks, she was admitted to the clinic with the classic HELLP syndrome and died on the 2nd day after delivery;
A pregnant woman was found to have asymptomatic bacteriuria at 13 weeks, leukocyturia at 16 weeks, and gestational pyelonephritis was diagnosed at 19 weeks (according to laboratory data). No kidney ultrasound was performed. There was no consultation with a urologist and no adequate therapy was administered;
In 12% of deceased women, the diagnosis of serious diseases and some pregnancy complications was made postmortem (chronic glomerulonephritis, chronic hepatitis, liver cirrhosis, purulent deciduitis, and chorioamnionitis).
3. Untimely diagnosis of severe PE.
Clinical example:
A 34-year-old pregnant woman presented as an emergency admission with signs of placental abruption at 36 weeks’ gestation. BP 80/60-70/40, swelling of the lower extremities. Antenatal fetal death. Diagnosis: Gravida 8, 36 weeks. Placental abruption. Massive hemorrhage. Hemorrhagic shock III. Antenatal fetal death. Intensive ITT was started, and the operating room was set up. After 5 minutes from the time of admission, generalized convulsions occurred (in the absence of a diagnosis of preeclampsia and administration of magnesium sulfate).
4. Doctors of related specialties lack the knowledge of preeclampsia and its complications when instead of the HELLP syndrome, the diagnosis was “acute viral hepatitis”, “acute cholecystopancreatitis”.
Clinical example:
A pregnant woman aged 27 (gravida 3, para 3) presented with complaints of nausea, vomiting, and pain in the right upper abdomen at 35 weeks’ gestation. On examination, skin and mucous membranes’ icterus, slight swelling. BP 140/90. The patient was admitted to the Central Hospital following arrival by ambulance. She was examined by a neurologist, urologist, infectious disease specialist, and gynecologist. Diagnosis: Pregnancy at 35 weeks’ gestation. Acute viral hepatitis? Acute cholecystopancreatitis? Moderate preeclampsia. The diagnosis of HELLP syndrome was made only in the postoperative period.
In this situation, a neurologist made a striking record: “noteworthy is an increase in blood magnesium level, which requires the discontinuation of magnesium sulfate infusion” (and this is in a pregnant woman with severe preeclampsia).
5. Errors in calculating blood loss volume followed by blood transfusion insufficient to compensate for the excessive loss of blood.
Clinical example:
A pregnant woman aged 34 (gravida 10, para 10). Bodyweight 94 kg. At 35-36 weeks gestation, she developed placental abruption with concurrent severe preeclampsia. BP 90/60. At 7.40 a.m., she was admitted to the hospital and at 7.53 a.m. underwent cesarean section and uterine amputation (Couvelaire uterus), Hb 62 g/l (initial Hb was 112 g/l). The estimated total blood loss was 1500 ml. In 13.07, she underwent re-laparotomy due to intra-abdominal bleeding (according to the surgery protocol “1500 ml of liquid blood in the abdominal cavity”). During the surgery, she went into cardiac arrest and died. The estimated total blood loss was 3000 ml. During these 5 hours from the admission to death, she was transfused with packed erythrocytes 460 ml, fresh frozen plasma 1200 ml, crystalloids 5300 ml, and 500 ml of 6% Venofundine. The pathologist reported that the cause of death was hemorrhagic shock and real blood loss was at least 4000 ml.
6. In the majority of the deceased with HELLP syndrome, no differential diagnosis was made to identify other forms of TMA amenable to specific therapy that can reduce mortality by a factor of ten, in particular for the atypical hemolytic-uremic syndrome (aHUS). In 2 patients, aHUS was diagnosed based only on clinical presentation, without further examination and appropriate therapy. Eculizumab was administered to 1 patient very late, on the 12th postoperative day (in this case, the diagnosis of TMA was not mentioned in her clinical record); the woman died on the 15th day from multiple organ failure. The activity of ADAMATS-13 to rule out thrombotic thrombocytopenic purpura was determined in only 1 patient.
7. Errors in treatment referral routing, lengthy transportation between primary, secondary, and tertiary care facilities when the antenatal fetal death occurs, and irreversible damage develops in maternal organs and systems.
Clinical example:
A pregnant woman aged 20 with chronic pyelonephritis. Gravida 2, para1. She was observed at a midwifery station since 9 weeks. At 22 weeks, she suffered headaches for 2 days and took citramon without effect; she had weakness, dizziness, swelling, and a paramedic was called. 07/11/2017, at 7.10 a.m., at the time of the paramedic arrival, she developed clonic convulsions and was administered 16 ml of magnesium sulfate intravenously. At7.20 a.m. the paramedic reported by telephone to the EMS on-call doctor. The EMS team departed at 7.45 and 8.05 arrived on the scene. The patient had “convulsive readiness,” BP was 145/100, and the fetal heartbeat was absent. The following measures were taken: a gastric tube was introduced, gastric lavage was performed, a peripheral venous catheter was placed, urinary bladder catheterization, sublingual nifedipine 10 mg, relanium 10 mg, magnesium sulfate 6 ml/hour. Under intravenous anesthesia, the patient was intubated and placed on mechanical ventilation. At 9.15 (after 2 hours!), she was transported to the intensive care unit of the Central District Hospital. The examination was performed (urine, blood, ECG, fetal ultrasound, abdominal ultrasound, kidney ultrasound, chest x-ray, ophthalmologist, neurologist, surgeon consultations). “In connection with the severity of the condition, it was decided to transfer the patient to a tertiary care facility by medical air transport and at 12.50, she was admitted to the republican hospital. At 13.03-13.48 (after 6 hours!) she underwent cesarean section. She died on the 2nd day after surgery (stroke, cerebral edema, multiple organ failure).
8. Anesthesia complications: bilateral perforation of the subclavian vein with a parietal pleural injury resulting in a massive 1750 ml mediastinal hematoma, which was not diagnosed during life.
Clinical example:
A primigravida aged 22 years was found to have a blood pressure of 140/90 at 34 weeks and was hospitalized with a diagnosis of gestational hypertension. At 37 weeks, she was admitted urgently to a hospital with blood pressure 180/105, proteinuria 6.6 g/l, and anuria. Ultrasound revealed a retroplacental hematoma. At 0.30 a.m., she underwent a cesarean section with hysterectomy due to bleeding and the formation of Couvelaire uterus. Total blood loss of 1500 ml. According to the record in her delivery note: “Given the difficulty in accessing the peripheral veins, catheterization of the central vein was performed.” At 15.00, the patient developed a hemodynamic collapse. Echocardiography revealed impaired left ventricle contractility, valvular insufficiency (aortic insufficiency I, mitral insufficiency II, pulmonary valve insufficiency I, tricuspid insufficiency I). Chest radiography showed “expansion of the upper third of the mediastinum, mediastinitis.”. The patient underwent re-laparotomy with abdominal organs’ exploration, but no source of bleeding was found in the abdominal cavity. Hb 26 g/l, RBC count 0.41×1012, platelets 37×109 (clearly inadequate infusion-transfusion therapy!). A day later, the patient died from hemorrhagic shock. The massive mediastinal hematoma was found postmortem.
A thorough analysis of all cases of maternal deaths made it possible to formulate and substantiate several recommendations aimed at reducing maternal mortality due to PE, eclampsia, and their complications:
1. An accurate diagnosis of hypertension and a reliable assessment of its control are of uppermost importance, including in the first trimester of pregnancy, when normally at 10-14 weeks there is a maximum decrease in TPR and blood pressure (to assess mean blood pressure it is necessary to take several BP measurements during 15-20 minutes and calculate the mean value). Blood pressure higher than 120/80 mm Hg cannot be considered normal. In this, we agree with our foreign colleagues, in particular, with the new 2017 High Blood Pressure Clinical Practice Guideline of the American College of Cardiology and the American Heart Association. They classify blood pressure of 120–129/80 mm Hg as elevated and 130-139/80-89 mm Hg as Stage 1 arterial hypertension, which, in the presence of risk factors, requires antihypertensive drug therapy [6];
2. Careful continuous monitoring of blood pressure in patients with preeclampsia to prevent a sharp rise in blood pressure. Antihypertensive therapy should be preventative (not after a rise in blood pressure, but before it and taking into account the drug half-life);
3. Drug-induced sharp fall in blood pressure should be avoided; its decrease should be gradual to maintain perfusion of the maternal organs and placenta in the antiangiogenic state with preeclampsia. Blood pressure monitoring and a reliable assessment of its control in many respects depend on the in-hospital emergency medical service;
4. Patients with pre-eclampsia and eclampsia are at the highest risk of stroke during delivery and on day 1 postpartum. It is necessary to predict the development of stroke in these periods in patients with severe hypertension, thrombocytopenia, congenital and acquired thrombophilia, cerebrovascular malformations, as well as with the development of the TMA;
5. Continued vigilance for preeclampsia should be exercised in women presenting with new-onset symptoms of the disease in the perinatal period (from 22 weeks);
6. Of utmost importance is an urgent multidisciplinary approach in the management of patients with severe forms of preeclampsia, eclampsia, and their complications when a prompt consultation can be initiated with all appropriate specialists including:
- a hematologist (in risk groups and for any hematological abnormalities, in all women with preeclampsia);
- a neurologist (all patients with preeclampsia, eclampsia and HELLP syndrome);
- nephrologist, ophthalmologist, and others;
- all patients admitted with suspected stroke must undergo a CT scan or MRI of the brain;
7. In patients with clinical manifestations of HELLP syndrome (microangiopathic hemolytic anemia, thrombocytopenia, microthrombosis, and multi-organ dysfunction), additional diagnostic tests can be performed to exclude other diseases and complications that may have clinical features of TMA.
In particular, early diagnosis of aHUS and specific therapy that blocks the formation of the end product of excessive activation of the complement system (the membrane attack complex) can reduce mortality from 90% to 10% [7, 8].
Platelet transfusions are contraindicated in thrombotic thrombocytopenic purpura because of an increased risk of microthrombosis, which may contribute to the worsening of the course of the diseases;
8. Improving the knowledge of doctors of related specialties regarding preeclampsia and its complications;
9. Introduction and use of advanced tools for predicting and early diagnosis of placental disorders and preeclampsia. Testing for the first-trimester maternal serum PIGF, free β-hCG, PAPP-A, PP-13, uterine artery Doppler allows for the prediction of preeclampsia before 37 weeks’ gestation with an accuracy of 76.6% [9];
10. Women considered to be at high risk of preeclampsia should be referred for obstetric care and delivery to tertiary-care centers.
Conclusion
A thorough analysis of all cases of maternal deaths made it possible to formulate and substantiate several recommendations aimed at reducing maternal mortality due to PE, eclampsia, and their complications.
References
- Филиппов О.С., Гусева Е.В., Малышкина А.И., Михайлов А.В., Крутова В.А., Зубенко Н.В.? и др. Материнская смертность в Российской федерации в 2017 году. Методическое письмо Министерства здравоохранения Российской федерации № 15-4/10/2-7236 от 07.11.2018. 90 с. [Filippov O.S., Guseva E.V., Malyshkina A.I., Mikhailov A.V., Krutova V.A., Zubenko N.V. i dr. Materinskaya smertnost’ v Rossiiskoi federatsii v 2017 godu / Metodicheskoe pis’mo Ministerstva zdravookhraneniya Rossiiskoi federatsii № 15-4/10/2-7339 ot 23.10. 2018. 90 p. (in Russ)].
- Ennaqui K., Makayssi A., Boufettal H., Samouh N. Haemorrhagic stroke of the brainstem secondary to postpartum eclampsia: about a case and literature review. Pan Afr Med J. 2017; 27: 266. doi: 10.11604/pamj.2017.27.266.12288
- Lanska D.J, Kryscio R.J. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000; 31(6): 1274–82.
- Ban L., Sprigg N., Abdul Sultan A., Nelson-Piercy C., Bath P.M., Ludvigsson J.F., Stephansson O., Tata L.J. Incidence of First Stroke in Pregnant and Nonpregnant Women of Childbearing Age: A Population-Based Cohort Study From England. J Am Heart Assoc. 2017; 6(4). pii: e004601. doi: 10.1161/JAHA.116.004601
- Cipolla M.J. The adaptation of the cerebral circulation to pregnancy: mechanisms and consequences. J Cereb Blood Flow Metab. 2013; 33(4): 465–78. doi: 10.1038/jcbfm.2012.210
- Whelton P.K., Carey R.M., Aronow W.S., Casey D.E. Jr., Collins K.J., Dennison Himmelfarb C., et al. 2017ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018; 71(6): 1269–1324. doi: 10.1161/HYP.0000000000000066
- Mancini A., Ardissino G., Angelini P., Giancaspro V., La Raia E., Nisi M., et al. HELLP syndrome and hemolytic uremic syndrome during pregnancy: two disease entities, same causation. Case report and literature review. G Ital Nefrol. 2019; 36(2). pii: 2019-vol2. 1. [Article in Italian].PMID: 30983177
- Макацария А.Д., Бицадзе В.О., Хизроева Д.Х., Акиньшина С.В. Тромботические микроангиопатии в акушерской практике. М.: ГЭОТАР-Медиа; 2017. 304 с. [Makatsariya AD, Bitsadze VO, Khizroeva D.Kh., Akinshina S.V. Thrombotic microangiopathies in obstetric practice. M.: GEOTAR-Media; 2017. 304 p. (in Russian)].
- Rolnik D.L., Wright D., Poon L.C.Y., Syngelaki A., O’Gorman N., de Paco Matallana C., et al. ASPRE trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol. 2017; 50(4): 492–5. doi: 10.1002/uog.18816
Received 18.06.2019
Accepted 21.06.2019
About the Authors
Iraida S. Sidorova, MD, Professor, Academician of RAS, Honoured Scientist of the Russian Federation, I.M. Sechenov First Moscow State Medical University, Institute of Clinical Medicine, Department of Obstetrics and Gynaecology № 1. E-mail: sidorovais@ yandex.ru119991, Russia, Moscow, Trubetskaya str. 8, bld. 2.
Natalya A. Nikitina, MD, Professor of the Chair of Obstetrics and Gynaecology № 1, I.M. Sechenov First Moscow State Medical University, Institute of Clinical Medicine, Chair of Obstetrics and Gynaecology № 1. E-mail: natnikitina@list.ru
119991, Russia, Moscow, Trubetskaya str. 8, bld. 2.
Yelena V. Guseva, Deputy Director of the Department - Head of the Department of Reproductive Health and the Implementation of Effective Obstetric and Gynecological Assistance of the Department of Medical Care for Children and Maternity Services of the Ministry of Health of Russia. Phone: +7(495)627-29-00
Address: 127994, Russia, GSP-4, Moscow, Rakhmanovsky per., 3.
For citation: Sidorova I.S., Nikitina N.A., Guseva E.V. The results of a confidential audit of maternal mortality due to preeclampsia and eclampsia in Russia in 2017–2018.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020;1: 119-27. (In Russian).
https://dx.doi.org/10.18565/aig.2020.1.119-127



