Characteristics of CACNA1C protein expression levels in the round ligaments of the uterus in patients with genital prolapse
Cheremin M.M., Smolnova T.Yu., Krasnyi A.M., Sadekova A.A., Chuprynin V.D.
Genital prolapse (GP) is a multifactorial disease that is influenced by various factors such as childbirth, obstetric trauma, age, and increased intra-abdominal pressure. However, not all individuals with these factors develop GP, highlighting the importance of gene and protein expression levels for understanding the underlying cause.
Objective: This study aimed to investigate the expression of the α1C subunit of the CaV1.2 calcium channel in the round ligaments of patients with GP.
Materials and methods: This study included 61 patients, with 31 in the GP group (group I, study group) and 30 with other gynecological conditions (group II, control group). The mean age of the patients was 55.6 and 46.5 years, respectively. In group I, 12/31 (38.7%) patients had grade II–III uterine and vaginal wall prolapse, whereas 15/31 (48.38%) and 4/31 (12.9%) patients had incomplete and complete GP, respectively. The control group did not have GP. The level of α1C protein expression in the round ligaments of the uterus was examined using Western blot.
Results: Group I exhibited lower α1C protein expression (3.034 [0.8108; 4.040] RU) than group II (4.098 [2.508; 6.543] RU) (p=0.0045). Reduced α1C protein expression in group I was associated with a sarcopenic phenotype, including muscle wasting and asthenia in 9/31 (29%), myopia in 7/31 (22%), hypotension in 16/31 (51%), constipation in 17/31 (54%), phlebopathy in 11/31 (35%), pelvic floor protrusion and relaxation in 16/31 (51%), apical GP in 24/31 (77%), hypermobility in 24/31 (77%), and flat feet in 15/31 (48%) patients.
Conclusion: Patients with GP had lower α1C protein expression than those in the control group. The low expression of α1C protein is indicative of a clinical symptom complex of connective tissue dysplasia (CTD), which aligns with the concept of a "sarcopenic phenotype." In patients with CTD and a sarcopenic phenotype, the labor process is associated with low α1C protein expression levels, which contributes to the development of GP. The specific form of GP (apical) is influenced by alterations in the calcium channel function, resulting in decreased α1C protein expression.
Authors' contributions: Cheremin M.M. – review of the relevant publications; Cheremin M.M., Smolnova T.Yu. – conception and design of the study; Cheremin M.M., Krasny A.M., Sadekova A.A. – obtaining data for analysis; Cheremin M.M.,
Smolnova T.Yu. – statistical analysis, drafting of the manuscript; Smolnova T.Yu., Krasny A.M., Chuprynin V.D. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Cheremin M.M., Smolnova T.Yu., Krasnyi A.M., Sadekova A.A., Chuprynin V.D. Characteristics of CACNA1C protein expression levels in the round ligaments of the uterus in patients with genital prolapse.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (7): 87-95 (in Russian)
https://dx.doi.org/10.18565/aig.2024.147
Keywords
The voltage-gated L-type calcium channel encoded by the pore-forming α1 subunit CaV1.2 (CACNA1C) is widely expressed in various body tissues, including the heart, brain, smooth muscle, endocrine system, and immune system. Impairment in its expression can lead to a range of clinical manifestations [1]. Calcium channels play a crucial role in cell function by facilitating the transport of Ca2+ ions into and out of the cell, thereby influencing processes such as excitation, rest, and cell function [2].
The activation of calcium channels is responsible for contraction of the myocardium, smooth muscles (such as those in the intestines, uterus, and blood vessels), striated (skeletal) muscles, pacemaker activity of cells in the cardiac conduction system, release of mediators by nerve cells, and secretion of enzymes and hormones [3]. Certain pathogenic variants of the CACNA1C gene, which encodes the L-type Ca2+ channel, can cause a multisystem disorder known as the Timothy syndrome. This disorder includes severe Long QT Syndrome (LQTS), congenital heart disease, dysmorphic facial features, syndactyly, abnormal immune function, and neuropsychiatric disorders [4]. The role of α1C in mental disorders, such as bipolar disorder, schizophrenia, and autism has also been identified [5]. Additionally, studies have shown a correlation between α1C gene expression and various types of cancer, including brain tumors, leukemia, and breast cancer [6].
In the field of obstetrics and gynecology, calcium channel dysfunction can play a significant role in the development of clinical conditions such as preterm labor and birth, abnormal labor activity, congenital cardiomyopathies, cardiac dysrhythmias in neonates, and arterial hypertension during pregnancy, delivery, and the postpartum period.
Our study in 2019 revealed the involvement of calcium channels in the development of genital prolapse (GP) [7]. Specifically, we hypothesized that one form of GP may involve the smooth muscle component [7, 8].
In addition to a reduction in the collagen component, relaxation and decreased tone of the smooth muscle in the central part of the pelvic diaphragm may contribute to the development of GP [7]. This hypothesis is based on observations that patients with the apical form of GP exhibit systemic smooth muscle "myopathy" (including organ diverticula, signs of venous phlebopathy, early myopia, constipation, and the need for manual assistance with defecation as well as primary and secondary labor dystocia) [7].
Therefore, the objective of our study was to further investigate the expression of the α1C protein in the round ligament of the uterus in patients with GP and clinical manifestations of generalized smooth muscle "relaxation".
Materials and methods
The study was conducted at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, and included 61 women aged 40–70 years (median age 53 (49.3; 60.8) years). All patients were divided into the study group (n=31, group I), including patients with GP, and 30 patients with other gynecological conditions (group II, control group). The inclusion criteria were age 40–70 years, presence of GP, informed consent for inclusion in the study, and absence of severe extragenital pathology. The exclusion criteria were age under 40 and over 70 years, oncological diseases, infectious diseases, mental disorders, and lack of informed consent.
The study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P.
The main surgical interventions for patients with GP were laparoscopic (LS) mesh hysteropexy, 10/31 (32.3%), LS mesh cervicopexy, 2/31 (6.5%), and LS mesh colpopexy, 2/31 (6.5%). These operations were supplemented by hysterectomy in 4/31 (12.9%) patients, MacCall procedure in 10/31 (32.3%) patients, and rectopexy with and without implants in 4/31 (12. 9%); sigmopexy with and without implant in 2/31 (6.5%); vaginal approach: anterior, posterior colporrhaphy, and perineolevatoroplasty in 20/31 (64.5%); Lefort–Neugebauer procedure in 1/31 (3.2%); and sphincteroplasty in 1/31 (3.2%). In the control group, surgical procedures using laparoscopic access for the underlying disease included hysterectomy, 3/31 (14.3%), myomectomy, 13/31 (61.9%), excision of endometriosis foci, 4/31 (19.1%), excision of adenomyosis foci, 2/31 (9.5%), oophorectomy, 4/31 (19.1%).
The level of α1C protein expression in round ligament tissue was studied using Western blot. Tissue samples measuring 0.5×0.5 cm from the round ligament were obtained during the laparoscopic stage of the operation to correct genital prolapse in patients in group I, as well as during laparoscopic operations during hysterectomy for various gynecological conditions (recurrent endometrial pathology, large uterine fibroids, diffuse nodular form of adenomyosis, etc.) in group II patients in the Department of Surgery of Kulakov NMRC for OG&P. Samples were immediately frozen in liquid nitrogen and stored at -80°C.
Before Western blot analysis, each sample was lysed on ice for 30 min with a solution of RIPA buffer (Pierce, USA) with the addition of protease inhibitors (Roche Applied Science, USA) until a homogeneous mass was obtained and then centrifuged at 13,000 rpm at 4°C for 10 min. The protein concentration of the sample was determined using the Bradford method with BSA as the protein standard using the BCA Quick Start Bradford Protein Assay kit (Bio-Rad, USA). Twenty micrograms of total protein and markers were loaded into the wells of a mini-SDS-PAGE gradient gel (5-20%) and separated electrophoretically, with visual monitoring of the separation front for markers. The proteins on the gel were then transferred to a PVDF membrane (Bio-Rad, USA) using a wet transfer system, according to the manufacturer's protocol (Bio-Rad, USA). The resulting membranes were blocked with 5% skim milk (Bio-Rad, USA) in phosphate-buffered saline with 0.5% Tween 20 (PBST) at 4°C overnight. Membranes were incubated with primary antibodies against α1C (Invitrogen, USA) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Invitrogen, USA) for 1.5 h at 37°C. The membranes were washed in PBST and stained with horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, USA) for 1 h at room temperature. Peroxidase activation for signal induction was performed using the Clarity Western ECL Substrate Kit (Bio-Rad, USA).
A ChemiDoc imaging system (Bio-Rad, USA) was used for detection, and the optical density of the bands was measured using Image Lab software (Bio-Rad, USA). The obtained data were normalized to GAPDH levels.
Statistical analysis
Continuous variables showing normal distribution were expressed as mean (M) and standard deviation (SD) and presented as M (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. Statistical significance of differences was determined using the parametric Student's t-test, assuming a normal distribution as determined by the Shapiro–Wilk test and equality of variances as determined by the Levine test. If these conditions were not met, the nonparametric Mann–Whitney test was used. Categorical variables are presented as counts and percentages and were compared using the chi-square or Fisher’s exact test. Differences were considered statistically significant at p<0.05. Statistical analysis and graph construction were performed using Compare2 and GraphPad Prism 8.4.3 software.
Results and discussion
The mean age of the patients was 56 (51; 61) and 52 (49; 54.5) years in the study and control groups, respectively (p=0.46). Body mass index (BMI) was 26.6 (24.8; 30.1) and 24.8 (21.9; 28.1) in groups I and II, respectively (p=0.173). There were no statistically significant differences between the groups in terms of age and BMI.
No GP were observed in the control group. Gynecological comorbidities in patients with GP were uterine fibroids in 6/31 (19.4%) and adenomyosis and ovarian cysts in 2/31 (6.5%) and 2/31 (6.5%) patients, respectively.
Gynecologic pathology in group II patients was represented by uterine fibroids in 21/30 (70%), diffuse nodular adenomyosis in 16/30 (53.3%), extragenital endometriosis in 7/30 (23.3%), endometrial pathology in 6/30 (20%), and ovarian cysts in 6/30 (20%).
Considering the similar ages of the patients, there were no differences in extragenital comorbidities. The only exceptions were diseases that could be considered in the context of connective tissue phenotype. Thus, in group I, myopia occurred in 18/31 (58.1%) women (in group II in 3/30 (10%)) (p<0.0001); vegetative-vascular dystonia of the hypotonic type at a young age in 16/31 (51.6%) (in group II in 3/30 (10%)) (p=0.001); chronic tonsillitis in 13/31 (42%) patients (in group II – in 6/30 (20%)) (p=0.097); varicose veins of the lower extremities and hemorrhoids in 11/31 (35.5%) (in group II in 9/30 (30%) and 1/30 (3.3%), respectively (p=0.786) (Fig. 1).
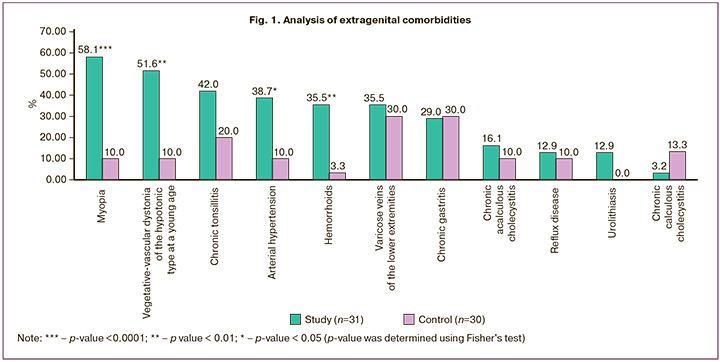
These findings are consistent with the morbidity associated with connective tissue dysplasia (CTD) [9].
All the patients in the study and control groups had a history of childbirth. The mean number of births per patient was 1.8 and 1.6 in groups I and II, respectively. In group I, 10/31 (32.3%) patients had a history of one birth and 21/31 (67.7%) had two or more births; in group II – 13/30 (43.35) and 17/30 (56.7%) women, respectively. The difference in the number of births between groups I and II was not statistically significant (p=0.434). However, PG developed only in the patients in group I. A high frequency of previous births does not always lead to PG development. Thus, according to the literature, in Asian and African countries, where women have an average of 5–8 births, GP occurs four times less frequently than in European countries, where the average number of births per woman does not exceed 1.8 [10–12]. The development of GP in nulliparous patients does not always fit the context of birth trauma [13, 14].
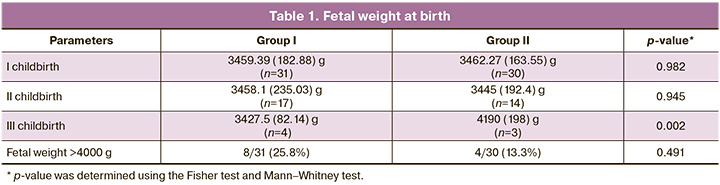
There were no significant differences in the birth weights of the first and second births in either group. The groups differed significantly only in the birth weight of newborns at the third birth. In group II, fetal weight was 1.2 times higher than that in group I (p=0.002) (Table 1). Despite this, no cases of ptosis or prolapse of the genital organs were observed in group II. This does not contradict the data in the domestic literature, which describes the formation of GP in patients within 1 year after the first uncomplicated birth with a fetus weighing up to 3400 g [15]. In addition, the birth of children with lower body weight, which is typical for patients with CTD, also casts doubt on birth trauma as a cause of GP development [16–18].
Perineal injuries in group I included 5/31 (16.1%) cases of grade I-II spontaneous perineal rupture and 10/31 (32.3%) cases of episiotomy; in group II they included 4/30 (13.3%) and 2/30 (6.6%) cases, respectively. There were no significant differences in spontaneous perineal ruptures between the groups (p>0.892).
The mean duration of the first labor in the study and control groups did not differ and was 9 (4.3; 12) and 9 (8; 14.3) hours, respectively, and the duration of the second labor was 7 (4; 9) and 7 (7; 10) hours, respectively, which did not differ from the characteristics of the course of labor in the population. However, in the group of patients with GP, 10/31 (32.3%) women had a tendency to increase the frequency of rapid and precipitous labor compared to 1/30 (3.3%) in group II (p=0.006), which does not contradict the data of other authors [19].
Despite the lack of differences in parity and birth injuries in the study groups, GP occurred in group I. Thus, in patients of group I, after a history of a single birth in history, GP was detected in 10/31 (32.3%) patients (after 16 (6; 18) years), after the second birth – in 17/31 (54.8%) (after 10.5 (4.5; 17.5) years), after the third – in 4/31 (12.9%) patients (after 8 (2; 10) years).
In some cases, GP is hereditary. Thus, heredity for GP was observed in 9/31 (29%) women in group I, whereas in group II, GP in relatives was not reported (p=0.007). The results obtained do not contradict those of other authors [15, 20, 21].
The apical form of GP in group I was represented by grade III uterine prolapse in 5/31 (16.1%) patients, incomplete uterine and vaginal wall prolapse in 15/31 (48.4%), and complete prolapse in 4/ 31 (12.9%) women.
In group I, 7/31 (22.6%) patients had local forms of vaginal wall prolapse in the form of grade II–III cysto- and rectocele.
In the study group, GP (apical form) was diagnosed at an average age of 42 (36.5; 47.3) years, with local forms of vaginal wall prolapse at an average age of 46 (34; 51.5) years. Of the apical forms, grade III uterine prolapse occurred at a relatively young age (49 (40.5; 56.5) years) and progressed to complete uterine and vaginal wall prolapse at 55 (51; 61) and 61.5 (58.8; 65.8) years, respectively. If the apical form of prolapse did not exceed 16.1% at the age of 49 years, it reached 61.3% at the age of 62 years. The average age at the time of surgery for apical prolapse was 56.5 (49.5; 61) years and for local prolapse 55 (51; 58) years, respectively.
Thus, the apical form of GP develops directly as an independent form and is not a consequence of the progression of local forms but only progresses with time. It is known that if GP is present in patients for > 5 years, the risk of recurrence after surgical correction increases by a factor of 3 [22].
Local forms do not progress so quickly, which is confirmed by the fact that patients with local forms of GP underwent surgery after an average of 16–18 years (after 12 years in apical forms).
The hereditary nature of the pathology may also be indicated by the CTD symptom complex. In addition to the above (myopia, varicose veins, etc.), the following clinical manifestations of CTD were noteworthy: joint hypermobility (JHM), dysfunction of the anorectal part of the pelvic diaphragm in the prenatal history (complaints of constipation, manual assistance, etc.), minor developmental anomalies, including according to echocardiographic data.
JHM was found in 24/31 (77.4%) patients in group I and 8/30 (26.7%) in group II (p<0.0001). If the population average JHM index score on the Beighton scale is 2.1 points, then this score in patients with GP was 4.4 (2.37) points compared to 2.0 (1.31) points in group II.
The most common complaints among patients in group I were from the anorectal part of the pelvic diaphragm, which reached 35% compared with 10% in group II (Table 2). Thus, complaints of a foreign body sensation in the vagina and a feeling of incomplete defecation were reported by 20/31 (64.5%) and 6/31 (19.4%) women, respectively; constipation by 17/31 (54.8%) patients; gas incontinence by 8/31 (25.8%); manual assistance during defecation by 11/31 (35. 5%) women in group I. In group II, the main complaints were infertility 2/30 (6.7%), lower abdominal pain 14/30 (46.7%), menorrhagia 18/30 (60%), recurrent endometrial pathology 4/30 (13.3%), growth of myomatous nodules 9/30 (30%), and constipation only in 3/30 (10%).
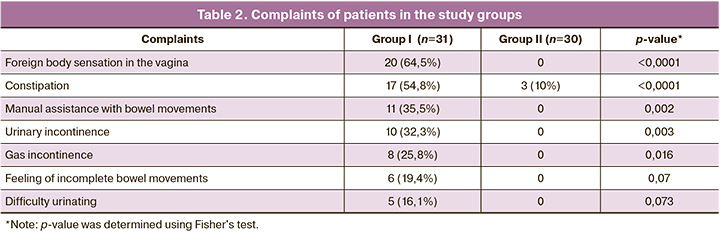
In group I, urinary incontinence was observed in 10/31 (32.3%) patients and difficulty in urinating in 5/31 (16.1%) patients (Table 2).
Anorectal symptoms appear to be due to GP; however, it was previously shown that the frequency of these symptoms in nulliparous primigravida with CTD syndrome did not differ from that in parous patients with GP [14]. Therefore, it was concluded that these symptoms were more likely to reflect pelvic floor dysfunction than to be a consequence of GP [14].
The high incidence of chronic gastritis, 9/31 (29%), and urolithiasis 4/31 (12.9%) in group I compared to group II could be due to the presence of latent gastro- and nephroptosis, as well as reflux esophagitis secondary to hiatal hernia.
CTD syndrome in women is characterized by flat feet in addition to joint hypermobility, skin elastosis, scoliosis, etc. [9]. The podometric index (PMI) of the feet was studied. Flat feet were found 3 times more often in group I (15/31 (48.4%)) than in group II (5/30 (16.7%)) (p=0.008). In the study group, the PMI was 27.48 (2.08), corresponding to grade 1–3 flatfoot, and in group II it was 28.98 (0.84), corresponding to a normal variant. This indicator was inversely correlated with the age of the patients (-0.509; p=0.01) and the presence of GP. The younger the age at which the flatfoot was detected, the more severe the form of the GP, which is consistent with the literature data [14, 23].
Hand dynamometry in group I showed a decrease in strength to 25.5 (23.75; 27) daN compared to group II – 30 (28.5; 31) daN (p<0.0001) and was directly correlated with flat feet (0.536; p=0.01). These phenotypic manifestations confirmed the "sarcopenic" phenotype.
In 2018, in patients with GP with low levels of α1C expression, we also showed such manifestations of the "sarcopenic phenotype" as asthenic (hypotrophic) body type (30%), muscular asthenia (29%), refractive error since childhood (22.2%), arterial hypotension (60%), functional isthmic-cervical insufficiency during pregnancy (23%), rapid and precipitous labor (37.6%), constipation (55%), varicose veins (39.5%), protrusion and relaxation of the pelvic floor (51%), apical forms of GP (60%), increased GMS (48.8%), grade 2 scoliosis (33%), grade 3 flatfoot (73.8%), hypotonic intestinal dysfunction (50–60%), and the presence of apical forms of GP [3]. It has been shown that the "sarcopenic" phenotype is characteristic of patients with GP, especially with apical forms [2, 3].
Calcium channels CaV1.2 are present throughout the smooth muscle system and are the main structure that forms the Ca2+ channel. Decreased tone and relaxation of the smooth muscle component of the central part of the pelvic diaphragm due to dysfunction of Ca2+ channels may play an important role in the development of GP [7, 8].
All patients with GP in this study underwent comparative analysis of α1C protein expression. In patients with GP (group I), the level of α1C protein expression was lower than that in the control group: 3.034 (0.8108; 4.040) RU compared to 4.098 (2.508; 6.543) RU (p=0.0045) (Fig. 2).
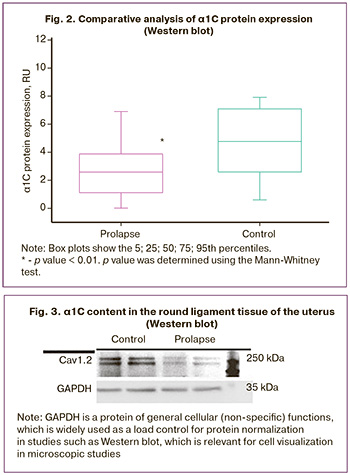
On the membrane, the α1C protein was detected as two bands of approximately 240 and 260 kDa (Fig. 3). We assumed that the 260 kDa protein was mature (glycosylated). The α1C expression was calculated as the sum of the two bands.
Considering that patients with GP have a symptom complex of the "sarcopenic phenotype", it is likely that local changes in microcirculation can also trigger the development of the initial forms of GP [24].
Impaired tone of the vascular wall was also confirmed by varicose veins in the lower extremities, which occurred in 35.5% of patients in group I in our study [3]. Our previous studies (laser Doppler) at the microcirculatory level showed that patients with GP are characterized at the systemic level by a hyperemic (stagnant) type of microcirculation with compensatory pacemaker activation of vascular tone [25, 26]. Therefore, the study of hemodynamics during echocardiography in relation to the characteristics of the microcirculation in patients with GP and the level of α1C protein expression is of particular interest.
Echocardiography was performed in all patients with low levels of α1C protein expression (Table 3).
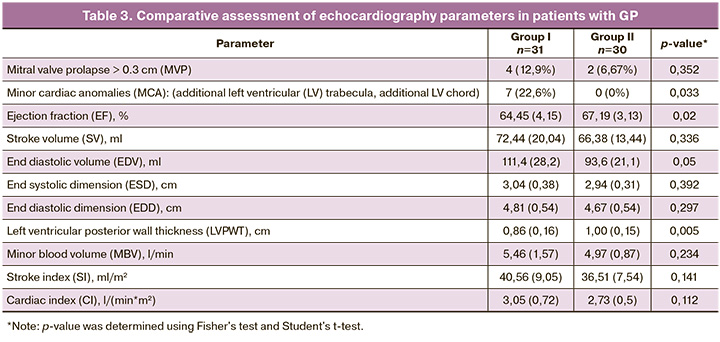
In the present analysis of echocardiograms in patients with GP, in addition to MVP and MCA, which are characteristic of CTD, there was a tendency towards a decrease in EF (p=0.02) and an increase in EDV (p=0.05) against the background of a thinner posterior wall of the LV (p=0.005), which confirms the "sarcopenic" phenotype in patients with GP.
Thus, a decrease in the expression of α1C protein in patients with CTD syndrome leads to changes in calcium channel function. This change not only results in the formation of a myasthenic phenotype but also affects the characteristic hemodynamics and hyperemic microcirculation. These mechanisms may serve as predictors of GP development.
These changes may reflect specific patterns of involvement of the stromal-muscular component, indirectly indicating the presence of a CTD.
Conclusions
- Patients with GP show a decrease in α1C protein expression compared to patients without GP.
- Patients with a low level of α1C protein expression exhibit a specific clinical symptom complex associated with CTD, which aligns with the concept of a "sarcopenic phenotype".
- The course of labor in patients with CTD and a sarcopenic phenotype is sometimes affected by a low level of α1C protein expression, indicating the potential formation of GP.
- In some cases, the apical form of GP can be attributed to a change in calcium channel function, resulting in a decrease in α1C protein expression.
References
- Zamponi G.W., Striessnig J., Koschak A., Dolphin A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015; 67(4): 821-70. https://dx.doi.org/10.1124/pr.114.009654.
- Смольнова Т.Ю., Красный А.М., Чупрынин В.Д., Садекова А.А.,Чурсин В.В., Тамбиева Ф.Р. Способ хирургической коррекции пролапса гениталий у пациентки со сниженным уровнем экспрессии гена CACNA1C в круглых связках матки. Акушерство и гинекология. 2020; 12: 234-41.[Smolnova T.Yu., Krasnyi A.M., Chuprynin V.D., Sadekova A.A., Chursin V.V., Tаmbieva F.R. Surgical procedure to correct genital prolapse in a patient with reduced CACNA1C gene expression in the round ligament of the uterus. Obstetrics and Gynecology. 2020; (12): 234-41. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.12.234-241.
- Смольнова Т.Ю., Красный А.М., Садекова А.А., Чупрынин В.Д. Роль экспрессии кальциевых каналов CAV1.2 в развитии некоторых патологических состояний в акушерстве и гинекологии. Акушерство и гинекология. 2020; 8: 5-11. [Smolnova T.Yu., Krasnyi A.M., Sadekova A.A., Chuprynin V.D. Role of CaV1.2 calcium channel expression in the development of some pathological conditions in obstetrics and gynecology. Obstetrics and Gynecology. 2020; (8): 5-11. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.8.5-11.
- Moon A.L., Haan N., Wilkinson L.S., Thomas K.L., Hall J. CACNA1C: association with psychiatric disorders, behavior, and neurogenesis. Schizophr. Bull. 2018; 44(5): 958-65. https://dx.doi.org/10.1093/schbul/sby096.
- Ehlinger D.G., Commons K.G. Cav1.2 L-type calcium channels regulate stress coping behavior via serotonin neurons. Neuropharmacology. 2019; 144: 282-90. https://dx.doi.org/10.1016/j.neuropharm.2018.08.033.
- Chang X., Dong Y. CACNA1C is a prognostic predictor for patients with ovarian cancer. J. Ovarian Res. 2021; 14(1): 88. https://dx.doi.org/10.1186/s13048-021-00830-z.
- Смольнова Т.Ю., Красный А.М., Чупрынин В.Д., Волгина Н.Е., Никитцева О.В. Влияние уровня экспрессии а-1 субъединицы потенциал-зависимого кальциевого канала Cav1.2 в гладкомышечной ткани у пациенток с пролапсом гениталий. В сборнике: XIII Международный конгресс по репродуктивной медицине 21-24 января 2019: 126-9. [Smolnova T.Yu., Krasnyi A.M., Chuprynin V.D., Volgina N.E., Nikittseva O.V. Effect of a-1 expression level subunit of voltage-dependent calcium channel Cav1.2 in smooth muscle tissue in patients with genital prolapse. In the collection: XIII International Congress on Reproductive Medicine January 21-24, 2019: 126-9. (in Russian)].
- Красный А.М., Озернюк Н.Д. Экспрессия генов, кодирующих субъединицы потенциал-зависимых Са2+-каналов L-типа в пролиферирующих и дифференцирующихся миобластах линии С2С12 мыши. Известия Российской академии наук. Серия биологическая. 2011; 3: 349-53. [Krasnyi A.M., Ozernyuk N.D. The expression of genes encoding the voltage-dependent L-type Ca2+ channels in proliferating and differentiating C2C12 myoblasts of mice. Izvestiya Rossiiskoi Akademii Nauk. Seriya biologicheskaya. 2011; (3): 349-53. (in Russian)].
- Клинические рекомендации Российского научного медицинского общества терапевтов по диагностике, лечению и реабилитации пациентов с дисплазиями соединительной ткани (первый пересмотр). Медицинский вестник Северного Кавказа. 2018; 13(1.2): 137-209. [Guidelines of the Russian Scientific Medical Society of Internal Medicine on the diagnosis, treatment and rehabilitation of patients with the connective tissue dysplasia (first edition). Medical News of North Caucasus. 2018; 13(1.2): 137-209.(in Russian)]. https://dx.doi.org/10.14300/mnnc.2018.13037.
- Åkervall S., Al-Mukhtar Othman J., Molin M., Gyhagen M. Symptomatic pelvic organ prolapse in middle-aged women: a national matched cohort studyon the influence of childbirth. Am. J. Obstet. Gynecol. 2020; 222(4): 356.e1-356.e14. https://dx.doi.org/10.1016/j.ajog.2019.10.007.
- Deeb M.E., Awwad J., Yeretzian J.S., Kaspar H.G. Prevalence of reproductive tract infections, genital prolapse, and obesity in a rural community in Lebanon. Bull. World Health Organ. 2003; 81(9):639-45.
- Marahatta R.K., Shah A. Genital prolapse in women of Bhaktapur, Nepal. Nepal Med. Coll. J. 2003; 5(1): 31-3.
- Gyhagen M., Al-Mukhtar Othman J., Åkervall S., Nilsson I., Milsom I.The symptom of vaginal bulging in nulliparous women aged 25-64 years: a national cohort study. Int. Urogynecol. J. 2019; 30(4): 639-47.https://dx.doi.org/10.1007/s00192-018-3684-5.
- Смольнова Т.Ю. Пролапс гениталий и дисплазия соединительной ткани. Клиническая и экспериментальная хирургия. Журнал им. акад. Б.В. Петровского. 2015; 2: 53-64. [Smolnova T.Yu. Women’s genital prolapse and connective tissue disease. Clin. Experiment. Surg. Petrovsky J. 2015; (2): 53-64. (in Russian)].
- Смольнова Т.Ю., Савельев С.В., Яковлева Н.И., Гришин В.Л., Барабанов В.М. Феномен генерализованной цитопатии у пациенток с опущением и выпадением внутренних половых органов как фенотипическое проявление синдрома дисплазии соединительной ткани на тканевом уровне. Медицинский вестник Северного Кавказа. 2008; 2: 44-9. [Smolnova T.Yu.,Savelyev S.V., Yakovleva N.I., Grishin V.L., Barabanov V.M. Phenomenon of generalized cytopathia in women with prolapse and loss of internal genitals - as phenotypical sign of the connective tissue dysplasia syndrome at the tissue level. Medical News of North Caucasus. 2008; (2): 44-9.(in Russian)].
- Смольнова Т.Ю., Адамян Л.В. Динамика фенотипических признаков синдрома дисплазии соединительной ткани в различные возрастные периоды. Актуальность проблемы в акушерстве и гинекологии. Акушерство и гинекология. 2013; 4: 74-9. [Smolnova T.Yu.,Adamyan L.V. Time course of changes in the phenotypic signs of connective tissue dysplasia at different ages: the urgency of the problem in obstetrics and gynecology. Obstetrics and Gynecology. 2013; (4): 74-9.(in Russian)].
- Жабченко И.А., Олешко В.Ф. Особенности течения беременности и родов, состояние плода и новорожденного у женщин с нарушениями обтурационной функции шейки матки при гестации. Охрана материнства и детства. 2016; 1(27): 5-9. [Zhabchenko I.A., Oleshko V.F. Peculiarities of pregnancy course and delivery, fetus and newborn condition in women with obstructive failure of cervix function. Maternal and child welfare. 2016; 1(27): 5-9. (in Russian)].
- Нечаева Г.И., Яковлев В.М., Конев В.П., Друк И.В., Морозов С.Л. Дисплазия соединительной ткани: основные клинические синдромы, формулировка диагноза, лечение. Лечащий врач. 2008; 2: 22-5. [Nechaeva G.I., Yakovlev V.M., Konev V.P., Druk I.V., Morozov S.L. Connective tissue dysplasia: the main clinical syndromes, diagnosis, treatment. Lechashchiy vrach. 2008; 2: 22-5. (in Russian)].
- Фотина Е.В., Закирова Р.Р., Алексеенкова М.В., Панина О.Б. Дисплазия соединительной ткани в генезе истмико-цервикальной недостаточности. Акушерство, гинекология и репродукция. 2021; 15(1): 41-50. [Fotina E.V., Zakirova R.R., Alekseenkova M.V., Panina O.B. Connective tissue dysplasia in the genesis of cervical incompetence. Obstetrics, Gynecology and Reproduction. 2021; 15(1): 41-50. (in Russian)]. https://dx.doi.org/10.17749/2313-7347/ob.gyn.rep.2020.131.
- Alcalay M., Stav K., Eisenberg V.H. Family history associated with pelvic organ prolapse in young women. Int. Urogynecol. J. 2015; 26(12): 1773-6. https://dx.doi.org/10.1007/s00192-015-2779-5.
- Samimi P., Jones S.H., Giri A. Family history and pelvic organ prolapse: a systematic review and meta-analysis. Int. Urogynecol. J. 2021; 32(4): 759-74. https://dx.doi.org/10.1007/s00192-020-04559-z.
- Richter H.E., Sridhar A.., Nager C.W., Komesu Y.M., Harvie H.S., Zyczynski H.M. et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Characteristics associated with composite surgical failure over 5 years of women in a randomized trial of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension. Am. J. Obstet. Gynecol. 2023; 228(1): 63.e1-63.e16. https://dx.doi.org/10.1016/j.ajog.2022.07.048.
- Смольнова Т.Ю., Адамян Л.В. Клинико-патогенетические аспекты опущения и выпадения внутренних половых органов при недифференцированных формах дисплазии соединительной ткани. Кубанский научный медицинский вестник. 2009; 6(111): 69-73. [Smol’nova T.J., Adamyan L.V. Clinico-pathogenetic respectives of genital prolapse in patients with nondifferencial connective tissue disease. Kuban Scientific Medical Bulletin. 2009; 6(111): 69-73.(in Russian)].
- Смольнова Т.Ю., Адамян Л.В., Сидоров В.В. Состояние микроциркуляторного русла у больных с пролапсом гениталий. В кн.: Кулаков В.И., Адамян Л.В., ред. Современные технологии в диагностике и лечении гинекологических заболеваний. М.: ПАНТОРИ; 2006: 170-2.[Smolnova T.Yu., Adamyan L.V., Sidorov V.V. State of the microvasculature in patients with genital prolapse. In: Kulakov V.I.,Adamyan L.V., ed. Modern technologies in the diagnosis and treatment of gynecological diseases. M.: PANTORI; 2006: 170-2.(in Russian)].
- Смольнова Т.Ю. Особенности гемодинамики и ее связь с некоторыми клиническими проявлениями у женщин при дисплазии соединительной ткани. Клиническая медицина. 2013; 10: 43-8. [Smol’nova T.Yu. Features hemodynamics and its relationship with some clinical manifestations in women with connective tissue dysplasia. Clinical Medicine. 2013; (10): 43-8.(in Russian)].
- Смольнова Т.Ю., Адамян Л.В., Сидоров В.В. Особенности микроциркуляции при опущении и выпадении внутренних половых органов у женщин репродуктивного возраста. Акушерство и гинекология. 2007; 1: 39-44. [Smolnova T.Yu., Adamyan L.V., Sidorov V.V. Microcirculatory features in descent and prolapse of the internal genitals in reproductive-aged females. Obstetrics and Gynecology. 2007; (1): 39-44. (in Russian)].
Received 24.06.2024
Accepted 05.07.2024
About the Authors
Mikhail M. Cheremin, Obstetrician-Gynecologist, PhD student, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,117997, Russia, Moscow, Ac. Oparin str., 4, mkhrznt@gmail.com, https://orcid.org/0000-0002-8600-068X
Tatyana Yu. Smolnova, Dr. Med. Sci., Senior Researcher at the Department of General Surgery, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, smoltat@list.ru, https://orcid.org/0000-0003-3543-651X
Alexey M. Krasnyi, PhD (Bio), Head of the Cytology Laboratory, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, a_krasnyi@oparina4.ru, https://orcid.org/0000-0001-7883-2702
Alsu A. Sadekova, Researcher at the Cytology Laboratory, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, a_sadekova@oparina4.ru
Vladimir D. Chuprynin, PhD, Head of the Department of Surgery, Academician V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia,
117997, Russia, Moscow, Ac. Oparin str., 4, v_chuprynin@oparina4.ru, https://orcid.org/0009-0003-7856-2863



