Amniotic fluid composition in pregnant women at high risk of preterm birth
Aim. To investigate the composition of the amniotic fluid in pregnant women at high risk of preterm birth.Khodzhaeva Z.S., Gorina K.A., Muminova K.T., Ivanets T.Yu., Kessler Yu.V., Priputnevich T.V., Belousov D.M.
Materials and methods. The study analyzed the composition of the amniotic fluid of 46 pregnant women aged 19 to 40 years who were at a high risk of preterm birth. The patients were categorized into two groups based on pregnancy outcomes, including 12 women with preterm birth (group I) and 34 women who had a full-term delivery (group 2). Amniotic fluid was collected using diagnostic transabdominal amniocentesis. The amniotic fluid analysis was carried out using hematological and biochemical automatic analyzers.
Results. The composition of the amniotic fluid differed statistically significantly between the study groups regarding the number of lamellar bodies (p=0.048), neutrophils (p=0.048), and total protein concentration (p=0.049). The presence of signs of an inflammatory process was associated with greater fetal lung maturity (r=0.33, p=0.046). Statistically significant correlations were found between the number of lamellar bodies and the cerebellum size (r=0.38, p=0.04), femur length (r=0.32, p=0.04), resistance index in the fetal MCA (r=-0.32, p=0.04).
Conclusion. Laboratory analysis of the amniotic fluid composition allows the detection of intra-amniotic inflammation and determination of the degree of fetal lung maturity, which is essential information for optimizing obstetric management.
Keywords
Amniotic fluid is the protective liquid that surrounds the fetus throughout gestation [1, 2]. AF contains nutrients and growth factors that facilitate fetal growth, provide mechanical cushioning, and antimicrobial effectors that protect the fetus [3]. AF is also used as a diagnostic substrate for assessing the karyotype and fetal status [4], fetal lung maturity [5–8], and is a useful marker for intraamniotic inflammation [9, 10].
Amniotic fluid contains soluble and cellular components [3, 11]. The soluble components include carbohydrates, proteins, peptides, lipids, electrolytes, enzymes, etc. [12]. The cellular components of the amniotic fluid include different cell types derived from exfoliating surfaces of the developing fetus, including the skin, respiratory system, urinary tract, and gastrointestinal tract as well as stem cells [13, 14]. Some cytological studies have shown that, even in the absence of infection, the amniotic fluid also contains innate immune cells, including macrophages, neutrophils, and other corpuscular elements [10, 15, 16]. These immune cell counts are an essential diagnostic marker that may help determine the management strategy for a complicated pregnancy. Besides, amniotic fluid is an indicator for the validation of the accuracy of possible non-invasive predictors that have been actively sought due to advances in molecular biological research [8, 17]. Currently, due to concerns about changes in fetal programming, resulting in increased susceptibility to diseases in adulthood, more focus is directed towards the prevention of neonatal respiratory distress syndrome (RDS) in controversial clinical cases including borderline survival of newborns receiving repeated courses of corticosteroids, late preterm labor [18–21], etc. In these cases, evaluation of the degree of fetal lung maturity by lamellar body (LB) count in the amniotic fluid may help specify the indications for the prevention of RDS. Since LBs are the storage form of surfactant and have the same size as platelets, a hematology analyzer can be used to count the number of lamellar bodies [22]. Besides, the absence of inflammation markers in AF may help avoid the irrational use of antibiotic therapy. Therefore, the feasibility of assessing the AF composition to optimize obstetric care of pregnant women at high risk of developing preterm birth is relevant to be addressed.
The present study was aimed to investigate the composition of the amniotic fluid in pregnant women at high risk of preterm birth.
Materials and methods
This prospective study analyzed the composition of the amniotic fluid of 46 pregnant women aged 19 to 40 years who were at a high risk of preterm birth and were managed at the 1st Department of Obstetric Pathology of Pregnancy. The patients were categorized into two groups based on gestational age at delivery, including 12 women with preterm birth at 35.1 (1.5) weeks (group I) and 34 women who had a full-term birth at 38.6 (0.9) weeks (group II).
Diagnostic workup included an obstetric and gynecologic history, somatic comorbidities, physical examination, pregnancy course, peripheral blood count, infection control of the genital tract, ultrasound fetometry, and Doppler ultrasonography. Mode of delivery and neonatal outcomes were also analyzed.
The inclusion criteria for both study groups were singleton pregnancy and gestational age of 280–366 weeks. High risk was defined based on anamnestic data (history of spontaneous preterm birth, pregnancy loss in the second trimester), as well as clinical and echographic signs of a shortened cervical length.
All patients signed informed consent to take part in the study and undergo transabdominal amniocentesis for amniotic fluid sampling. The criteria for exclusion were multiple pregnancy, placenta previa, severe non-obstetric, and obstetric pathology. Also excluded from the study were patients with preterm rupture of membranes, contamination of amniotic fluid during sampling (with blood, meconium, etc.), symptoms of acute infectious and exacerbation of chronic diseases, and refusal to participate in the study. The study (protocol No. 9 of November 16, 2017) was approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
The amniotic fluid analysis was carried out using the XS-800i hematology analyzer (SYSMEX, Japan) and BA-400 automatic biochemistry analyzer (BioSystems, Spain). To avoid confounders to obtain reliable results, pre-analytical sample preparation was not performed, including the centrifugation stage.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 8.3 and IBM SPSS Statistics 22 software in compliance with the general recommendations for medical and biological research. Quantitative variables showing normal distribution were expressed as means and standard deviation; otherwise, the median and the quartiles Q1 and Q3 were reported. The distribution of continuous variables was tested for normality using the D'Agostino–Pearson test. Statistical analysis was performed using the Yates corrected Chi-square test for 2x2 contingency tables and the Fisher test, and the Student's t-test for independent samples. Variable not meeting normality assumptions were analyzed with the U-Mann-Whitney test. Diagnostic accuracy of the studied parameters was assessed by ROC analysis; the data are presented as an area under the curve with a 95% confidence interval (CI). Correlation between two quantitative variables was analyzed using the Pearson correlation coefficient. Differences between the groups were considered statistically significant at p<0.05.
Results
The study included 46 high-risk pregnant women with intact fetal membranes. The patients were categorized into two groups based on gestational age at delivery (preterm and full-term birth). They were no statistically significant differences between the groups regarding age, gestational age at the inclusion in the study, body weight, height, and body mass index. The clinical characteristics of the patients are presented in the Table 1.
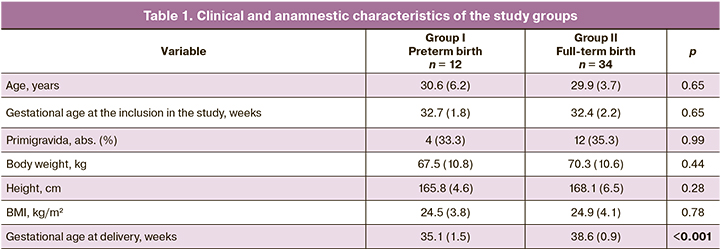
To identify potential predictors of preterm birth, a detailed comparative analysis of the amniotic fluid composition was carried out. The results are presented in the Table. 2.
The composition of the amniotic fluid differed statistically significantly between the study groups regarding the lamellar body counts (p = 0.048), neutrophil counts (p = 0.048), and total protein concentration (p = 0.049). ROC analysis was performed to identify the neutrophil count threshold level for predicting preterm birth (Fig. 1). A cutoff value of neutrophil count >64.2% was associated with full-term birth with a sensitivity of 91.7% and a specificity of 67.6%. Area under the curve (AUC) was 0.69 [95% CI 0.53 - 0.86; p = 0.047].
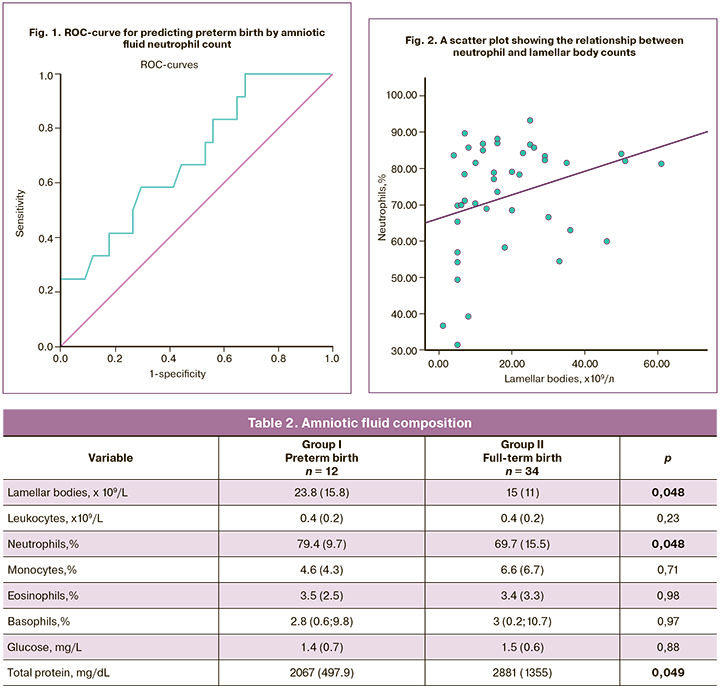
To evaluate the association between the inflammatory process and fetal lung maturity, Pearson correlation analysis was performed between neutrophil and lamellar body counts, which showed a positive correlation (r = 0.33, p = 0.046). This observation may indicate the potentiating effect of inflammation on the production of surfactant by type II pneumocytes, which is consistent with the published evidence [23]. Figure 2 shows the relationship between neutrophil lamellar body counts.
To identify non-invasive predictors of fetal maturity, we carried out a correlation analysis between the lamellar body count and the results of ultrasound fetometry and Doppler ultrasonography (Table 3).
We also carried out a Pearson correlation analysis of the same parameters of amniotic fluid and maternal peripheral blood. We analyzed the counts of leukocytes (r = 0.26, p = 0.09), neutrophils (r = -0.27, p = 0.07) and a serum glucose level (r = 0.27, p = 0.07), which showed no statistically significant correlations.
At the same time, there was a correlation between leukocyte counts in maternal AF and the peripheral blood of preterm newborns in the group of preterm birth (r = 0.63; p = 0.03). However, no such statistically significant relationships were seen in full-term newborns (Table 4).
Discussion
Amniotic fluid neutrophils can be considered as a predictor of preterm birth. Their increase indicates the development of this complication (p = 0.048). The total protein level of (p = 0.049) can be regarded as a less significant marker. A decrease in the amniotic fluid glucose concentration is known to be an indirect marker of intraamniotic infection [9, 23], but in our study, there was no statistically significant difference. This fact is most likely due to the absence of intraamniotic microbial invasion according to the data of an extensive microbiological study, which was carried out in parallel withthebiochemicalanalysisofamnioticfluid. Assessment of fetal lung maturity based on the measurement of LB count is a cost-effective and effective method of objectifying clinical information. Our findings suggest that the presence of signs of an inflammatory process is associated with greater fetal lung maturity (r = 0.3; p = 0.046). Ultrasound criteria such as cerebellar size (r = 0.38; p = 0.04), femur length (r = 0.32; p = 0.04), fetal MCA resistance index can be considered as non-invasive predictors of fetal lung maturity (r = -0.32; p = 0.04). No significant associations were found between markers of inflammation (leukocytes, neutrophils) in amniotic fluid and peripheral blood of pregnant women. As mentioned above, an increased number of leukocytes in the AF was associated with leukocytosis in premature infants (in the group of premature birth – r = 0.63; p = 0.03).
Conclusion
Amniotic fluid is a dynamic and complex mixture that reflects the physiological status of the developing fetus with a negligible influence of maternal factors. The invasiveness of amniotic fluid sampling limits its use for research purposes. However, the absence of correlations with maternal peripheral blood and current trends towards the demedicalization of maternal care, especially in terms of antibiotic therapy and repeated courses of antenatal prophylaxis of RDS, indicate the advisability of a reasonable expansion of indications for transabdominal amniocentesis. The use of amniotic fluid testing as an inexpensive point-of-care method is warranted in perinatal centers. Its results may help answer many important questions, including the presence of intra-amniotic inflammation, the degree of fetal lung maturity, and predict perinatal outcomes with subsequent correction of obstetric management.
References
- Orczyk-Pawilowicz M., Jawien E., Deja S., Hirnle L., Zabek A., Mlynarz P. Metabolomics of human amniotic fluid and maternal plasma during normal pregnancy. PLoS One. 2016; 11(4): e0152740. https://dx.doi.org/10.1371/journal.pone.0152740.
- Gomez-Lopez N., Romero R., Xu Y., Miller D., Leng Y., Panaitescu B. et al. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am. J. Reprod. Immunol. 2018; 79(4): e12827. https://dx.doi.org/10.1111/aji.12827.
- Underwood M.A., Gilbert W.M., Sherman M.P. Amniotic fluid: Not just fetal urine anymore. J. Perinatol. 2005; 25(5): 341-8. https://dx.doi.org/10.1038/sj.jp.7211290.
- Tarui T., Kim A., Flake A., McClain L., Stratigis J.D., Fried I. et al. Amniotic fluid transcriptomics reflects novel disease mechanisms in fetuses with myelomeningocele. Am. J. Obstet. Gynecol. 2017; 217(5): 587. e1-587. e10. https://dx.doi.org/10.1016/j.ajog.2017.07.022.
- Tsuda H., Takahashi Y., Iwagaki S., Kawabata I., Hayakawa H., Kotani T. et al. Intra-amniotic infection increases amniotic lamellar body count before 34 weeks of gestation. J. Matern. Neonatal Med. 2010; 23(10): 1230-6. https://dx.doi.org/10.3109/14767051003615442.
- Štimac T., Petrović O., Krajina R., Prodan M., Bilić-Zulle L. Lamellar body count as a diagnostic test in predicting neonatal respiratory distress syndrome. Croat. Med. J. 2012; 53(3): 234-8. https://dx.doi.org/10.3325/cmj.2012.53.234.
- Beamon C., Carlson L., Rambally B., Berchuck S., Gearhart M., Hammett-Stabler C., Strauss R. Predicting neonatal respiratory morbidity by lamellar body count and gestational age. J. Perinat. Med. 2016; 44(6): 677-83. https://dx.doi.org/10.1515/jpm-2014-0310.
- Welch R.A., Recanati M.A., Welch K.C., Shaw M.K. Maternal plasma LPCAT 1 mRNA correlates with lamellar body count. J. Perinat. Med. 2018; 46(4): 429-31. https://dx.doi.org/10.1515/jpm-2017-0057.
- Chaemsaithong P., Romero R., Korzeniewski S.J., Martinez-Varea A., Dong Z., Yoon B.H. et al. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J. Matern. Neonatal Med. 2016; 29(3): 349-59. https://dx.doi.org/10.3109/14767058.2015.1006620.
- Yoon B.H., Romero R., Park J.Y., Oh K.J., Lee J.H., Conde-Agudelo A., Hong J.S. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 2019; 221(2): 142. e1-142. e22. https://dx.doi.org/10.1016/j.ajog.2019.03.018.
- Volante E., Gramellini D., Moretti S., Kaihura C., Bevilacqua G. Alteration of the amniotic fluid and neonatal outcome. Acta Biomed. 2004; 75(Suppl. 1): 71-5.
- Perluigi M., Di Domenico F., Cini C., Coccia R., Giorlandino F.R., Giorlandino M. et al. Proteomic analysis for the study of amniotic fluid protein composition. J. Prenat. Med. 2009; 3(3): 39-41.
- Casadei R., D’Ablaing G., Kaplan B.J., Schwinn C.P. A cytologic study of amniotic fluid. Acta Cytol. 1973; 17(4): 289-98.
- Dziadosz M., Basch R.S., Young B.K. Human amniotic fluid: A source of stem cells for possible therapeutic use. Am. J. Obstet. Gynecol. 2016; 214(3): 321-7. https://dx.doi.org/10.1016/j.ajog.2015.12.061.
- Kim M.J., Romero R., Gervasi M.T., Kim J.S., Yoo W., Lee D.C. et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab. Investig. 2009; 89(8): 924-36. https://dx.doi.org/10.1038/labinvest.2009.49.
- Gomez-Lopez N., Romero R., Garcia-Flores V., Xu Y., Leng Y., Alhousseini A. et al. Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. Am. J. Reprod. Immunol. 2017; 78(4): 10.1111/aji.12723. https://dx.doi.org/10.1111/aji.12723.
- Welch R.A., Shaw M.K., Welch K.C. Amniotic fluid LPCAT1 mRNA correlates with the lamellar body count. J. Perinat. Med. 2016; 44(5): 531-2. https://dx.doi.org/10.1515/jpm-2015-0008.
- Преждевременные роды. Клинические рекомендации (протокол лечения). М.; 2011. [Preterm birth. Clinical recommendations (treatment Protocol). 2011. (in Russian)].
- ACOG; Committee on Obstetric Practice. Antenatal corticosteroid therapy for fetal maturation. Obstet. Gynecol. 2017; 130(2): 102-9. https://dx.doi.org/10.1016/S0029-7844(02)02023-9.
- Mitsiakos G., Kovacs L., Papageorgiou A. Are antenatal steroids beneficial to severely growth restricted fetuses? J. Matern. Neonatal Med. 2013; 26(15): 1496-9. https://dx.doi.org/10.3109/14767058.2013.789852.
- Ходжаева З.С., Горина К.А. Антенатальная профилактика респираторного дистресс-синдрома плода: взгляд в будущее. Акушерство и гинекология. 2019; 5: 12-8. [Khodzhaeva Z.S., Gorina K.A. Antenatal prevention of fetal respiratory distress syndrome: a look into the future. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; 5: 12-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.5.12-18.
- Lu J., Gronowski A.M., Eby C. Lamellar body counts performed on automated hematology analyzers to assess fetal lung maturity. Lab. Med. 2008; 39(7): 419-23. https://dx.doi.org/10.1309/TPM3HYJE475RYMA2.
- Westover A.J., Moss T.J.M. Effects of intrauterine infection or inflammation on fetal lung development. Clin. Exp. Pharmacol. Physiol. 2012; 39(9): 824-30. https://dx.doi.org/10.1111/j.1440-1681.2012.05742.x.
Received 07.02.2020
Accepted 17.07.2020
About the Authors
Zulfiya S. Khodzhaeva, Dr.Med.Sci., Professor, Deputy Director for Research, Institute of Obstetrics, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.Tel.: +7(916)407-75-67. E-mail: zkhodjaeva@mail.ru. 4, Ac. Oparina str., Moscow, 117997, Russian Federation.
Ksenia A. Gorina, Junior Researcher at the Department of Pathology of Pregnancy, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(926)649-77-32. E-mail: k_gorina@oparina4.ru. 4, Ac. Oparina str., Moscow, 117997, Russian Federation.
Kamilla T. Muminova, Ph.D., Junior Researcher at the Department of Pathology of Pregnancy, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(916)373-77-07. Е-mail: k_muminova@oparina4.ru. 4, Ac. Oparina str., Moscow, 117997, Russian Federation.
Tatyana Yu. Ivanets, Dr.Med.Sci., Head of the Clinical Diagnostic Laboratory, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(910)404-26-69. Е-mail: t_ivanets@oparina4.ru. 4, Ac. Oparina str., Moscow, 117997, Russian Federation.
Yulia V. Kessler, Clinical Pathologist at the Biochemistry Laboratory, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(916)439-70-01. Е-mail y_kessler@oparina4.ru. 4, Ac. Oparina str., Moscow, 117997, Russian Federation.
Tatyana V. Priputnevich, Dr.Med.Sci., Head of the Department of Microbiology and Clinical Pharmacology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(903)264-12-57. Е-mail: priputl@gmail.com. 4, Ac. Oparina str., Moscow, 117997, Russian Federation.
Dmitry M. Belousov, Diagnostic Medical Sonographer, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Tel.: +7(925)506-51-46. E-mail: d_belousov@oparina4.ru.
4, Ac. Oparina str., Moscow, 117997, Russian Federation.
For citation: Khodzhaeva Z.S., Gorina K.A., Muminova K.T., Ivanets T.Yu., Kessler Yu.V., Priputnevich T.V., Belousov D.M. Amniotic fluid composition in pregnant women at high risk of preterm birth.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 8: 82-87 (in Russian)
https://dx.doi.org/10.18565/aig.2020.8.82-87



