The content of different forms of E-cadherin in blood plasma and placental tissue in preeclampsia
Objective: To determine the content of the adhesion molecule E-cadherin in the plasma and placenta of women with preeclampsia (PE) and assess its involvement in the development of PE. Materials and methods: This study included 55 pregnant women. Thirty-five of them had pregnancies complicated by PE (Group I), and 20 had uncomplicated pregnancies (Group II, control). Blood plasma levels of the soluble form of E-cadherin were determined using enzyme immunoassay. Placental E-cadherin expression was studied by the Western blot method. Results: The plasma soluble form of E-cadherin was higher in patients with PE than in controls, 8.0 ng/ml and 3.87 ng/ml, respectively (p<0.001). Placental tissue analysis showed a significantly higher content of the soluble form of E-cadherin in PE. Conclusion: The increased level of the soluble form of E-cadherin in the plasma and placental tissue in PE reflects the abnormal proliferation and differentiation of trophoblasts, which is typical of this pathology. Authors’ contributions: Shelekhin A.P., Baev O.R., Sadekova A.A., Kokoeva D.N., Krasnyi A.M. – conception and design of the study, data collection, review of relevant literature, statistical analysis, manuscript drafting. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors’ Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Shelekhin A.P., Baev O.R., Sadekova A.A., Kokoeva D.N., Krasnyi A.M. The content of different forms of E-cadherin in blood plasma and placental tissue in preeclampsia. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (3): 36-40 (in Russian) https://dx.doi.org/10.18565/aig.2022.282Shelekhin A.P., Baev O.R., Sadekova A.A., Kokoeva D.N., Krasnyi A.M.
Keywords
Preeclampsia (PE) is a major pregnancy complication and a leading cause of maternal and perinatal morbidity and mortality. Currently, there is no single specific marker for the diagnosis of PE, and various multivariate screening methods based on history, ultrasound, and quantification of non-specific markers in serum and urine have been used [1, 2].
One of the factors leading to the development of PE may be an excessive maternal immune response to pregnancy, resulting in impaired placentation, placental ischemia, and consequently, increased trophoblast cell death [3]. The placenta is the link between the mother and fetus, providing transport, endocrine, and barrier functions. In addition, the placenta suppresses excessive maternal immune responses caused by trophoblasts and semi-allogeneic fetal tissues with the help of human leukocyte antigen (HLA-G) molecules [4]. Reduced immunological tolerance and expression of HLA-G molecules lead to activation of T-lymphocytes and damage to the placental barrier.
Cell adhesion molecules (CAMs), including E-cadherin (E-cad), are involved in maintaining placental barrier integrity [5]. These cell adhesion molecules are an important binding part of the adhesive contacts that form the cell cytoskeleton and ensure the structural integrity of tissues. When cells aggregate and adhere to each other, intercellular permeability and the degree of migration of substances and molecules through the tissues are reduced. Owing to intercellular adhesion, only certain molecules and proteins can pass through the placenta. E-cad is a calcium-dependent molecule that mediates cellular adhesion through the participation of calcium ions [6]. E-cad exists in two forms: a membrane-bound form and a soluble form. Membrane-bound E-cad is a glycoprotein with a molecular weight of 120 kDa and consists of an extracellular region, a transmembrane region, and an intracellular region. The N-terminal extracellular region interacts with the N-terminus of E-cad of another cell to form an E-cadherin–E-cadherin intercellular bond that allows trophoblast cells to maintain the integrity of the placental barrier. The disruption of intercellular contacts formed by E-cad molecules affects not only intercellular adhesion but also the transmission path of Wnt and some growth factors [7, 8]. Disruption of E-cad leads to increased levels of the soluble form of E-cad (sE-cad) in plasma, which is a manifestation of loss of intercellular adhesive contacts of the trophoblast and increased permeability of the placental barrier. Soluble E-cad (sE-cad) is an extracellular fragment of E-cad, has a molecular weight of 80 kDa, and is formed directly from E-cad with the participation of matrix metalloproteinases (MMPs), ADAMs molecules, α-secretase, B. Fragilis toxin, and HtrA proteases. sE-cad activates the EGFR, Wnt/β-catenin, and IGF-1R signalling pathways and inhibits Hippo signalling. Alterations in these pathways may also activate ADAMs and MMPs, further enhancing the production of sE-cad. This molecule performs largely opposite functions to those of E-cad. In addition, sE-cad inhibits the formation of new adhesive junctions by interacting with the extracellular domain of E-cad, thus blocking intercellular contact.
When the immunological balance and permeability of the placental barrier are compromised, antibodies and NK cells enter the bloodstream and cytotoxic lymphocyte activity is increased, leading to endothelial dysfunction, systemic inflammatory response, and the development of PE [9].
This study aimed to determine the content of soluble E-cadherin in the plasma and placenta of women with preeclampsia and to assess its involvement in the development of PE.
Materials and methods
This case-control study included 55 pregnant women who were observed in the Research and Outpatient Department and delivered at the V.I. Kulakov Scientific Research Center for Obstetrics, Ministry of Health of Russia. The women were divided into the study and control groups. The study group included 35 pregnant women diagnosed with PE. PE was defined as an elevated blood pressure after 20 weeks of gestation (≥ 140 mmHg systolic or ≥ 90 mmHg diastolic) plus proteinuria (> 0.3 g/24 hours). The control group comprised 20 women with uncomplicated pregnancies, without a poor obstetric history, who delivered at term. This study was approved by the local research ethics committee. The inclusion criteria were pregnant women at 27 to 40 weeks of gestation, aged 18 to 45 years, and without severe non-obstetric comorbidities. The exclusion criteria were rhesus conflict, chronic inflammatory diseases in the acute phase, autoimmune diseases, cancer, and fetal malformations. sE-cad in plasma was measured by ELISA immunoassay using a Human E-cadherin ELISA Kit (Cusabio, USA). Maternal peripheral blood (5 ml) was collected in tubes containing ethylenediaminetetraacetic acid (EDTA) and processed within 1 hour after sampling. Blood samples were centrifuged in two steps at 4ºC: first step, 10 min, 200 g; second step, 10 min, 4500 g. The samples were stored at -80ºC until further processing. Placental tissue samples were collected midway between the edge and center of the placenta within 1 h of delivery and examined using western blotting. Proteins were denatured, separated by gel electrophoresis, and transferred to polyvinylidene fluoride membranes (PVDF). Immunostaining was performed to visualize the proteins on the membrane.
Statistical analysis
Data with non-normal distribution are reported as the median and interquartile range Me (Q1; Q3). The Student’s t-test was used to compare two groups with a normal distribution. The distribution of continuous variables was tested for normality using the Shapiro–Wilk test, and equality of variance was assessed using Levene's test. For non-normally distributed parameters, the Mann–Whitney U test was used for comparisons. Categorical variables were compared using the Fisher’s exact test. Differences were considered statistically significant at p<0.05. Statistical analysis and graphing were performed using Stattech (Russia) and Statistica 10 software (USA).
Results
The main clinical and anamnestic findings and significant pregnancy complications of the study participants are presented in Table.
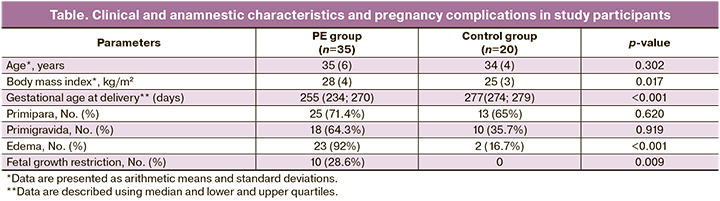
The women’s ages ranged from 26 to 45 years, and were 35 (6) and 34 (4) years in the study and control groups, respectively, with no significant difference. There were no significant differences in parity between the groups. The gestational age at delivery was significantly lower in the PE group (p<0.001), with an average of 35 weeks in the study group and 39 weeks in the control group. Analysis of anthropometric data revealed no statistically significant differences between the groups.
Patients in the PE group had a significantly lower birth weight (2372 (898 g)) than women with uncomplicated pregnancies (3204 g (345 g)) (p<0.001). Ten women had fetal growth restriction. There were 18 preterm births in the PE group.
The concentration of sE-cad in the peripheral blood was higher in patients with PE, with sE-cad levels of 3.87 (2.61; 5.79) ng/ml in controls and 8.001 (4.99; 11.85) ng/ml in women with PE (p<0.001) (Fig. 1).
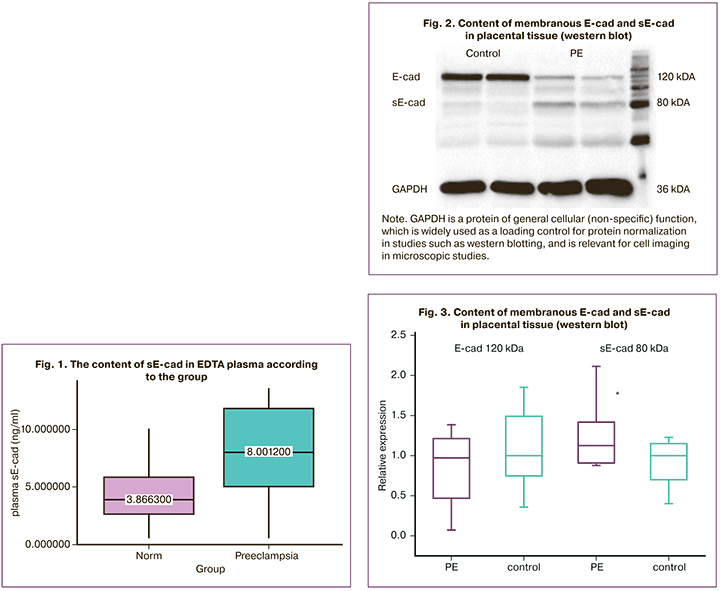
The next step of our study was to determine the content of sE-cad and E-cad in placental tissue using western blotting. We found that in the PE group the sE-cad content was higher (1.13 (0.91; 1.42) r.u.) compared to the sE-cad content in the women who had uncomplicated pregnancies (1 (0.7; 1.15) r.u.) (Fig. 2, 3).
The expression of E-cad in the PE group (0.98 (0.47; 1.21) r.u.) was not statistically different when compared to the control group (1 (0.7; 1.15) r.u.).
Note. GAPDH is a protein of general cellular (non-specific) function, which is widely used as a loading control for protein normalization in studies such as western blotting, and is relevant for cell imaging in microscopic studies.
Discussion
A review of the relevant literature shows that different studies have produced conflicting results. A study by Blechschmidt K. et al. (2007) compared placental tissue from pregnant women with PE and uncomplicated pregnancies using immunohistochemical staining. The analysis showed a significant decrease in the immunoreactivity of membranous E-cad in placentas from pregnant women with PE, reflecting damage to the placental barrier. A disadvantage of this study is the small sample size [10]. A similar study, but with a larger sample size, was conducted by Peksa M. et al. (2022), who found reduced E-cad expression in the placentas of pregnant women with PE [11]. This study also highlights the importance of E-cad in maintaining the integrity of the placental barrier and discusses the possibility of using E-cad as a marker of PE.
Contrary results in studies of the placenta have been reported by Li H.W. et al. (2003) [12] and Li X.L. et al. (2014) [13], who observed increased E-cad expression in pregnancies complicated by PE. Similar findings were reported by Du et al. (2017) [14]. However, the current literature lacks sufficient coverage of studies analyzing the relationship between membranous and soluble E-cadherin.
In our study, we found elevated levels of soluble E-cad in the plasma and placenta in pregnancies complicated by PE. High E-cad expression is associated with increased cytotrophoblast proliferation and inhibition of differentiation into syncytiotrophoblast, and impaired differentiation leads to apoptosis of cytotrophoblasts and syncytiotrophoblast [13]. Apoptosis is associated with the destruction of trophoblast cell adhesion bonds, increased levels of soluble E-cad in the bloodstream, and increased permeability of the placental barrier. Thus, the level of soluble E-cad reflects pathological changes in trophoblasts in PE.
Conclusion
Our study has established a possible role for E-cadherin in the pathogenesis of PE. Further studies are required to clarify the diagnostic and prognostic significance of this molecule in PE.
References
- Karapetian А.О., Baev О.R., Sadekova А.А., Krasnyi А.М., Sukhikh G.T. Cell-free foetal DNA as a useful marker for preeclampsia prediction. Reprod. Sci. 2021; 28(5): 1563-9. https://dx.doi.org/10.1007/s43032-021-00466-w.
- MacDonald T.M., Walker S.P., Hannan N.J., Tong S., Kaitu’u-Lino T.J. Clinical tools and biomarkers to predict preeclampsia. EBioMedicine. 2022; 75: 103780. https://dx.doi.org/10.1016/j.ebiom.2021.103780.
- Miller D., Motomura K., Galaz J., Gershater M., Lee E.D., Romero R.,Gomez-Lopez N. Cellular immune responses in the pathophysiology of preeclampsia. J. Leukoc. Biol. 2022; 111(1): 237-60. https://dx.doi.org/10.1002/JLB.5RU1120-787RR.
- Ander S.E., Diamond M.S., Coyne C.B. Immune responses at the maternal-fetal interface. Sci. Immunol. 2019; 4(31): eaat6114. https://dx.doi.org/10.1126/SCIIMMUNOL.AAT6114.
- Шелехин А.П., Баев О.Р., Красный А.М. Роль молекул клеточной адгезии в патогенезе преэклампсии. Акушерство и гинекология. 2021; 6: 22-8. [Shelekhin A.P., Baev O.R., Krasnyi A.M. The role of cell adhesion molecules in the pathogenesis of preeclampsia. Obstetrics and Gynecology. 2021; (6): 22-8 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.6.22-28.
- Zhou W., Santos L., Dimitriadis E. Characterization of the role for cadherin 6 in the regulation of human endometrial receptivity. Reprod. Biol. Endocrinol. 2020; 18(1): 66. https://dx.doi.org/10.1186/S12958-020-00624-W.
- Qiuling J., Fei S., Qi L., Juan Z., Yunjian W., Huamei Y. et al. Downregulated ribosomal protein L39 inhibits trophoblast cell migration and invasion by targeting E-cadherin in the placenta of patients with preeclampsia. FASEB J. 2021; 35(4): e21322. https://dx.doi.org/10.1096/FJ.202002061R.
- Fedorova L., Gatto-Weis C., Smaili S., Khurshid N., Shapiro J.I.,Malhotra D., Horrigan T. Down-regulation of the transcription factor snail in the placentas of patients with preeclampsia and in a rat model ofpreeclampsia. Reprod. Biol. Endocrinol. 2012; 10: 15. https://dx.doi.org/10.1186/1477-7827-10-15.
- Luo F., Yue J., Li L., Mei J., Liu X., Huang Y. Narrative review of the relationship between the maternal-fetal interface immune tolerance and the onset of preeclampsia. Ann. Transl. Med. 2022; 10(12): 713.https://dx.doi.org/10.21037/ATM-22-2287.
- Blechschmidt K., Mylonas I., Mayr D., Schiessl B., Schulze S., Becker K.F., Jeschke U. Expression of E-cadherin and its repressor snail in placental tissue of normal, preeclamptic and HELLP pregnancies. Virchows Arch. 2007; 450(2): 195-202. https://dx.doi.org/10.1007/S00428-006-0343-X.
- Pęksa M., Kamieniecki A., Gabrych A., Lew‐tusk A., Preis K., Świątkowska‐freund M. Loss of E-cadherin staining continuity in the trophoblastic basal membrane correlates with increased resistance in uterine arteries and proteinuria in patients with pregnancy-induced hypertension. J. Clin. Med. 2022; 11: 668. https://dx.doi.org/10.3390/JCM11030668.
- Li H.W., Cheung A.N.Y., Tsao S.W., Cheung A.L.M., O W.S. Expression of e-cadherin and beta-catenin in trophoblastic tissue in normal and pathological pregnancies. Int. J. Gynecol. Pathol. 2003; 22(1): 63-70.https://dx.doi.org/10.1097/00004347-200301000-00013.
- Li X.L., Dong X., Xue Y., Li C.F., Gou W.L., Chen Q. Increased expression levels of E-cadherin, cytokeratin 18 and 19 observed in preeclampsia were not correlated with disease severity. Placenta. 2014; 35(8): 625-31.https://dx.doi.org/10.1016/j.placenta.2014.04.010.
- Du L., Kuang L., He F., Tang W., Sun W., Chen D. Mesenchymal-to-epithelial transition in the placental tissues of patients with preeclampsia. Hypertens. Res. 2017; 40(1): 67-72. https://dx.doi.org/10.1038/hr.2016.97.
Received 25.11.2022
Accepted 10.02.2023
About the Authors
Artemiy P. Shelekhin, Postgraduate Student, I.M. Sechenov First MSMU (Sechenov University), Ministry of Health of Russia, dr.shelekhin@gmail.com,https://orcid.org/0000-0002-7682-1329, 8 Trubetskaya str., Moscow, 119991, Russia.
Oleg R. Baev, Dr. Med. Sci., Professor, Head of the 1st Maternity Department, Academician V.I. Kulakov NMRС for OG&P, Ministry of Health of Russia,
o_baev@oparina4.ru, https://orcid.org/0000-0001-8572-1971, 4 Ac. Oparina str., Moscow, 117997, Russia.
Alsu A. Sadekova, PhD, Senior Researcher at the Cytology Laboratory, Academician V.I. Kulakov NMRС for OG&P, Ministry of Health of Russia, +7(495)438-22-72,
sialsad@gmail.com, https://orcid.org/0000-0003-4726-7477, 4 Ac. Oparina str., Moscow, 117997, Russia.
Diana N. Kokoeva, Junior Researcher at the Cytology Laboratory, Academician V.I. Kulakov NMRС for OG&P, Ministry of Health of Russia, +7(495)438-22-72,
Dikokoeva@mail.ru, 4 Ac. Oparina str., Moscow, 117997, Russia.
Alexey M. Krasnyi, PhD, Head of the Cytology Laboratory, Academician V.I. Kulakov NMRС for OG&P, Ministry of Health of Russia, a_krasnyi@oparina4.ru,
https://orcid.org/0000-0001-7883-2702, 4 Ac. Oparina str., Moscow, 117997, Russia.



