Characteristics of production and reception of sex hormones in patients with chronic endometritis
Objective. To determine the level of sex hormones and evaluate endometrial receptivity in patients with chronic endometritis. Materials and methods. There was an assessment of sex hormone levels, the expression of estrogen (ER) and progesterone (PR) receptors, and the membrane component of the progesterone receptor (PGRMC1) in patients with chronic endometriosis and in the patients of the control group. Results. Patients with immunohistochemical signs of endometritis had significantly higher levels of progesterone in the blood (p=0.006). The analysis of the expression of sex hormone receptors in the endometrium revealed a decrease in the expression of PR and PGRMC1, which indicates an impairment of hormone reception at the molecular level (p<0.001) Conclusion. Patients with chronic endometritis can be administered progestogen therapy only in case of progesterone deficiency.Lyzikova Yu.A.
Keywords
A steady increase in the number of chronic inflammatory diseases of the female genital organs and, in particular, chronic endometritis has been registered recently [1–6]. Due to the absence of a standardized approach to the diagnosis of chronic endometritis, the frequency of its detection ranges from 2 to 70% among the patients of reproductive age [7–10]. The highest incidence of the disease is observed in infertile women, since the chronic inflammatory process of the endometrium leads to impaired endometrial receptivity and implantation processes [11, 12]. Complete implantation is normally possible only in the presence of the receptive endometrium, which largely determines the outcome of pregnancy [13, 14]. At the initial stages of chronic endometritis, the implantation capacity of the endometrium is preserved, which is due to the compensatory mechanism; its gradual depletion results in a critical impairment of receptivity [15–22]. The understanding of the processes that cause endometrial susceptibility can lead to an increase in the frequency of implantations and improve the outcomes of pregnancies [23, 24]. Since the impaired synthesis of hormones directly affects fertility, the assessment of hormone levels in patients with chronic inflammatory process in the uterine mucosa is of scientific interest; moreover, it is also important to determine their receptivity in the endometrium.
Materials and Methods
The concentration of hormones, namely follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone, prolactin, estradiol, progesterone, and 17-hydroxyprogesterone was assessed in the blood serum by the method of enzyme-linked immunosorbent assay (ELISA), AO Vector-Best and the Xema Corporate Group (Russia).
Immunohistochemical examination of the endometrium was performed using antibodies to CD56 (Diagnostic Biosystems, USA), FoxP3 (Diagnostic Biosystems, USA), estrogen receptor (ER) (Diagnostic Biosystems, USA), progesterone receptor (PR) (Diagnostic Biosystems, USA), progesterone receptor membrane component 1 (PGRMC1) (Abcam, UK). The concentrate of primary antibodies to FoxP3 (Abcam, UK) was diluted in a ratio of 1:100 before its use; the concentrate of antibodies to PGRMC1 was diluted in a ratio of 1:150 in the solution of Antibody Universal Diluent (Abcam, UK).
The examination of the endometrium was carried out in five high power fields, magnification 400×. When CD56 positive NK lymphocytes and FoxP3-positive T lymphocytes were revealed, they were counted in the five non-overlapping high power fields described above and the arithmetic mean was calculated. Receptor expression was determined in the surface epithelium, glands, and stroma as a percentage of positive cells and expressed as an arithmetic mean for five non-overlapping high power fields.
Statistical analysis
In statistical processing, the normal distribution of the values was proved by the Kolmogorov-Smirnov test. The distribution of variable parameters was different from the normal distribution, therefore, the comparative analysis between the groups was carried out using nonparametric statistical methods. Data processing was performed using the following methods: Mann–Whitney U-test with Bonferroni correction and χ2-test for the comparison of categorical variables. To identify the threshold values of hormone levels and receptor expression, the ROC curve method was used. The model quality was assessed using a scale for the values of the area under the curve (AUC): 0.9-0.1 refers to excellent level, 0.8–0.9 refers to very good, 0.6–0.7 refers to average, and 0.5–0.6 refers to unsatisfactory level. The null hypothesis was rejected at the value of p=0.05. The statistical analysis of the results was performed using the Statistica (version 10.0) and MedCalc (version 12.0.0) software packages.
Results
Immunohistochemical examination of the endometrium was performed in 340 patients of the reproductive age. The patients did not show any statistically significant differences in their age. The main group included 230 (67.65%) women diagnosed with chronic endometritis, and the comparison group included 110 (32.35%) patients whose parameters of immunohistochemical and histological studies corresponded to the normal endometrium. The results of the endometrial examination are presented in Table 1.
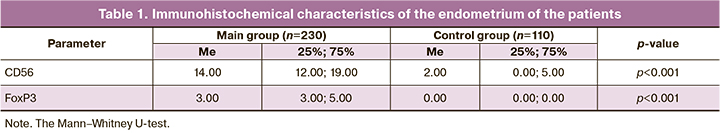
The clinical characteristics and anamnestic data of the patients are presented in Table 2.
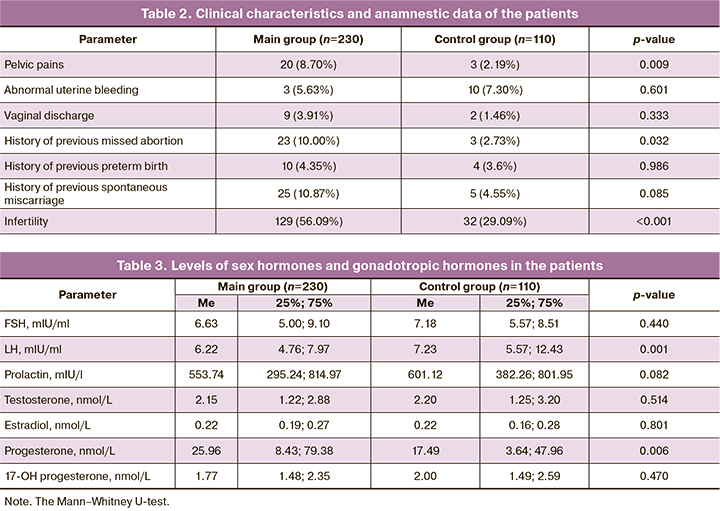
The comparison of hormone levels showed a statistically significantly lower level of LH in patients with immunohistochemical signs of endometritis (p=0.001) and a higher level of progesterone (p=0.006) (Table 3).
In order to identify the threshold value of the level of sex hormones which can be present in patients with chronic endometritis, ROC analysis was performed. At the LH level<8.57 mIU/ml, the likelihood ratio of chronic endometritis is 1.55, the area under the curve (AUC) was 0.624 [95% CI 0.569–0.676; p<0.001]. When the level of progesterone prevailed 2.22 nmol/L, the likelihood ratio of chronic endometritis was 1.22, the area under the curve (AUC) was 0.590 [95% CI 0.534–0.644; p=0.005].
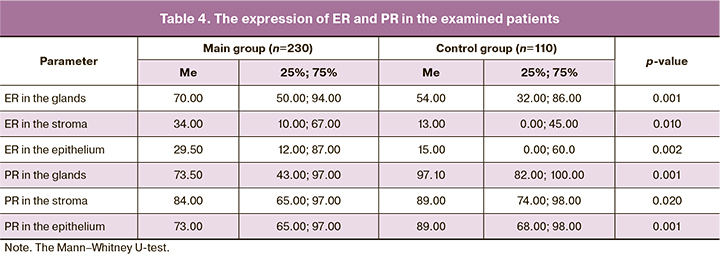
The expression of ER and PR in the endometrium was evaluated in patients of both groups (Table 4).
In order to identify the threshold value of receptor expression which can be present in the patients with chronic endometritis, ROC analysis was performed. Chronic endometritis is likely to occur in the following situations: when the level of ER in the glands prevailed or was equal to 44.00%, the likelihood ratio for a positive test was 1.48, and AUC was 0.663 [95% CI 0.587–0.733; p<0.001]; when the level of ER in the endometrial stroma prevailed or was equal to 42.00%, the likelihood ratio for a positive test was 1.61, AUC was 0.613 [95% CI 0.536–0.686; p=0.009]; when the level of ER in the superficial epithelium exceeded or was equal to 2.00%, the likelihood ratio for a positive test was 1.41, and AUC was 0.638 [95% CI 0.561–0.709; p=0.001]; when the level of PR in the glands was less than 76.00%, the likelihood ratio for a positive test was 3.34, then AUC was 0.693 [95% CI 0.619–0.761; p<0.001]; when the level of PR in the stroma was less than 95.00%, the likelihood ratio for a positive test was 1.65, and AUC was 0.602 [95% CI 0.525–0.676; p=0.023]; when the level of PR in the epithelium was less than 76.00%, the likelihood ratio for a positive test was 1.93, and AUC was 0.618 [95% CI 0.541–0.691; p=0.008].
PGRMC1 was found in 18 (7.83%) patients of the main group; this indicator was revealed statistically significantly more often in the patients of the control group, namely, in 65 (59.09%) patients (χ2=105.98, p<0.001).
Discussion
Chronic endometritis in most cases is asymptomatic, patients more frequently experience pelvic pain (p=0.009), which cannot be referred to a specific sign of the disease. Patients with chronic endometritis show a statistically significantly lower level of LH (p=0.001); however, a higher level of progesterone (p=0.006) in these patients indicates that this level of LH is sufficient for ovulation and stimulation of progesterone synthesis. Since patients with chronic endometritis have a high level of progesterone, it can be assumed that the development of the disease does not result from the systemic level of the hormone, but it is due to an impaired mechanism of its reception. The analysis of data on the expression of ER and PR in the endometrium revealed that the expression of PR in the glands, stroma and epithelium was statistically significantly lower in patients with chronic inflammatory process in the uterine cavity, while the expression of ER in the similar structures was higher. The action of progesterone in tissues is provided by its interaction with PGRMC1. Due to the fact that this type of receptor is involved in the regulation of steroid synthesis and catabolism, patients with chronic endometritis demonstrate the impaired processes of production and elimination of progesterone.
Therefore, patients with chronic endometritis have increased progesterone secretion at the systemic level, but they have reduced endometrial receptivity. Perhaps these changes are due to the inflammatory process of the uterine mucosa, so chronic endometritis cannot be diagnosed on the basis of these changes. Since the level of hormones in the blood does not show endometrial receptivity to them, chronic endometritis should be diagnosed using immunohistochemical methods that will determine the degree of hormonal disorders and the need for hormonal correction.
Conclusion
Elevated progesterone levels can inhibit the expression of receptors in the endometrium, so hormone therapy of chronic endometritis using progestogens should be performed only after determining progesterone deficiency. The absence of PGRMC1 and a decrease in PR expression in patients with chronic endometritis indicate that the mechanism of interaction between the hormone and target organs is impaired in case of inflammation, so anti-inflammatory therapy should be administered at the first stage of treatment of patients with chronic endometritis.
References
- Омапаршаева М.И., Дикке Г.Б., Абусуева З.А., Хашаева Т.Х.-М. Вос-становление рецептивности эндометрия у женщин после несостоявшегося выкидыша. Акушерство и гинекология. 2019; 1: 109-16. [Omaparshaeva M.I., Dikke G.B., Abusueva Z.A., Hashaeva T.Kh.-M. Restoration of endometrial receptivity in women after miscarriage. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2019; 1: 109-16. (in Russian)]. https://dx.doi.org/10.18656/aig.2019.1.109-116.
- Кебурия Д.К., Смольникова В.Ю., Припутневич Т.В., Муравьева В.В. Микробиота полости матки и ее влияние на репродуктивные исходы Акушерство и гинекология. 2019; 2: 22-7. [Keburiya D.K., Smol'nikova V.Yu., Priputnevich T.V., Murav'eva V.V. Microbiota of the uterine cavity and its influence on reproductive outcomes. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2019; 2: 22-7. (in Russian)]. https://dx.doi.org/10.18656/aig.2019.2.22-27.
- Доброхотова Ю.Э., Ганковская Л.В., Боровкова Е.И., Зайдиева З.С., Скальная В.С. Модулирование локальной экспрессии факторов врожден-ного иммунитета у пациенток с хроническим эндометритом и бесплодием. Акушерство и гинекология. 2019; 5: 125-32. [Dobrohotova Yu.E., Gankovskaya L.V., Borovkova E.I., Zajdieva Z.S., Skal'naya V.S. Modeling of local expression of innate immune factors in patients with chronic endometritis and infertility. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2019; 5: 125-32. (in Russian)]. https://dx.doi.org/10.18656/aig.2019.5.125.132.
- Доронина О.К., Дейлидко Э.Н. Применение аутоплазмы, обогащенной растворимыми факторами тромбоцитов, при проведении программ экстракорпорального оплодотворения пациенток с бесплодием и хроническим эндометритом. Акушерство и гинекология. 2019; 7: 72-6. [Doronina O.K., Dejlidko E.N. The use of autoplasma, enriched with platelet-derived soluble factors, when carrying out the programs of in vitro fertilization (IFV) in patients with infertility and chronic endometritis. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2019; 7: 72-6. (in Russian)]. https://dx.doi.org/10.18656/aig.2019.7.72-76.
- Эфендиева З.Н., Аполихина И.А., Калинина Е.А. «Тонкий» эндометрий в аспекте репродуктивных неудач: современная проблема или гипер-диагностика? Акушерство и гинекология; 2019: 9: 32-9. [Efendieva Z.N., Apolihina I.A., Kalinina E.A. Thin endometrium in the aspect of reproductive failures: a modern problem or overdiagnosis? Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2019: 9: 32-9. (in Russian)]. https://dx.doi.org/10.18656/aig.2019.9.32-39.
- Cicinelli E., Matteo A., Tinelli R., Lepera A., Alfonso R., Indraccolo U. et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum. Reprod. 2015; 30(2): 323-30. https://dx.doi.org/10.1093/humrep/deu292.
- Ищенко А.И., Унанян А.Л., Коган Е.А., Демура Т.А., Коссович Ю.М. Клинико-анамнестические, иммунологические, эхографические и гистероскопические особенности хронического эндометрита, ассоциированного с нарушением репродуктивной функции. Вестник Российской академии медицинских наук. 2018; 73(1): 5-15. [Ishchenko A.I., Unanyan A.L., Kogan E.A., Demura T.A., Kossovich Yu.M. Clinical-anamnestic, immunological, echographic and hysteroscopic features of chronic endometritis associated with reproductive dysfunction. Bulletin of the RAMS. 2018; 73(1): 5-15. (in Rus-sian)]. https://dx.doi.org/10.15690/vramn927.
- Johnston-MacAnanny E.B., Hartnett J., Engmann L.L., Nulsen J.C., Sanders M.M., Benadiva C.A. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil. Steril. 2010; 93(2): 437-41. https://dx.doi.org/10.1016/j.fertnstert.2008.12.131.
- Kasius J.C., Broekmans F.J.M., Sie-Go D.M.D.S., Bourgain C., Eijke-mans M.J.C., Fauser B.C. et al. The reliability of the histological diagnosis of endometritis in asymptomatic IVF cases: a multicenter observe study. Hum. Reprod. 2012; 27(1): 153-8. https://dx.doi.org/10.1093/humrep/der341.
- Feng Y., Ma X., Deng L., Yao B., Xiong Y., Wu Y. et al. Role of selectins and their ligands in human implantation stage. Glycobiology. 2017; 27(5): 385-91. https://dx.doi.org/10.1093/glycob/cwx009.
- Di Spiezio Sardo A., Di Carlo C., Minozzi S., Spinelli M., Pistotti V., Alviggi C. et al. Efficacy of hysteroscopy in improving reproductive outcomes of infertile couples: a systematic review and meta-analysis. Hum. Reprod. Update. 2016; 22(4): 479-96. https://dx.doi.org/10.1093/humupd/dmw008.
- Romero R., Espinoza J., Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil. Steril. 2004; 82(4): 799-804. https://dx.doi.org/10.1016/j.fertnstert.2004.05.076.
- Moffett A., Colucci F. Uterine NK cells: active regulators at the maternal-fetal interface. J. Clin. Invest. 2014; 124(5): 1872-9. https://dx.doi.org/10.1172/JCI68107.
- Ledee-Bataille N., Dubanchet S., Coulomb-L’hermine A., Durand-Gasselin I., Frydman R., Chaouat G. A new role for natural killer cells, interleukin (IL)-12, and IL-18 in repeated implantation failure after in vitro fertiliza-tion. Fertil. Steril. 2004; 81(1): 59-65. https://dx.doi.org/10.1016/j.fertnstert.2003.06.007.
- Бурдули А.Г. Андрогеновый статус у пациенток в программах ЭКО. Акушерство и гинекология. 2019; 3: 40-4. [Burduli A.G. Androgen status in patients in IVF programs. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2019; 3: 40-4. (in Russian)]. https://dx.doi.org/10.18656/aig.2019.3.40-44.
- Mulac-Jericevic B., Mullinax R.A., DeMayo F.J., Lydon J.P., Conneely O.M. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000; 289(5485): 1751-4. https://dx.doi.org/10.1126/science.289.5485.1751.
- Fleisch M.C., Chou Y.C., Cardiff R.D., Asaithambi A., Shyamala G. Overexpression of progesterone receptor A isoform in mice leads to endome-trial hyperproliferation, hyperplasia and atypia. Mol. Hum. Reprod. 2009; 15(4): 241-9. https://dx.doi.org/10.1093/molehr/gap013.
- Szekeres-Bartho J., Barakonyi A., Par G., Polgar B., Palkovics T., Szereday L. Progesterone as an immunomodulatory molecule. Int. Immunopharmacol. 2001; 1(6): 1037-48. https://dx.doi.org/10.1016/s1567-5769(01)00035-2.
- Yie S.M., Xiao R., Librach C.L. Progesterone regulates HLA-G gene expression through a novel progesterone response element. Hum. Reprod. 2006; 21(10): 2538-44. https://dx.doi.org/10.1093/humrep/del126.
- Choi B.C., Polgar K., Xiao L., Hill J.A. Progesterone inhibits in vitro embryotoxic Th1 cytokine production to trophoblast in women with recurrent pregnancy loss. Hum. Reprod. 2000; 15(Suppl. 1): 46-59. https://dx.doi.org/ 10.1093/humrep/15.suppl_1.46.
- Venetis C.A., Kolibianakis E.M., Bosdou J.K., Lainas G.T., Sfontouris I.A., Tarlatzis B.C. et al. Basal serum progesterone and history of elevated progesterone on the day of hCG administration are significant predictors of late follicular progesterone elevation in GnRH antagonist IVF cycles. Hum. Reprod. 2016; 31(8): 1859-65. https://dx.doi.org/10.1093/humrep/dew141.
- Venetis C.A., Kolibianakis E.M., Bosdou J.K., Tarlatzis B.C. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60000 cycles. Hum. Reprod. Update. 2013; 19(5): 433-57. https://dx.doi.org/10.1093/humupd/dmt014.
- Kasius A., Smit J.G., Torrance H.L., Eijkemans M.J., Mol B.W., Opmeer B.C. et al. Endometrial thickness and pregnancy rate after IVF: a systematic review and meta-analysis. Hum. Reprod. Update. 2014: 20(4): 530-41. https://dx.doi.org/10.1093/humupd/dmu011.
- Mahajan N. Endometrial receptivity array: clinical application. J. Hum. Reprod. Sci. 2015: 8(3): 121-9. https://dx.doi.org/10.4103/0974-1208.165153.
Received 20.02.2020
Accepted 18.11.2020
About the Authors
Yuliya A. Lyzikova, MD, PhD, Associate Professor, Gomel State Medical University. Tel.: +37596688876. E-mail: Lyzikovayulia@yandex.by. ORCID: 0000-0002-8465-9368.246000, Republic of Belarus, Gomel, Lange str., 5.
For citation: Lyzikova Yu.A. Characteristics of production and reception of sex hormones in patients with chronic endometritis.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 12: 144-148 (in Russian)
https://dx.doi.org/10.18565/aig.2020.12.144-148



