Characteristics of the embryological stage of infertility treatment using assisted reproductive technologies depending on the total antioxidant capacity of native ejaculate
Objective: To investigate the impact of ejaculate total antioxidant capacity of the ejaculate on fertilization and embryo development in couples with different types of infertility and determine the value of the total antioxidant capacity as a biomarker for predicting the outcomes of assisted reproductive technology (ART).Agadzhanyan D.S., Lobanova N.N., Smolnikova V.Yu., Makarova N.P., Krasnyi A.M., Shchipitsyna V.S., Sadekova A.A., Kokoeva D.N., Kalinina E.A.
Materials and methods: The study included 50 infertile couples who underwent in vitro fertilization using intracytoplasmic sperm injection (ICSI). On the day of transvaginal punctures, native ejaculate was collected from their spouses to test for total antioxidant capacity using the FORM 3000 device using the FORD kit.
Results: Embryos obtained with sperm from the ejaculate with higher total antioxidant capacity reached the late blastocyst stage on day 5. Embryos obtained with sperm from the ejaculate with lower total antioxidant capacity by day 5 developed to morulae or early blastocyst stage (p=0.03). There was a negative correlation between the total antioxidant capacity of the ejaculate and the percentage of fertilized oocytes. No correlation was found between total antioxidant capacity and ART outcomes in couples with different types of infertility.
Conclusion: Total ejaculate antioxidant capacity of native ejaculate before in vitro fertilization affects the characteristics of the embryological stage of ART. A high level of oxidative stress was significantly associated with a decrease in the fertilization rate. At the same time, the pregnancy rate was not associated with the level of oxidative stress in male germ cells. Careful pre-pregnancy care and conscious parenting are necessary to improve the effectiveness of ART in men with high levels of oxidative stress in the ejaculate through an increase in the number of zygotes.
Keywords
Infertility is defined as the failure to achieve pregnancy after 12 months of regular unprotected sexual intercourse. Infertility is attributable to an identifiable cause in about 85% of women and couples, which include tubal obstruction or absence, ovulatory dysfunction, and spermatogenesis disorders. Remaining couples have no identified cause of infertility [1–3]. Currently, male infertility contributes to up to 50% of all cases [4, 5]. It should be noted that about 25 years ago, the male factor infertility constituted only 40%, i.e. there is a trend towards deterioration of male health and growth of male infertility.
One of the factors of male infertility, in particular idiopathic infertility, is oxidative stress [6–8]. Oxidative stress arises from an imbalance between reactive oxygen species (ROS) and protective antioxidants, which is responsible for removing excess free radicals [2]. There are both external and internal factors that influence the appearance of oxidative stress. External factors include toxic environment, stress, alcohol consumption, and smoking. Internal factors causing oxidative stress are varicocele, inflammatory processes in the urogenital tract, infection or systemic diseases such as diabetes mellitus, atherosclerosis and others [9].
The influence of ROS on the function and structure of spermatozoa is a subject of long-standing debate in the field [10]. As early as 1946, the scientific journal Nature reported that mature spermatozoa are among the first cells to produce large amounts of ROS, due to their high metabolic rate to maintain motility [11].
Main sources producing ROS in sperm are believed to be leukocytes and immature spermatozoa [12]. ROS levels correlate positively with the concentration of leukocytes in sperm. Immature spermatozoa are germ cells with excess cytoplasm. The preserved cytoplasm activates the nicotinamide adenine dinucleotide phosphate (NADPH)-system, which initiates ROS formation through a cascade of reactions [13]. At the same time, small concentrations of ROS are necessary for normal functioning of spermatozoa, their hyperactivation and acrosome reaction [4].
ROS excess is known to cause damage to various components of the cell wall and sperm organelles, as well as nuclear and mitochondrial DNA, resulting in apoptosis and a decrease in sperm motility and fertilizing ability. The most significant adverse effects of ROS interaction with germ cells include lipid peroxidation (LPO) and DNA fragmentation [14]. Unlike somatic cells, gametes are more vulnerable to LPO due to the absence of the necessary system of cytoplasmic enzyme repair. Besides, the cytoplasmic membrane contains a large amount of polyunsaturated fatty acids and membrane-bound NADPH oxidase 5, which makes germ cells susceptible to ROS [15, 16].
Hence, antioxidants are critical to inactivate ROS continuously to maintain only the small amount required to normal sperm function [17]. Therefore, the role of the antioxidant system is essential to cope with excessively produced ROS. Antioxidants are compounds capable of scavenging and inhibiting the formation of ROS [18]. Male ejaculate contains a group of enzymatic and non-enzymatic antioxidants. Superoxide dismutase, catalase, glutathione reductase and peroxidase are among the enzymatic antioxidants that are responsible for protection of spermatozoa from oxidative stress. The function of non-enzymatic antioxidants, such as taurine, pyruvate, etc., which come from outside the body, is to bind free radicals. These two antioxidant defense systems prevent oxidative damage to cellular structures only by functioning together [19, 20]. Various studies show that the ejaculate of fertile men has a higher antioxidant capacity than that of infertile men [21, 22]. This makes it possible to use the level of antioxidants in the ejaculate for diagnostic purposes and for quality control during male infertility treatment. In various studies, both individual antioxidants and total antioxidant capacity (TAC) have been studied in the ejaculate. Given that a decrease in one antioxidant can be offset by an increase in another antioxidant, measurement of TAC provides more reliable information than individual measurements [23].
Ejaculate quality has a direct impact on the outcome of egg fertilization, but there have been no studies linking TAC or ejaculate oxidative stress levels to the outcomes of assisted reproductive technology (ART).
This study aimed to investigate the impact of TAC in native ejaculate on the embryological stage of ART in couples with different types of infertility.
Materials and methods
The study included 50 infertile couples referred to the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility of the V.I. Kulakov NMRC for OG&P. Each couple provided signed informed consent to take part in the study. All patients underwent a complete examination before the ART program, which included detailed medical history taking, clinical, laboratory, and functional examination in accordance with the Order of the Russian Ministry of Health No. 803n of July 31, 2020. "On the procedure for the use of assisted reproductive technologies, contraindications and limitations to their use" [24]. The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Ovarian function stimulation was performed on day 2–5 of the menstrual cycle according to a standard protocol depending on the patients' ovarian reserve using gonadotropin preparations and gonadotropin-releasing hormone antagonists. The ovulatory trigger was administered 35–36 hours before transvaginal puncture as soon as ≥1 follicle reached ≥17 mm in diameter.
Ovulation was triggered by a single human chorionic gonadotropin (hCG) injection of 10,000 IU. At risk of ovarian hyperstimulation syndrome, a gonadotropin-releasing hormone agonist was administered as a trigger for final oocyte maturation at a dose of 0.2 mg. On the day of the transvaginal puncture, native ejaculate was collected from the spouses of the patients. Ejaculate samples were obtained by masturbation after sexual abstinence for 3–5 days. Standard sperm examination was performed according to the WHO laboratory manual for the examination and processing of human semen (2010) [25]. All patients were fertilized by intracytoplasmic sperm injection (ICSI). Embryos were cultured for up to 5 days in G-TL medium (VitroLife, Sweden) in table-top COOK incubators (Ireland) at reduced oxygen content (5%O2). All patients underwent the transfer of a better quality embryo into the uterine cavity on day 5 of culture. Morphological evaluation of the embryos was performed according to the criteria presented in Table 1. On day 12–14 after embryo transfer, testing of blood for hCG was used to confirm pregnancy.
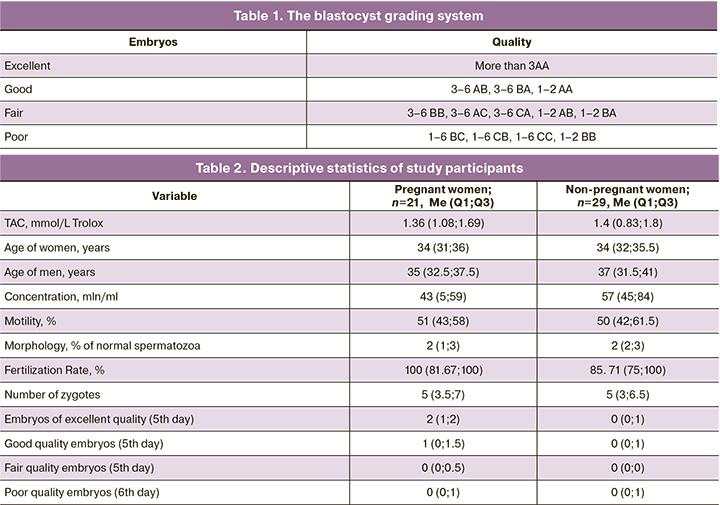
The TAC of the native ejaculate was measured using FORM 3000 (Callegari, Italy) according to the manufacturer's recommendations. TAC in the ejaculate w compared to the water-soluble vitamin E analog Trolox (mmol/L Trolox) using the FORD kit. For this purpose, one μL of ejaculate diluted in 49 μL of 0.9% aqueous sodium chloride solution was taken. Data are presented as relative units.
The FORD assay is based on the use of preformed free radicals and a decrease in absorbance which is proportional to the concentration of antioxidants in the ejaculate in the presence of acidic buffer (pH=5.2) and a suitable oxidant (FeCl3). The amino derivative (Chromogen) forms a stable colored product, the cation being determined photometrically at 505 nm. The antioxidants present in the samples reduce the cation content, resulting in an eventual discoloration of the solution, proportional to the amount of antioxidants in the sample. The FORD assay reflects the levels of antioxidants in the blood, and in healthy individuals has a value in the 1.07–1.53 mmol/L Trolox range. [26].
To identify differences in the mean values of the main clinical and embryological indices, all patients were divided into two groups classified by ART outcome (occurrence or non-occurrence of pregnancy). Of the 50 couples, 21 women were pregnant (positive hCG test results), while 29 had a negative hCG test result.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 22 and Microsoft Excel 15.0. It was found that numerical variables were not normally distributed and groups were compared using the nonparametric Mann–Whitney U-test and the Spearman Rank Test was used for the correlation analysis.
Results
The descriptive statistics of the study participants are presented in Table 2. Variables not meeting normality assumptions were reported as the median (Me) and interquartile range (Q1; Q3). The critical level of significance when testing statistical hypotheses was considered at p<0.05.
The concentration and motility of spermatozoa in the study group were within normal values. Evaluation of sperm morphology showed that the proportion of ideally shaped spermatozoa in the study group was 2 (1; 3)%, which is less than the reference value (4%).
Spearman correlation analysis was performed to identify a possible association of ejaculate TAC with the main clinical and embryological characteristics of ART. The results are presented in Table 3.
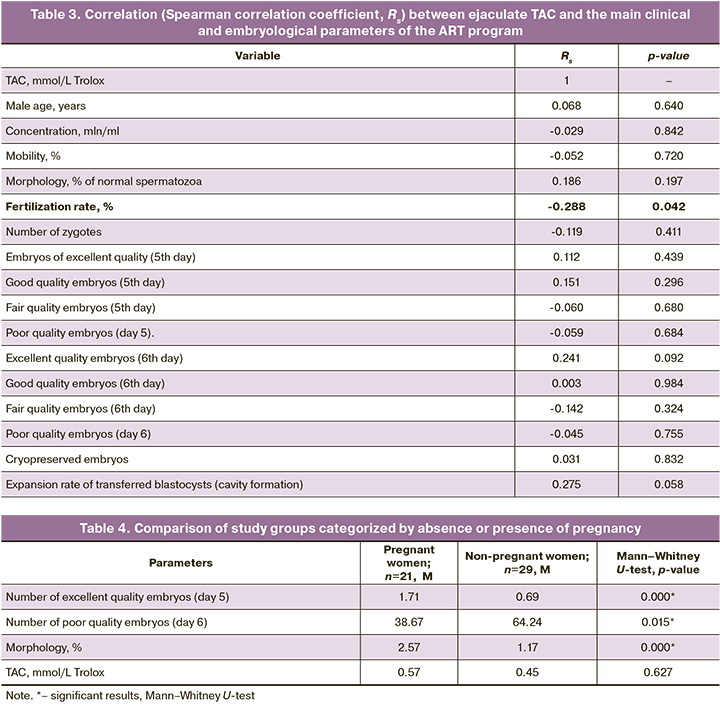
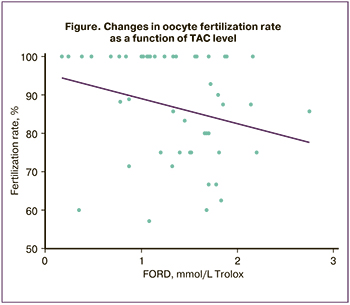
Correlation analysis revealed a negative correlation between ejaculate TAC and oocyte fertilization rate (r=-0.288; p=0.042), that is, fertilization rate decreased with increasing TAC (Figure).
There was also a trend for a positive correlation between the number of excellent quality cryopreserved embryos at 6 days of development and TAC (r=0.241; p=0.092). The higher the antioxidant capacity in the ejaculate, the higher the likelihood of obtaining excellent quality embryos.
Besides, there was a positive correlation between the degree of expansion (cavity formation) of the blastocyst transferred into the uterine cavity and TAC (r=0.275; p=0.058).
To assess the factors role of the studied in IVF program outcomes, we compared the mean values in the two groups (patients with and without pregnancy) using the Mann–Whitney U test. The results of the analysis are presented in Table 4.
The mean number of excellent quality embryos (day 5) was 1.71 in pregnant women, whereas in the non-pregnant women it was 0.69 (U=128.5, p<0.001). Also, the mean number of unsatisfactory embryos on day 6 in pregnant women was 38.67, while in the other group it was 64.24 (U=180.5, p=0.015). Male ejaculate parameters of the two groups were also compared. Significant differences (U=119.5, p<0.001) were obtained when comparing the mean sperm morphology parameters. It averaged 2.57% in the group with an onset of pregnancy and 1.17% in the group without onset.
No association was found between the TAC ejaculate and ART program outcomes. The mean TAC level was 0.57 in pregnant patients and 0.45 in nonpregnant women (U=285, p=0.627). There was no association between TAC in the ejaculate and the acquisition of excellent quality embryos.
Discussion
The incidence of male infertility remains high, and hence the relevance of investigating its causes. Oxidative stress is considered as one of such causes [27, 28]. There are a number of studies proving the importance of proper functioning of antioxidant system in male ejaculate. It has been shown that in the non-obstructive form of azoospermia, LPO processes increase in seminal plasma and serum, the concentration of reduced glutathione decreases, and the activity of glutathione antioxidant system enzymes (glutathione peroxidase and glutathione transferase) decreases [29].
Sharma et al. found that an imbalance between ROS and TAC production in the seminal fluid indicates oxidative stress and correlates with male infertility. Composite ROS/TAC may be more strongly correlated with infertility than ROS or TAC alone [30].
Meanwhile, studies by Mahfouz et al. showed that the measurement of TAC by colorimetric assays is a reliable and simple test for the diagnosis and treatment of male infertility [31]. Animal studies indicate that decreased antioxidant capacity and increased protein peroxidation in seminal plasma in dogs are associated with poor sperm quality and infertility [32].
Our study did not aim to link TAC levels with male infertility, as this topic has been well studied. We focus on the impact of TAC level in the ejaculate on the parameters of the embryological stage of infertility treatment using ART. Our findings showed a significant decrease in the oocyte fertilization rate with increasing TAC in the ejaculate. Studies by other authors have shown that one of the main pathophysiological effects of free radicals is cell membrane damage of sperm and oocytes [33]. Spermatozoa are particularly susceptible to oxidative stress due to the high content of omega-3 polyunsaturated fatty acids in the membrane and the rather limited ability to protect against oxidation in their cytoplasm. During fertilization, changes in the physiological properties of the male germ cell membrane can cause disruption of capacitation, acrosomal reaction, and gamete fusion. During in vitro fertilization by ICSI, a spermatozoon with a membrane is placed in the oocyte, which can, indeed, disrupt the release of male genetic material and the formation of pronuclei. Altered properties of the male germ cell membrane, especially regarding lipid fluidity, can lead to blocking of pronucleus formation and, as a consequence, the absence/reduction of fertilization rate. In the present study, the analysis of the embryological stage parameters of the infertility treatment using ART and confirmed that fact. The question of changes in the lipid composition of the sperm membrane under the influence of oxidative stress remains open. It is planned to investigate this in the next research projects.
Conclusion
Standard analysis of sperm parameters, such as morphology, sperm count and motility, is important for predicting the fertility of large populations, but they are not sufficient to reliably determine the ability of male sperm to fertilize an egg. The ejaculate also contains components that may affect the fertilizing ability of spermatozoa and that remains poorly understood. Determination of pro-oxidant-antioxidant balance parameters, such as TAC and ROS in male ejaculate may help to guide medical treatment decisions to improve the outcomes of ART infertility treatment. Therapy as part of the preconception care of couples undergoing IVF is currently actively debated in the medical literature and among specialists.
References
- Carson S.A., Kallen A.N. Diagnosis and management of infertility: a review. 2021; 326(1): 65-76. https://dx.doi.org/10.1001/jama.2021.4788.
- Сыркашева А.Г., Коротченко О.Е. Окислительный стресс. Антиоксидантная терапия при прегравидарной подготовке и/или при бесплодии. Медицинский совет. 2017; 13: 150-56. [Syrkasheva A.G., Korotchenko O.Е. Oxidizing stress antioxidant therapy in pregravidar training and/or infertility Meditsinskiy sovet/Medical Council. 2017; 13: 150-6 (in Russian)]. https://dx.doi.org/10.21518/2079-701X-2017-13-150-156.
- Adeoye O., Olawumi J., Opeyemi A., Christiania O. Review on the role of glutathione on oxidative stress and infertility JBRA Assist. Reprod. 2018; 22(1): 61-6. https://dx.doi.org/10.5935/1518-0557.20180003.
- Barati E., Nikzad H., Karimian M. Oxidative stress and male infertility: current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020; 77(1): 93-113. https://dx.doi.org/10.1007/s00018-019-03253-8.
- Agarwal A., Baskaran S., Parekh N., Cho C.L., Henkel R., Vij S. et al. Male infertility. Lancet. 2021; 397(10271): 319-33. https://dx.doi/org/10.1016/S0140-6736(20)32667-2.
- Martins A.D., Agarwal A. Oxidation reduction potential: a new biomarker of male infertility. Panminerva Med. 2019; 61(2): 108-17. https://dx.doi.org/10.23736/S0031-0808.18.03529-2.
- Смольникова В.Ю., Агаджанян Д.С., Красный А.М. Активные формы кислорода и компоненты системы антиоксидантной защиты как маркеры прогнозирования качества эмбрионов у супружеских пар с различными типами бесплодия. Акушерство и гинекология. 2020; 11: 55-60. [Smolnikova V.Yu., Agadzhanyan D.S., Krasnyi A.M. Reactive oxygen species and components of the antioxidant defense system as markers for prediction of embryo quality in married couples with different infertility types. Obstetrics and Gynecology. 2020; 11: 55-60. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.11.55-60.
- Божедомов В.А., Ушакова И.В., Липатова Н.А., Спориш Е.А. Роль гиперпродукции активных форм кислорода в мужском бесплодии и возможности антиоксидантной терапии. Consilium Medicum. 2012; 14(7): 51-5. [Bozhedomov V.A., Ushakova I.V., Sporish E.A. Role of hyperproduction of reactive oxygen species in male infertility and the possibility of antioxidant therapy (in Russian)].
- Agarwal A., Rana M., Qiu E., AlBunni H., Bui AD., Henkel R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018; 50(11): e13126. https://dx.doi.org/10.1111/and.13126.
- Drevet J.R., Hallak J., Nasr-Esfahani M.H., Aitken R.J. Reactive oxygen species and their consequences on the structure and function of mammalian spermatozoa. Antioxid. Redox Signal. 2022 Mar 7. https://dx.doi.org/10.1089/ars.2021.0235.
- Tosic J., Walton A. Formation of hydrogen peroxide by spermatozoa and its inhibitory effect of respiration. Nature. 1946; 158: 485. https://dx.doi.org/10.1038/158485a0.
- Walczak-Jedrzejowska R., Wolski J.K., Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent. European J. Urol. 2013; 66(1): 60-7. https://dx.doi.org/10.5173/ceju.2013.01.art19.
- Oborna I., Fingerova H., Novotny J., Brezinova J., Svobodova M., Aziz N. Reactive oxygen species in human semen in relation to leukocyte contamination. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2009; 153(1): 53-8. https://dx.doi.org/10.5507/bp.2009.009.
- Gharagozloo P., Gutiérrez-Adán A., Champroux A., Noblanc A., Kocer A., Calle A. et al. A novel antioxidant formulation designed to treat male infertility associated with oxidative stress: promising preclinical evidence from animal models. Hum. Reprod. 2016; 31(2): 252-62. https://dx.doi.org/10.1093/humrep/dev302.
- Dorostghoal M., Kazeminejad S.R., Shahbazian N., Pourmehdi M, Jabbari A. Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia. 2017; 49(10). https://dx.doi.org/10.1111/and.12762.
- Евдокимов В.В., Жуков О.Б., Кастрикин Ю.В., Байжуманов А.А., Туровецкий В.Б., Пирутин С.К. Оксидативный стресс и патозооспермия. Андрология и генитальная хирургия. 2017; 18(2): 2432. [Evdokimov V.V., Zhukov O.B., Kastrikin Yu.V., Baizhumanov A.A., Turovetskiy V.B., Pirutin S.K. Oxidative stress and sperm pathologies. Andrology and Genital Surgery. 2017; 18(2): 27-32. (in Russian)]. https://dx.doi.org/10.17650/2070-9781-2017-18-2-27-32.
- Asadi N., Bahmani M., Kheradmand A., Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. J. Clin. Diagn. Res. 2017; 11(5): IE01-IE05. https://dx.doi.org/10.7860/JCDR/2017/23927.9886.
- Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 2015; 97: 55-74. https://dx.doi.org/10.1016/j.ejmech.2015.04.040.
- Гамидов С.И., Шатылко Т.В., Ли К.И., Гасанов Н.Г. Роль антиоксидантных молекул в терапии мужского бесплодия и подготовке мужчины к зачатию ребенка. Медицинский совет. 2020; 3: 122-9. [Gamidov S.I., Shatylko T.V., Li K.I., Gasanov N.G. Role of antioxidant molecules in the treatment of male infertility and preparation of a man for conception. Meditsinskiy Sovet/Medical Council. 2020; 3: 122-9. (in Russian)]. https://dx.doi.org/10.21518/2079-701X-2020-3-122-129.
- Gupta S., Finelli R., Agarwal A., Henkel R. Total antioxidant capacity-relevance, methods and clinical implications. Andrologia. 2021; 53(2): e13624. https://dx.doi.org/10.1111/and.13624.
- Subramanian V., Ravichandran A., Thiagarajan N., Govindarajan M., Dhandayuthapani S., Suresh S. Seminal reactive oxygen species and total antioxidant capacity: Correlations with sperm parameters and impact on male infertility. Clin. Exp. Reprod. Med. 2018; 45(2): 88-93. https://dx.doi.org/10.5653/cerm.2018.45.2.88.
- Макарова Н.П., Романов А.Ю., Долгушина Н.В., Паркер М.М., Красный А.М. Сравнительный анализ экспрессии генов глутатионпероксидазы и глутатионредуктазы в сперматозоидах человека при криоконсервации. Клеточные технологии в биологии и медицине. 2018; 1: 58-62. [Makarova N.P., Romanov A.Yu., Dolgushina N.V., Parker M.M., Krasnyi A.M. Comparative analysis of the expression of glutathione peroxidase and glutathione reductase genes expression in human sperm after cryopreservation. Bulletin of Experimental Biology and Medicine. 2018; 1: 58-62. (in Russian)].
- Chen S.S., Chang L.S., Wei Y.H. Oxidative damage to proteins and decrease of antioxidant capacity in patients with varicocele. Free Radic. Biol. Med. 2001; 30(11): 1328-34. https://dx.doi.org/10.1016/s0891-5849(01)00536-6.
- Приказ Министерства здравоохранения РФ от 31 июля 2020 г. N 803н "О порядке использования вспомогательных репродуктивных технологий, противопоказаниях и ограничениях к их применению" [Order No. 803н of the Ministry of Healthcare of Russia dated 31 July 2020 “On the Procedure for the Use of Assisted Reproductive Technologies, Contraindications and Restrictions to their Application" (in Russian)].
- World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. WHO; 2010.
- Găman M.A., Epîngeac M.E., Diaconu C.C., Găman A.M. Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters. World J. Diabetes. 2020; 11(5): 193-201. https://dx.doi.org/10.4239/wjd.v11.i5.193.
- Cannarella R., Crafa A., Barbagallo F., Mongioì L.M., Condorelli R.A., Aversa A. et al. Seminal plasma proteomic biomarkers of oxidative stress. Int. J. Mol. Sci. 2020; 21(23): 9113. https://dx.doi.org/10.3390/ijms21239113.
- Ahmadi S., Bashiri R., Ghadiri-Anari A., Nadjarzadeh A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016; 14(2): 729-36.
- Vorobets M.Z., Melnyk O.V., Kovalenko I.V., Fafula R.V., Borzhievsky A.T., Vorobets Z.D. Сondition of urogenital tract microbiotes and pro-and antioxidant system in male azoospermia. Regul. Mech. Biosyst. 2021; 12(4): 696-701.
- Sharma R.K., Pasqualotto F.F., Nelson D.R., Thomas A.J. Jr, Agarwal A. The reactive oxygen species – total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum. Reprod. 1999; 14(11): 2801-7. https://dx.doi.org/10.1093/humrep/14.11.2801.
- Mahfouz R., Sharma R., Sharma D., Sabanegh E., Agarwal A. Diagnostic value of the total antioxidant capacity (TAC) in human seminal plasma. Fertil. Steril. 2009; 91(3): 805-11. https://dx.doi.org/10.1016/j.fertnstert.2008.01.022.
- Domoslawska A., Zdunczyk S., Franczyk M., Kankofer M., Janowski T. Total antioxidant capacity and protein peroxidation intensity in seminal plasma of infertile and fertile dogs. Reprod. Domest. Anim. 2019; 54(2): 252-7. https://dx.doi.org/10.1111/rda.13345.
- Brody S.A. Мужское бесплодие и окислительный стресс: роль диеты, образа жизни и пищевых добавок. Андрология и генитальная хирургия. 2014; 15(3): 33-41. [Brody S.A. Male factor infertility and oxidative stress: role of diet, lifestyle and nutritional supplements. Andrology and Genital Surgery. 2014; 15(3): 33-41. (in Russian)]. https://dx.doi.org/10.17650/2070-9781-2014-3-33-41.
Received 09.03.2022
Accepted 28.04.2022
About the Authors
Diana S. Agadzhanyan, MD, Postgraduate Student, B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. KulakovNational Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, agadzhanyand@inbox.ru,
117997, Russia, Moscow, Academician Oparin str., 4.
Natalia N., Lobanova, Junior Researcher, B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, n_lobanova@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.
Veronika Yu. Smolnikova, Dr. Med. Sci., Associate Professor, Leading Researcher, B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation,
v_smolnikova@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Natalya P. Makarova, Dr. Bio. Sci., Leading Researcher, B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov
National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, np_makarova@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.
Aleksey M. Krasnyi, PhD (Bio), Head of the Laboratory of Cytogy, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, alexred@list.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Alsu A. Sadekova, PhD (Bio), Researcher at the Laboratory of Cytogy, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of the Russian Federation, sialsad@gmail.com, 117997, Russia, Moscow, Academician Oparin str., 4.
Valeriya S. Shchipitsyna, Junior Researcher at the Laboratory of Cytogy, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of the Russian Federation, v_shchipitsyna@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Diana N. Kokoeva, PhD, Junior Researcher at the Laboratory of Cytogy, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of the Russian Federation, d_kokoeva@oparina4.ru, 117997, Russia, Moscow, Academician Oparin str., 4.
Elena A. Kalinina, Dr. Med. Sci., Professor, Head of the B.V. Leonov Department of Assisted Technologies for the Treatment of Infertility, Academician V.I. Kulakov
National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation, e_kalinina@oparina4.ru,
117997, Russia, Moscow, Academician Oparin str., 4.
Authors' contributions: Agadzhanyan D.S. – collection and analysis of literature, manuscript drafting; Smolnikova V.Yu., Makarova N.P., Lobanova N.N. – conception and design of the study, patient selection, manuscript editing; Shchipitsyna V.S., Kokoeva D.N. – measuring oxidative stress parameters; Sadekova A.A. – measuring oxidative stress parameters, manuscript editing; Krasnyi A.M. – manuscript editing; Kalinina E.A. – manuscript editing and approval.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Agadzhanyan D.S., Lobanova N.N., Smolnikova V.Yu., Makarova N.P., Krasnyi A.M., Shchipitsyna V.S., Sadekova A.A., Kokoeva D.N., Kalinina E.A.
Characteristics of the embryological stage of infertility treatment using assisted reproductive technologies depending on the total antioxidant capacity of native ejaculate.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 5: 101-108 (in Russian)
https://dx.doi.org/10.18565/aig.2022.5.101-108



