Specific features of the expression of syndecan-1 and heparanase in the epithelium of Stage III ovarian endometrioid cysts
Objective. To investigate the expression of syndecan-1 and heparanase in the lining of the ovarian endometrioma capsule in reproductive-aged women in the proliferative phase of the cycle, by assessing the severity of inflammatory infiltration and the correlation with pain syndrome.Marinkin I.O., Timofeeva Yu.S., Kuleshov V.M., Volchek A.V., Makarov K.Yu., Omigov V.V., Aidagulova S.V.
Subjects and methods. Twenty patients aged 27.7 ± 5.3 years with Stage III ovarian endometrioid cysts were examined using a semiquantitative immunohistochemical assessment of the expression of syndecan-1 and heparanase in the capsule of resected endometriomas, as well as a statistical analysis.
Results. The expression of heparanase in the epithelial nuclei with sufficient statistical significance (p < 0.0001) shows a strong positive correlation with a visual analog scale pelvic pain scores of 3–5 (r = 0.902) and with subepithelial inflammatory infiltration (r = 0.887), as well as a strong negative correlation with the manifestations of cytogenic stromal fibrosis (r = -0.902).
Conclusion. The pronounced heparanase expression in the epithelium of ovarian endometriomas is associated with inflammatory cell infiltration and the presence of pain syndrome; no significant regularities were found for syndecan-1.
Keywords
External genital endometriosis (NGE) affects up to 22% of infertile women in their reproductive age and is associated with estrogen-dependence, pelvic pain, persistent activity and some fundamental characteristics resembling malignancy [1 – 3]. Among the latter are an increased proliferative activity of epithelial cells and cytogenic stroma, and the so-called “metastasis” i.e., the spread of proliferates, or extrauterine endometrial tissue heterotopia, indicating abnormalities of cell adhesion and cell-cell contacts. In most tissues, cell-cell and cell-matrix interactions occur due to a specific spectrum of biologically active compounds with a prominent role of large heavily glycosylated protein molecules – proteoglycans [4, 5]. One of the proteoglycans, transmembrane heparan sulfate syndecan-1 (SDC1, also known as CD138) is found on plasma cells in endometrial tissue during the proliferative phase of the menstrual cycle and thought to be the most reliable marker of chronic endometritis [6]. The biological role of SDC1 consists, first of all, in using its extracellular carbohydrate chains to bind some growth factors, in particular, vascular endothelial growth factor (VEGF) [7]. In various cells expressing SDC1, the cleavage of its carbohydrate chains triggers a cascade of molecular signals, which provides a powerful mechanism to enhance tumor growth, angiogenesis, invasion and metastasis [8, 9]. The function of cleaving carbohydrate chains from heparan sulfates is performed by heparanase (HPSE) – the only endoglycosidase in mammals, which expression is significantly increased in aggressive cancers and tissues with active proliferation [5]. We have demonstrated that high expression of HPSE in foci of adenomyosis in patients with multiple uterine fibroids the size of a 12-week pregnancy has a negative correlation with total heparan sulfates expression [10].
Despite the growth in research on endometriosis using full-genome methods, reliable biomarkers for non-invasive diagnosis of endometriosis are still not available [11 – 13]. In recent years, studies addressing this issue have been increasingly focused on gene expression in the eutopic endometrium, and the differences can be used as biomarkers for minimally invasive diagnostics of EGE.
In recent years, there have been some domestic studies investigating gene expression profiles in eutopic endometrium [14, 15]. Laparoscopy with biopsy and histological verification of the disease remain the “gold standard” of diagnostics [16]. In the concepts of pathomorphogenesis of ovarian endometriosis, the role of inflammation has been noted [13].
This study was aimed to investigate the expression of syndecan-1 and heparanase in the ovarian endometrioma inner lining in women of reproductive age in the proliferative phase of the menstrual cycle and estimate the severity of inflammatory infiltration and correlation with pain syndrome.
Material and methods
This is a clinical and morphological study of 20 patients aged 27.7 ± 4.3 years with stage III endometriotic ovarian cysts (according to the criteria of L.V. Adamyan et al. [17]), who underwent surgery from 2014 to 2016 at the Department of Gynecology of Novosibirsk Regional Clinical Hospital affiliated with the Department of Obstetrics and Gynecology of the Novosibirsk State Medical University. Nineteen patients underwent endometrioma enucleation, and one had ovarian resection; in all women, an excision of pelvic endometriotic lesions and separation of the adhesions was carried out.
The inclusion criteria for the study patient selection were as follows: elective surgery during the proliferative phase of the menstrual cycle, the reproductive age up to 35 years, the level of follicle-stimulating hormone (FSH) less than 15 mIU/ml, the level of anti-Müllerian hormone (AMH) at least 2 ng/ml, histologically verified ovarian endometriosis and patients’ informed consent prior to their inclusion in the study. Exclusion criteria were as follows: pregnancy, hormone therapy at least three months before surgery, cancer, immunodeficiency states, and decompensated extragenital comorbidities.
Samples of resected endometriotic tissue were fixed in buffered formalin and embedded in the Histomix paraffin medium; paraffin sections were stained with hematoxylin and eosin. The expression of SDC1 and HPSE was examined using a two-step immunohistochemical (IHC) staining technique, monoclonal antibodies to SDC1 (Dako, ready to use) and to HPSE (Abnova) were used as primary antibodies at a dilution of 1: 200; the reaction products were visualized with diaminobenzidine (DAB), the cell nuclei were stained with Mayer’s hematoxylin. For negative control, 5% bovine serum was used instead of primary antibodies. The area of intra – and extracellular products of the IHC reaction was evaluated semi-quantitatively (1–3 points) using an Axio Scope.A1 microscope and photographed with an AxioCam MRc5 camera (S. Zeiss); each parameter was evaluated by 20–25 images with a magnification of 63×12.
Statistical analysis was performed using the STATISTICA v.6.0 software. The Spearman correlation test was used to assess the association between the expression of SDC1 and HPSE by DAB-positive products of IHC reaction and the severity of pain on a visual analog scale (VAS). Depending on the presence or absence of subepithelial inflammatory infiltration, 18 patients were divided into two equal subgroups to analyze the differences in the expression of SDC1 and HPSE. Next, 18 patients were allocated into two subgroups categorized by the presence or absence of the fibrosis of cytogenic stroma (9 in each subgroup) to study differences in the expression of HPSE and SDC1. When comparing pairs of subgroups categorized by the presence of subepithelial inflammatory infiltration or by the fibrosis of cytogenic stroma, the Mann-Whitney test was used. Differences were considered statistically significant at p <0.05.
Results and discussion
The most common complaints among all 20 patients were pain score of 2–5 on the VAS (n = 13, 65%) and infertility (n = 13, 65%). At the same time, five patients were almost asymptomatic (pain score of 1–2 on the VAS), despite the verified stage III of endometriotic ovarian cysts [17]. Fifteen patients (75%) did not realize their reproductive function; of them, two had an early reproductive loss, and the others had no pregnancies.
Concomitant gynecological diseases included cervical conditions (n = 12, 60%), uterine fibroids (n = 7.35%) and pelvic inflammatory diseases (n = 6, 30%) with obstruction of the Fallopian tubes, which were diagnosed by chromosalpingoscopy. Diagnostic hysteroscopy before surgery was performed in 4 patients, who were found to have an endometrial polyp (n = 2) and adenomyosis (n = 2). Before surgery, only one patient was diagnosed with chronic endometritis verified by the histological examination of pipelle endometrial biopsy specimens.
Concentrations of CA125 in blood samples ranged from 23 to 69 U/ml and with a mean value (40.33 ± 16.9 U/ml) elevated slightly above the reference range. The main indicators of ovarian reserve – FSH and AMH – were within the reference range of 7.33 ± 1.2 mIU/ml and 3.79 ± 1.61 ng/ml, respectively.
The findings of IHC analysis of the ovarian endometrioma inner lining showed that SDC1 was located mainly in the basal and, to a lesser extent, on the basolateral plasmalemma of epithelial cells and was poorly expressed in the nuclei of epithelial cells (Figure, a). However, an increase in CA125 correlated even with minimal expression of SDC1 in the epithelium (r = 0.646; p = 0.0037). In the cytogenic stroma, the expression of SDC1 was located in the extracellular matrix and had a negative correlation with the expression of epithelial cells in the cytoplasm (r = –0.671; p = 0.0023); it was also detected in the cytolemma of solitary plasma cells.
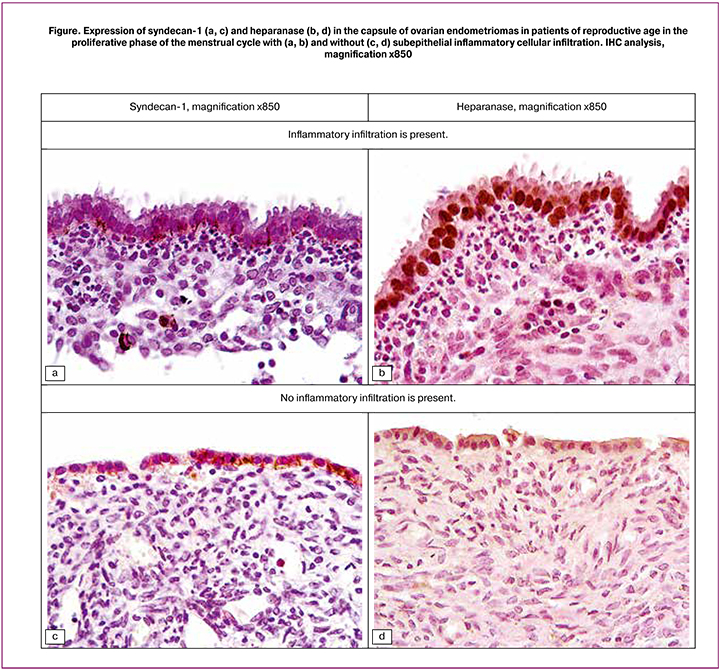
The expression of HPSE in the same samples was different from the expression of SDC1. In all cases, most or all of the epithelial nuclei were highly or moderately DAB-positive (Figure, b), but there were significant fluctuations in the response of the cell cytoplasm – from no response to very marked one. The intensity of SDC1 expression in the nucleus and cytoplasm of endometrioma epithelial cells was generally weak, but had a higher correlation coefficient (r = 0.707; p = 0.001), compared with the expression of HPSE, the intensity of which in the nuclei and cytoplasm of epithelial cells had a moderate positive correlation (r = 0.527; p = 0.0246). Compared with observations with pronounced subepithelial cell infiltration, with mild infiltration and absence of neutrophils, the expression of SDC1 in the epithelium and endometriomas was similar (Figure, c), but the expression of HPSE was reduced (Figure, d).
High expression of HPSE in the nuclei of endometrial epithelial cells directly correlated with the presence of subepithelial inflammatory infiltration (r = 0.949; p <0.0001) and with more intense pain on the VAS (r = 0.902; p <0.0001), but negatively correlated with expression HPSE in the cytogenic stroma (r = –0.949; p <0.0001) and with its fibrosis (r = –1.0; p <0.0001). The presence of subepithelial inflammatory infiltration was positively correlated with pain score of 3–5 on the VAS (r = 0.887; p <0.0001) and negatively correlated with fibrosis (r = –0.949; p <0.0001), as well as with the expression of HPSE in the cytogenic stroma (r = –1.0; p <0.0001).
In general, pelvic pain score of 3–5 on the VAS in patients with stage III ovarian endometriomas had a statistically significant (p <0.0001) positive correlation with HPSE expression in epithelial nuclei (r = 0.902) and inflammatory infiltration (r = 0.887), and negative correlation with the presence of cytogenic stroma fibrosis (r = –0.902).
Next, using the Mann-Whitney test, we compared the indicators of the expression of heparan sulfate SDC1 and the enzyme of its biodegradation HPSE in endometriomas depending on the presence or absence of subepithelial inflammatory infiltration (9 clinical observations). For SDC1, no significant differences were found between the groups (Table 1). At the same time, the groups differed significantly in the expression of HPSE in the nuclei (p = 0.0001) and cytoplasm (p = 0.0354) of the epithelium and cytogenic stroma (p = 0.0002) of ovarian endometrioma, with pain score of 3–5 on the VAS (p = 0.0003), and the absence of the fibrosis of cytogenic stroma (p = 0.0001).
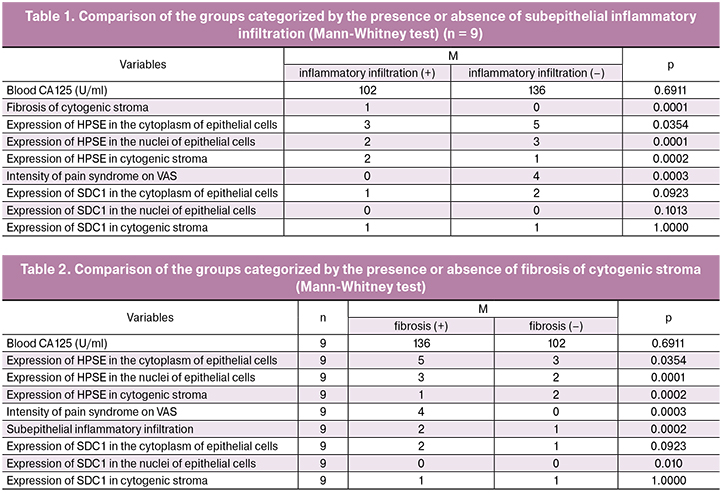
There were differences between the subgroups when they were categorized by the presence or absence of fibrosis of the cytogenic stroma (Table 2). Significant between-subgroup differences were found in the presence of inflammatory infiltration (p = 0.0002), pain syndrome (p = 0.0003) and higher expression of HPSE in the nuclei (p = 0.0001), and cytoplasm of the endometrial epithelium (p = 0.0354). At the same time, the presence of fibrosis was accompanied by a higher expression of HPSE by subepithelial stromal cells of cysts (p = 0.0002), which indicates a high probability of an impaired intercellular contact, release of growth factors and activation of connective tissue cell migration, i.e., maintaining growth potential.
As a rule, in tumors, the amounts of heparan sulfate proteoglycan SDC1 and the enzyme of its metabolism HPSE are inversely proportional; however, it has recently become clear that these two molecules work in concert forming the so-called heparanase/syndecan-1 axis [9]. HPSE drives tumor progression, promoting the expression and bioactivity of several key growth factors, including vascular endothelial growth factor (VEGF), which abounds in the tumor microenvironment [18]. At the same time, activating the ERK (Extracellular signal-Regulated Kinase) signaling pathway contributes to survival, proliferation, and motility of the cells, and increases cell expression of growth factors and matrix metalloproteinase-9 (MMP-9) [19].
Besides, due to the activity of HPSE on the cell surface, carbohydrate chains are cleaved from SDC1, which enhances the enzymatic degradation of the core protein of the heparan sulfate molecule with the participation of MMP-9. The detached SDC1 joins VEGF secreted by the tumor and other growth factors, concentrating them in the tumor microenvironment and potentiating their signaling properties. In general, the synchronous activity of HPSE, SDC1, and inflammation, is a powerful mechanism for the progression of tumor growth, angiogenesis, invasion, and metastasis [9, 20].
Therefore, it is important to note that the absence of a significant negative correlation between the expression of SDC1 and HPSE in the epithelium of stage III ovarian endometriomas may be additional evidence of the fundamental difference between endometriotic heterotopies and tumor proliferates.
Conclusion
The findings of IHC analysis of the ovarian endometrioma inner lining in patients of early reproductive age with stage III endometriotic ovarian cysts suggest that the marked expression of HPSE in the epithelium is associated with subepithelial inflammatory cell infiltration and the presence of pain score of 3–5 on the VAS. The expression of heparan sulfate proteoglycan SDC1 in the epithelium of endometriomas is reduced and does not correlate with the high expression of HPSE.
References
1. Machairiotis N., Stylianaki A., Dryllis G., Zarogoulidis P., Kouroutou P., Tsiamis N. et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn. Pathol. 2013; 8: 194. doi: 10.1186/1746-1596-8-194
2. Becker C.M., Gattrell W.T., Gude K., Singh S.S. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil. Steril. 2017; 108(1): 125-136. doi: 10.1016/j.fertnstert.2017.05.004
3. Amalinei C., Păvăleanu I., Lozneanu L., Balan R., Giuşcă S.E., Căruntu I.D. Endometriosis — insights into a multifaceted entity. Folia Histochem. Cytobiol. 2018; 1(2): 61-82. doi: 10.5603/FHC.a2018.0013
4. Суховских А.В., Григорьева Э.В. Тканеспецифичность экспрессии протеогликанов в различных типах опухолей человека. Успехи молекулярной онкологии. 2016; 3 (1): 53-60. doi: 10.17650/2313-805X-2016-3-1-53-60х
5. Karamanos N.K. Matrix pathobiology — central roles for proteoglycans and heparanase in health and disease. FEBS J. 2017; 284(1): 7-9. doi: 10.1111/febs.13945
6. Vidyavathi K., Harendra K.M.L., Venigalla S. Evaluation of endometrium for chronic endometritis by using syndecan-1 in abnormal uterine bleeding. J. Lab. Physicians. 2012; 4: 69-73. doi: 10.4103/0974-2727.105584
7. Afratis N.A., Nikitovic D., Multhaupt H.A., Theocharis A.D., Couchman J.R., Karamanos N.K. Syndecans — key regulators of cell signaling and biological functions. FEBS J. 2017; 284(1): 27-41. doi: 10.1111/febs.13940
8. Sukhovskih A.V., Mostovich L.A., Kunin I.S., Boboev M.M., Nepomnyashchikh G.I., Aidagulova S.V., Grigorieva E.V. Proteoglycan expression in normal human prostate tissue and prostate cancer. ISRN Oncology. 2013; doi: 10.1155/2013/680136
9. Ramani V.C., Purushothaman A., Stewart M.D., Thompson C.A., Vlodavsky I., Au J.L., Sanderson R.D. The heparanase/syndecan-1 axis in cancer: mechanisms and therapies. FEBS J. 2013; 280(10): 2294-306. doi: 10.1111/febs.12168
10. Маринкин И.О., Пивень Л.А., Соляников Д.А., Тимофеева Ю.С., Кулешов В.М., Соколова Т.М., Макаров К.Ю., Омигов В.В., Айдагулова С.В. Особенности экспрессии эндогликозидазы в очагах аденомиоза у пациенток репродуктивного возраста с миомой тела матки. Акушерство и гинекология. 2016; 11: 79-85. doi: https://dx.doi.org/10.18565/aig.2016.11.79-85
11. Hirsch M., Duffy J.M.N., Davis C.J., Nieves Plana M., Khan K.S. International collaboration to harmonise outcomes and measures for endometriosis. Diagnostic accuracy of cancer antigen 125 for endometriosis: a systematic review and meta-analysis. BJOG. 2016; 123: 1761-1768. doi: 10.1111/1471-0528.14055
12. Gupta D., Hull M.L., Fraser I., Miller L., Bossuyt P.M., Johnson N., Nisenblat V. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016; 4: CD012165. doi: 10.1002/14651858.CD012165
13. Klemmt P.A.B., Starzinski-Powitz A. Molecular and Cellular Pathogenesis of Endometriosis. Curr. Womens Health Rev. 2018; 14(2): 106-116. doi: 10.2174/1573404813666170306163448
14. Тихончук Е.Ю., Асатурова А.В., Адамян Л.В. Молекулярно-биологические изменения эндометрия у женщин с наружным генитальным эндометриозом. Акушерство и гинекология. 2016; 11: 42-48. doi: https://dx.doi.org/10.18565/aig.2016.11.42-8
15. Кузнецова М.В., Пшеничнюк Е.Ю., Бурменская О.В., Асатурова А.В., Трофимов Д.Ю., Адамян Л.В. Исследование экспрессии генов в эутопическом эндометрии женщин с эндометриоидными кистами яичников. Акушерство и гинекология. 2017; 8: 93-102. doi: https://dx.doi.org/10.18565/aig.2017.8.93-102
16. Fassbender A., Vodolazkaia A., Saunders P., Lebovic D., Waelkens E., De Moor B., et al. Biomarkers of endometriosis. Fertil. Steril. 2013; 99: 1135-45. doi: 10.1016/j.fertnstert.2013.01.097
17. Адамян Л.В., Куликов В.И., Андреева Е.Н. Эндометриозы: Руководство для врачей. М.: Медицина, 2006. 416 с.
18. Pisano C., Vlodavsky I., Ilan N., Zunino F. The potential of heparanase as a therapeutic target in cancer. Biochem. Pharmacol. 2014; 89 (1): 12-9. doi: 10.1016/j.bcp.2014.02.010
19. Jana S., Chatterjee K., Ray A.K., DasMahapatra P., Swarnakar S. Regulation of Matrix Metalloproteinase-2 Activity by COX-2-PGE2-pAKT Axis Promotes Angiogenesis in Endometriosis. PLoS ONE. 2016; 11(10): e0163540. doi: 10.1371/journal.pone.0163540
20. Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002; 420(6917): 860-867. doi: 10.1038/nature01322
Received 13.08.2018
Accepted 21.09.2018
About the Authors
Marinkin, Igor’ O., doctor of medical sciences, professor, the head of the Obstetrics and Gynecology Department of Novosibirsk State Medical University (NSMU) and rector of NSMU, +7(383)222-32-04, rector@ngmu.ruTimofeyeva, Yulia S., assistent of the Obstetrics and Gynecology Department of NSMU, +79061947755, julie_srg@mail.ru
Kuleshov, Vitaliy M., doctor of medical sciences, professor, professor of the Obstetrics and Gynecology Department of NSMU, +7(383)341-04-36, kuleshov_vm@mail.ru
Volchek, Alexandr V., physician-gynecologist of the State Budget Health Foundation of Novosibirsk region «Maternity house № 2», +79130031722, alexander@volcheck.ru
Makarov, Konstantin Yu., doctor of medical sciences, docent, professor of the Obstetrics and Gynecology Department of NSMU, +7(383)222-93-12, fdpngma@mail.ru
Omigov, Vladimir V., candidate of medical sciences, senior researcher of laboratory of cellular biology and fundamental basis of reproduction of NSMU’s Central scientific laboratory, +7(383)226-35-60, omigov@vector.nsc.ru
Aidagulova, Svetlana V., doctor of biological sciences, professor, head of laboratory of cellular biology and fundamental basis of reproduction of NSMU’s Central scientific laboratory, +79139092251, s.aydagulova@gmail.com
ORCID 0000-0001-7124-1969
For citations: Marinkin I.O., Timofeeva Yu.S., V.M. Kuleshov1, Volchek A.V., Makarov K.Yu., Omigov V.V., Aidagulova S.V. Specific features of the expression of syndecan-1 and heparanase in the epithelium of Stage III ovarian endometrioid cysts
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2018; (11): 86-91. (in Russian)
https://dx.doi.org/10.18565/aig.2018.11.86-91



