Placental insufficiency in pregnant women with high body mass index and without gestational diabetes
Objective: To investigate blood levels of two adipokines in the first trimester and expression of placental vascular endothelial growth factor (VEGF) in the third trimester in women with a high body mass index (BMI) and without gestational diabetes. Materials and methods: The prospective observational study included 70 patients with grade I–III obesity (24, 24, and 22 women, respectively) who gave birth at full term to newborns with an Apgar score of 7–8. The control group consisted of 22 postpartum women with a normal BMI. Blood adiponectin and omentin were determined using an enzyme-linked immunosorbent assay (ELISA) before nine weeks of gestation. The expression of vascular endothelial growth factor (VEGF) was examined in 24 placenta samples. Results: At the gestational age of 8–9 weeks, blood adiponectin level in women with grade I-III obesity was statistically significantly lower than in the controls. Omentin levels in the control group and those with grade I obesity did not differ and exceeded those with grade II and III obesity. There was a moderate negative correlation between the BMI of women of all groups and the blood omentin levels (rs=-0.565, p<0.0001). The IHCA showed that the total VEGF expression in terminal villi and capillaries of the fetal placentae in obesity is reduced compared to the control group. In a pairwise comparison of groups with high BMI, the highest VEGF expression was found in obesity grade I relative to grades II and III, which did not differ from each other. Conclusion: In the first trimester of pregnancy, maternal pregravid obesity is associated with decreased adiponectin and omentin concentrations and placental VEGF expression. A high BMI, starting with II grade obesity, can be considered a risk factor for placental insufficiency.Datsenko N.S., Volchek A.V., Yakimova A.V., Pozdnyakov I.M., Ageeva T.A., Marinkin I.O., Aidagulova S.V.
Keywords
The number of women of reproductive age who are overweight or obese continues to increase, becoming a global medical and social issue [1]. Obesity in pregnancy is associated with an increased incidence of adverse maternal and fetal outcomes, including gestational diabetes, preeclampsia, and fetal growth restriction [2, 3].
The placenta of pregnant women with obesity is characterized by changes in several functions associated with inflammation and oxidative stress [4, 5]. In the placenta and other organs, many factors regulate normal angiogenesis, including vascular endothelial growth factor (VEGF) [6]. A comparative immunohistochemical analysis (IHCA) of the placenta of pregnant women with gestational and type 1 diabetes mellitus showed an increase in expression levels of VEGF and its receptors (VEGFR-1, -2, -3) in the placental terminal and stem villi, which had higher expression in type 1 diabetes mellitus [7]. According to [8], the development and increase in the severity of preeclampsia were associated with gestational endotheliosis and a statistically significant decrease in serum VEGF.
Adipokines, such as omentin, are a group of cytokines secreted by the adipose tissue and the placenta exerting positive effects on metabolic processes and organization of the placental barrier. In obese mothers in the terminal villi of the placenta, the expression of leptin and adiponectin was studied along with the analysis of DNA methylation [9]. The authors showed that maternal obesity was associated with the downregulation of leptin and adiponectin systems in term placenta and thus a loss of the beneficial effects of these two adipokines on placental development. Maternal obesity was also associated with epigenetic changes in leptin and adiponectin systems.
When comparing 96 pregnant women with gestational diabetes and 96 without this pathology, there were no statistically significant differences in the blood omentin-1 levels; however, omentin-1 levels were significantly lower in the offspring of mothers with gestational diabetes, which increased the risk of developing insulin resistance later in life [10].
Maternal adiponectin may be a significant predictor of fetal growth and birth weight regardless of BMI and insulin resistance [11]. Results of a randomized, double-blind, placebo-controlled trial of the effects of metformin from 12-18 weeks of gestation to delivery in non-diabetic pregnant women with a BMI greater than 35 kg/m2 showed that metformin did not significantly affect birth weight and the risk of gestational diabetes [12].
In general, there are conflicting and controversial results regarding the role of adipokines in reproductive dysfunction in obese women, which necessitates further research [13].
The present study aimed to investigate blood levels of adipokines in the first trimester and expression of placental vascular endothelial growth factor (VEGF) in the third trimester in women with a high body mass index (BMI) and without gestational diabetes.
Materials and methods
Patients. This is an observational descriptive one-stage study including 70 obese pregnant women with a BMI before pregnancy > 30 kg/m2 who gave birth at 37–41 weeks of gestation to a live infant without asphyxia, with an Apgar score of 7–8. Groups 1 (n=24), 2 (n=24), and 3 (n=22) included women aged 30.3 (0.5), 30.0 (0.9), and 33.5 (0.8) years with grade I [BMI 31.88 (1.40)], II [BMI 36.60 (1.07)], and III [BMI 42.20 (1.90)] obesity, respectively. Control group included 22 women aged 28.5 (0.7) with a normal BMI [21.0 (1.88)]. Multiparous women predominated in all four groups. Exclusion criteria were chronic arterial hypertension, gestational diabetes, preeclampsia, and other vascular extragenital pathology. Patients signed informed consent to participate in the study per the legislation of the Russian Federation and the ethical guidelines of the "World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects" adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and amended by the 59th WMA General Assembly, Seoul, South Korea, October 2008. The study was reviewed and approved by the Research Ethics Committee of the NSMU (Protocol No. 1 dated June 24, 2019).
Enzyme-linked immunosorbent assay (ELISA)
To study concentrations of adipokines (adiponectin and omentin) at 8–9 weeks of gestation, fasting blood samples (10 ml) were drawn from the antecubital vein into a dry test tube and centrifuged at 1500 rpm for 10 min. The supernatant was frozen at -70°C in plastic tubes. The studies were performed using commercial test systems Human Adiponectin ELISA and RayBio Human Omentin ELISA (Germany) according to the manufacturer's instructions. The ELISA results were recorded on a vertical photometer Uniplan (Russia) at a wavelength of 450 nm. To determine the concentration of each of the adipokines in the analyzed samples, calibration plots were built according to the average readings of the optical density of each standard solution.
Immunohistochemical (IHCA) study
Analysis of VEGF expression was performed on paraffin sections of the placenta of 6 randomly selected women from each of 4 groups using ready-to-use rabbit monoclonal antibodies (cat. No. RM-9128-R7) using UltraVision Quanto Detection System HRP kit (ThermoScientific) with diaminobenzidine (DAB). Cell nuclei were counterstained with hematoxylin. The products of IHC reactions were studied in 15 fields of view (i.e., 90 per group) with the densest localization of villi using an Axio Scope.A1 microscope with an AxioCam MRc5 camera and a Zen blue image analysis program (C. Zeiss) at a magnification of 400, separately focusing on the total expression (VEGFSUMM), in syncytiotrophoblast (VEGFSTB) and fetal capillaries (VEGFCAP), which were expressed in µm2.
Statistical analysis
Continuous variables were compared between two groups with a Student’s t-test. (critical level of significance p<0.05) using Microsoft Excel 2019 and MedCalc Statistical Software, version 18.9.1 (http://www.medcalc.org; 2018 ) based on the normality of the data assessed by the Shapiro-Wilk test (Shapiro–Wilk test for BMI: W=0.94; p<0.001). The correlation between VEGF expression in the structural compartments of the placenta and blood adipokines with BMI was tested with Spearman's rank correlation. Quantitative variables are presented as M (SD). Categorical variables were compared using the χ2 test. P-values=0.008 were considered statistically significant, adjusted for multiple comparisons using the Bonferroni correction.
Results and discussion
Body mass index (BMI) was [M (SD)] 31.51 (1.13) 36.70 (1.27), and 41.86 (1.89) kg/m2 in groups 1, 2, and 3, which was statistically significantly different from the control group [20.90 (1.93)] and differed in all-pairwise comparisons between groups with obesity (p<0.0001).
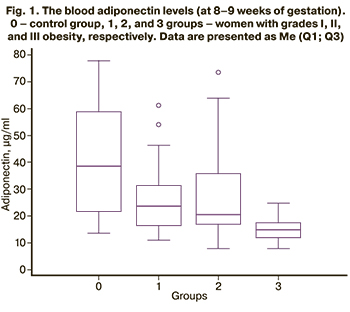
The blood adiponectin levels of women in the control group (at the gestational age of 8–9 weeks) was 41.17 (20.86) μg/ml, which was statistically significantly higher than in each of the obese groups, including [(26.66 (13.90), p=0.003)], [(26.93 (16.55), p=0.003)], and [(17.41 (9.82), p<0.0001)] μg/ml in groups 1,2, and 3. The blood adiponectin levels in group 1was higher than in group 3 (p=0.02) and in group 2 was higher than in group 3 (p=0.03); however, there was no difference between groups 1 and 2 (p=0.95) (Fig. 1).
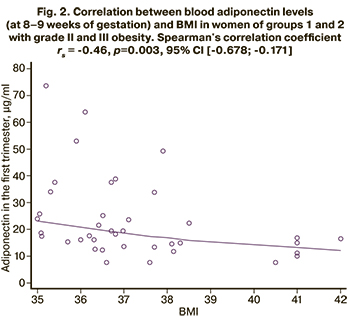
A correlation analysis was performed to evaluate the relationship between blood adiponectin levels (at 8–9 weeks of gestation) and BMI. Statistically significant results were obtained only for groups 1 and 2 with grades II and III obesity (Fig. 2): the adiponectin concentration decreased with BMI increase. Spearman's correlation coefficient rs = -0.46, p=0.003, 95% CI (-0.678; -0.171).
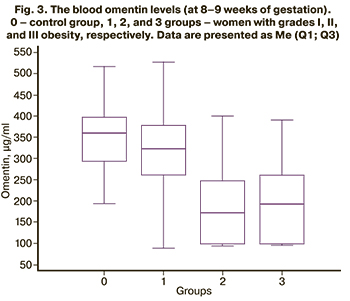
Blood concentrations of adipokine-omentin (at 8–9 weeks of gestation) in women in the control group was 346.49 (78.26) μg/ml and did not differ statistically significantly from group 1 (308.72 (110.88 ) μg/ml, p=0.08), while the mean values of both the control and group 1 exceeded those in the groups with obesity [group 2 (179.63 (88.25), p<0.0001 for both comparisons and group 3 (191.16 (90.26) μg/ml, p<0.0001 and p=0.0006, respectively)]. At the same time, the blood omentin level in women with grades II and III obesity of II and III did not differ, p=0.67 (Fig. 3).
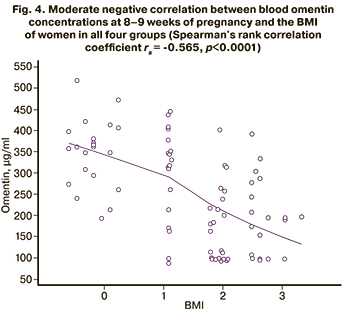
A moderate negative correlation was found between the BMI of women in all four groups and the omentin concentration: rs = -0.565, p<0.0001 (Fig. 4).
Analysis of obstetric clinical parameters (table) revealed statistically significant differences in placenta weight with a gradual decrease from the control group to group 3 (pregnant women with the highest BMI).
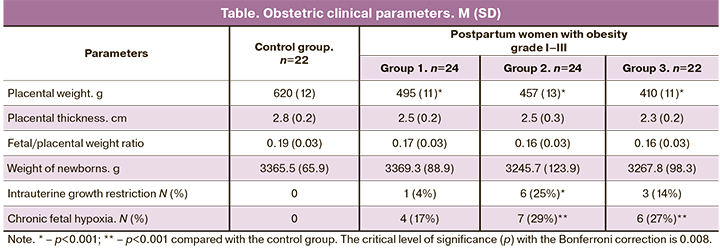
In the control group (Table), there were no clinical manifestations of chronic placental insufficiency – intrauterine growth restriction (IUGR) and chronic fetal hypoxia (CFH). At the same time, there were statistically significant differences with the control group in the number of IUGR cases in the 2nd group (p<0.05); in addition, in terms of CFH, the 2nd and 3rd groups differed from the control (p<0.01).
In obese puerperas, histological examination of placenta samples revealed involutive-dystrophic changes in villi of varying severity in combination with compensatory-adaptive reactions: expressed syncytio-capillary membranes, vascular congestion, a large number of chorionic symplasts, which can be regarded as manifestations of the types of cellular adaptation [14]. Along with this, in the morphometric study, an increase in BMI was accompanied by a progressive decrease in the density of capillaries in the terminal villi of the placentas: 2-fold in group 1, 2.2-fold in group 2, 2.5-fold in group 3, compared with the control group [15], which indicates a reduction in vasculogenesis.
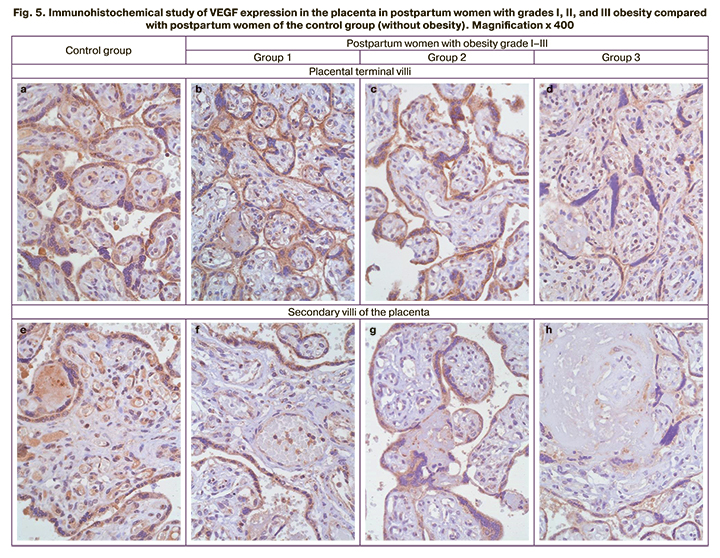
IHCA study of placenta samples at 37–41 weeks showed a variation in VEGF expression in terminal and secondary villi with a decrease in DAB-positive staining with the progression of obesity, including more severe atrophy and fibrosis (Fig. 5).
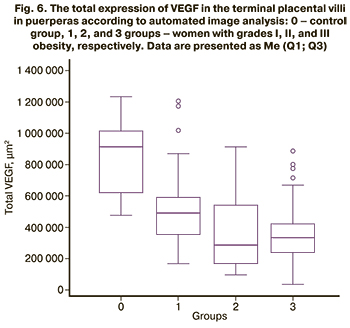
The study of placental VEGF expression in postpartum women using automated image analysis (Fig. 6) revealed a statistically significant (p<0.0001) decrease in the area of DAB-positive zones in obese women [508809 (228264) μm2 in group 1, 368556 (225529) μm2 in group 2, and 349441 (148609) μm2 in group 3] compared with the control group (864662 (230605) μm2). In contrast, groups 2 and 3 did not differ from each other (p=0.46), having lower VEGF expression compared to group 1 (p<0.0001).
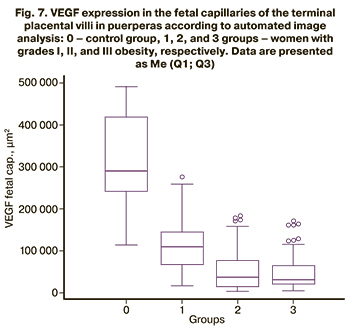
When the zones of fetal capillaries were identified, and the area of VEGF expression was automatically estimated, the results were similar to the total expression in 4 groups (Fig. 7). Thus, compared with the control group [311810 (107857) μm2], there was a statistically significant (p<0.0001) decrease in the area of DAB-positive zones in obese puerperas [114607 (58286) μm2 in group 1, 56906 (41221) μm2 in group 2 and 51531 (45571) μm2 in group 3]. In contrast, groups 2 and 3 did not differ from each other (p=0.50), but were less in comparison with group 1 (p<0.0001).
Since VEGF was intensely expressed in the syncytiotrophoblast (Fig. 5), we separately analyzed the patterns of its expression obtained by subtracting the indices of each visual field (VEGFSTB = VEGFSUM - VEGFFETALCAP). VEGFSTB expression in the control group (552853 (208553) μm2) was statistically significantly (p<0.0001) higher than in each of the groups of obese parturient women: group 1 [394203 (200193)], group 2 [311649 (193567)], and group 3[297910 (134180)] μm2. At the same time, there were no differences between groups 2 and 3 (p=0.54), but it was lower compared to group 1 (p<0.001). Similar patterns were observed when studying the ratio of VEGFFET CAP/VEGFSUM.
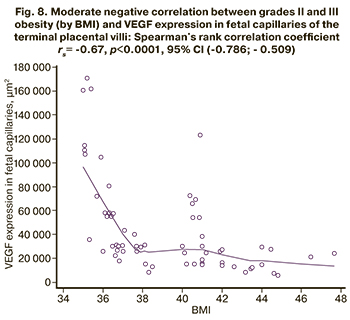
There was a moderate negative correlation in groups 2 and 3 with obesity between BMI and VEGF expression in fetal capillaries of the terminal placental villi: rs = -0.67, p<0.0001, 95% CI (-0.786; -0.509) (Fig. 8).
Grade II and III obesity in pregnant women contributes to the development of placental insufficiency, as evidenced by the structural changes in the terminal villi and fibrotic changes in the secondary villi compared with the control group and grade I obesity (Fig. 5). Obesity of a pregnant woman without comorbidities can cause placental insufficiency since at the beginning of pregnancy; pregravid obesity contributes to the formation of systemic inflammation throughout pregnancy with a decrease in the total antioxidant capacity, which is not accompanied by oxidative damage to the placenta [16]. High adaptive capabilities of this extraembryonic organ have been demonstrated in various pathological processes [17, 18].
Apparently, in cases where obesity is the only underlying disease of a pregnant woman, even a significant decrease in VEGF expression in the placental villi does not always lead to placental insufficiency, as evidenced by clinical data (Table).
Previously, it has been shown that VEGF expression increased in the placenta of women with gestational diabetes and type I diabetes mellitus, which was regarded as a compensatory response [7] to hypoxia. VEGF usually is highly expressed in the placenta, and this growth factor stimulates the growth, survival, and proliferation of vascular endothelial cells. VEGF mechanism of action is well known: tissue hypoxia/ischemia triggers the production of the transcription factor HIF, which stimulates the release of VEGF-A. Circulating VEGF-A then binds to VEGF receptors on endothelial cells, activating tyrosine kinase and molecular mechanisms of angiogenesis. Along with this, VEGF-A acts as a vasodilator (indirectly, through an increase in nitric oxide synthesis) [19].
Healthy postpartum women and women with arterial hypertension had similar levels of VEGF expression in fetal capillaries and other placental compartments. Women with arterial hypertension had an increased VEGF expression in the syncytiotrophoblast and a more significant number of syncytial knots, which is considered a marker of arterial hypertension in puerperas [14].
Our results demonstrating a decrease in VEGF expression with a synchronous increase in the degree of obesity in pregnant women possibly indicates a change in the type of placental compensatory-adaptive reactions, suppression of the vascular type of adaptation (which is considered the most effective) [20] with its replacement with a cell type.
Two blood serum adipokines were found to positively affect pregnant women with grade I–III pregravid obesity without gestational diabetes, assessed by ELISA in the first trimester of pregnancy. Higher concentrations of adiponectin and omentin in the first trimester were associated with less clinically relevant uncompensated placental insufficiency and VEGF expression in terminal placental villi during term labor.
According to our study findings, a decrease in some blood serum adipokine concentrations in the first trimester of pregnancy, which is characteristic of pregnant women with grades II and III obesity, may serve as a predictor of a decrease in VEGF expression in the third trimester of pregnancy in women with pregravid obesity.
Conclusion
In women with grade I–III obesity at 8–9 weeks gestation, blood adiponectin concentration is statistically significantly lower than women with normal body weight. Omentin concentrations did not differ between women in the control group and those with grade I obesity but were higher in women with grade II and III obesity. Concentrations of both adipokines in grade II and III obesity were minimal and did not differ. A moderate negative correlation was found between the BMI of women in all four groups and the blood omentin concentration.
IHCA analysis at 37–41 weeks showed that the total expression of VEGF in placental terminal villi and fetal capillary expression in obese parturient women were statistically significantly lower than in the control group. In a pairwise comparison in groups with high BMI, the highest VEGF expression was found in grade I obesity compared with grades II and III, which did not differ from each other.
Therefore, comparing women with varying degrees of pregravid obesity revealed that a decrease in adiponectin and omentin concentrations in the first trimester of pregnancy was associated with a reduction in placental VEGF expression in the third trimester. Therefore, a high BMI, starting from grade II obesity, even without gestational diabetes, can be considered a risk factor for the development of placental insufficiency.
References
- Аганезова Н.В., Аганезов С.С. Ожирение и репродуктивное здоровье женщины. Акушерство и гинекология. 2016; 6: 18-25. [Aganezova N.V., Aganezov S.S. Obesity and reproductive health of woman. Obstetrics and Gynecology. 2016; 6: 18-25. (in Russian)]. http://dx.doi.org/10.18565/aig.2016.6.18-25.
- Myatt L., Maloyan A. Obesity and placental function. Semin. Reprod. Med. 2016; 34(1): 42-9. https://dx.doi.org/10.1055/s-0035-1570027.
- Wallace J.G., Bellissimo C.J., Yeo E., Fei Xia Y., Petrik J.J., Surette M.G. et al. Obesity during pregnancy results in maternal intestinal inflammation, placental hypoxia, and alters fetal glucose metabolism at mid-gestation. Sci. Rep. 2019; 9(1): 17621. https://dx.doi.org/10.1038/s41598-019-54098-x.
- Чабанова Н.Б., Матаев С.И., Василькова Т.Н., Шевлюкова Т.П. Роль системного воспаления в развитии осложнений беременности у женщин с ожирением. Акушерство и гинекология. 2017; 10: 12-8. [Chabanova N.B., Mataev S.I., Vasilkova T.N., Shevlyukova T.P. The role of systemic inflammation in the development of pregnancy complications in obese women. Obstetrics and Gynecology. 2017; 10: 12-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.10.12-18.
- Roberts K.A., Riley S.C., Reynolds R.M., Barr S., Evans M., Statham A. et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta. 2011; 32(3): 247-54. https://dx.doi.org/10.1016/j.placenta.2010.12.023.
- Pietro L., Daher S., Rudge M.V.C., Calderon I.M.P., Damasceno D.C., Sinzato Y.K. et al. Vascular endothelial growth factor (VEGF) and VEGF-receptor expression in placenta of hyperglycemic pregnant women. Placenta. 2010; 31(9): 770-80. https://dx.doi.org/10.1016/j.placenta.2010.07.003.
- Щеголев А.И., Дубова Е.А., Павлов К.А., Есаян Р.М., Сухих Г.Т., Шестакова М.В. Сравнительная иммуногистохимическая оценка фактора роста эндотелия сосудов и его рецепторов в ворсинах плаценты при гестационном и сахарном диабете 1-го типа. Архив патологии. 2013; 75(5): 13-8. [Shchegolev A.I., Dubova E.A., Pavlov K.A., Esayan R.M., Shestakova M.V., Sukhikh G.T. Comparative immunohistochemical evaluation of vascular endothelial growth factor and its receptors in the placental villi in gestational diabetes mellitus and type 1 diabetes. Arkh. Patol. 2013; 75(5): 13-8. (in Russian)].
- Сидорова И.С., Никитина Н.А. Особенности патогенеза эндотелиоза при преэклампсии. Акушерство и гинекология. 2015; 1: 72-8. [Sidorova I.S., Nikitina N.A. Features of the pathogenesis of endotheliosis in preeclampsia. Akusherstvo i Gynekologiya. 2015; 1: 72-8. (in Russian)].
- Nogues P., Dos Santos E., Jammes H., Berveiller P., Arnould L., Vialard F. et al. Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta. Clin. Epigenetics. 2019; 11(1): 20. https://dx.doi.org/10.1186/s13148-019-0612-6.
- Bellos I., Fitrou G., Pergialiotis V., Perrea D.N., Daskalakis G. Serum levels of adipokines in gestational diabetes: a systematic review. J. Endocrinol. Invest. 2019; 42(6): 621-31. https://dx.doi.org/10.1007/s40618-018-0973-2.
- Lekva T., Roland M.C.P., Michelsen A.E., Friis C.M., Aukrust P., Bollerslev J. et al. Large reduction in adiponectin during pregnancy is associated with large-for-gestational-age newborns. J. Clin. Endocrinol. Metab. 2017; 102(7): 2552-9. https://dx.doi.org/10.1210/jc.2017-00289.
- Balani J., Hyer S., Syngelaki A., Akolekar R., Nicolaides K.H., Johnson A. et al. Association between insulin resistance and preeclampsia in obese non-diabetic women receiving metformin. Obstet. Med. 2017; 10(4): 170-3. https://dx.doi.org/10.1177/1753495X17725465.
- Ведзижева Э.Р., Кузнецова И.В., Успенская Ю.Б., Гитель Е.П., Васильева И.В. К вопросу о патогенезе репродуктивных нарушений у женщин с ожирением. Акушерство и гинекология. 2017; 6: 18-24. [Vedzizheva E.R., Kuznetsova I.V., Uspenskaya Yu.B., Gitel' E.P., Vasil'eva I.V. On the pathogenesis of reproductive disorders in obese women. Obstetrics and Gynecology. 2017; 6: 18-24. (in Russian)]. http://dx.doi.org/10.18565/aig.2017.6.18-24.
- Azliana A.F., Zainul-Rashid M.R., Chandramaya S.F., Farouk W.I., Nurwardah A., Wong Y.P. et al. Vascular endothelial growth factor expression in placenta of hypertensive disorder in pregnancy. Indian J. Pathol. Microbiol. 2017; 60(4): 515-20. https://dx.doi.org/10.4103/IJPM.IJPM_376_16.
- Даценко Н.С., Никитенко Е.В., Агеева Т.А., Якимова А.В. Клинико-морфологические проявления плацентарной недостаточности у женщин с ожирением. Уральский медицинский журнал. 2020; 3: 48-51. [Datsenko N.S., Nikitenko E.V., Ageeva T.A., Yakimova A.V. Clinical and morphological manifestations of placental insufficiency in obese women. Ural Medical Journal. 2020; 3: 48-51. (in Russian)]. https://dx.doi.org/10.25694/URMJ.2020.03.12.
- Santos-Rosendo C., Bugatto F., González-Domínguez A., Lechuga-Sancho A.M., Visiedo F. Placental adaptive changes to protect function and decrease oxidative damage in metabolically healthy maternal obesity. Antioxidants (Basel). 2020; 9(9): 794. https://dx.doi.org/10.3390/antiox9090794.
- Айдагулова С.В., Жорник Т.М., Непомнящих Д.Л., Маринкин И.О., Виноградова Е.В., Нохрина Ж.В. Ультраструктурные модификации эндотелия при плацентарной недостаточности и микроангиопатиях. Бюллетень экспериментальной биологии и медицины. 2009; 147(5): 583-8. [Aidagulova S.V., Zhornik T.M., Nepomnyashchikh D.L., Marinkin I.O., Vinogradova E.V., Nokhrina Zh.V. Ultrastructural modifications of the endothelium in placental insufficiency and microangiopathies. Bulletin of Experimental Biology and Medicine. 2009; 147(5): 650-4. (in Russian)]. http://dx.doi.org/10.1007/s10517-009-0590-3.
- Илизарова Н.А., Жорник Т.М., Маринкин И.О., Айдагулова С.В., Непомнящих Д.Л. Патоморфологическое исследование ворсин при плацентарной недостаточности инфекционного генеза. Бюллетень Сибирского отделения Российской академии медицинских наук. 2009; 29(5): 92-6. [Ilizarova N.A., Zhornik T.M., Marinkin I.O., Aidagulova S.V., Nepomnyashchikh D.L. Pathomorphological investigation of placental villi in the placental insufficiency of infectious genesis. Bulletin of the Siberian Branch of Russian Academy of Medical Sciences. 2009; 29(5): 92-6. (in Russian)].
- Ribatti D. The crucial role of vascular permeability factor/vascular endothelial growth factor in angiogenesis: a historical review. Br. J. Haematol. 2005; 128(3): 303-9. https://dx.doi.org/10.1111/j.1365-2141.2004.05291.
- Якимова А.В., Шкурупий В.А., Маринкин И.О. Морфологические изменения плаценты человека при туберкулезе легких. Бюллетень Сибирского отделения Российской академии медицинских наук. 2014; 34(2): 34-8. [Yakimova A.V., Shkurupiy V.A., Marinkin I.O. Morphological changes in placenta of lung tuberculosis mothers. Bulletin of the Siberian Branch of Russian Academy of Medical Sciences. 2014; 34(2): 34-8. (in Russian)].
Received 17.05.2021
Accepted 31.05.2021
About the Authors
Natalya S. Datsenko, Teaching Assistant at the Department of Obstetrics and Gynecology, Novosibirsk State Medical University, Ministry of Health of Russia,+7(913)918-94-40, datsenko.natasha@mail.ru, 630091, Russia, Novosibirsk, Krasny Ave., 52.
Alexander V. Volchek, Junior Researcher at the Laboratory of Cellular Biology and Fundamental Basis of Reproduction, Central Scientific Laboratory, Novosibirsk State Medical University, Ministry of Health of Russia, +7(913)003-17-22, alexander@volcheck.ru, 630091, Russia, Novosibirsk, Krasny Ave., 52.
Anna V. Yakimova, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology, Novosibirsk State Medical University, Ministry of Health of Russia,
+7(952)927-52-26, yakimova@ngmu.ru, https://orcid.org/0000-0001-6590-8149, 630091, Russia, Novosibirsk, Krasny Ave., 52.
Ivan M. Pozdnyakov, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology, Novosibirsk State Medical University, Ministry of Health of Russia,
+7(383)267-93-11, ngpc@nso.ru, 630091, Russia, Novosibirsk, Krasny Ave., 52.
Tatyana A. Ageeva, Dr. Med. Sci., Professor, Professor at the Department of Anatomic Pathology, Novosibirsk State Medical University, Ministry of Health of Russia,
+7(903)937-50-51, ageta@mail.ru, 630091, Russia, Novosibirsk, Krasny Ave., 52.
Igor O. Marinkin, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, Rector of Novosibirsk State Medical University, Ministry of Health of Russia, +7(383)222-32-04, rector@ngmu.ru, 630091, Russia, Novosibirsk, Krasny Ave., 52.
Svetlana V. Aidagulova, Dr. Bio. Sci., Professor, Head of the Laboratory of Cellular Biology and Fundamental Basis of Reproduction, Central Scientific Laboratory,
Novosibirsk State Medical University, Ministry of Health of Russia, +7(913)909-22-51, s.aydagulova@gmail.com, https://orcid.org/0000-0001-7124-1969,
630091, Russia, Novosibirsk, Krasny Ave., 52.
Authors' contributions: Datsenko N.S. – patient management, enzyme immunoassay; Volchek A.V. – patient management, statistical analysis; Yakimova A.V. – conception of the study, manuscript editing; Pozdnyakov I.M. – patient counseling; Ageeva T.A. – analysis of the placenta structural changes, immunohistochemical analysis; Marinkin I.O. – manuscript editing; Aydagulova S.V. – manuscript preparation, interpreting the results.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Datsenko N.S., Volchek A.V., Yakimova A.V., Pozdnyakov I.M., Ageeva T.A., Marinkin I.O., Aidagulova S.V. Placental insufficiency in pregnant women
with high body mass index and without gestational diabetes.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 10: 68-75 (in Russian)
https://dx.doi.org/10.18565/aig.2021.10.68-75



