Результаты медико-биологических исследований доказали многофакторность больших акушерских синдромов, к которым также относятся состояния, связанные с прерыванием беременности в различные сроки: привычный выкидыш, преждевременные роды [1–3]. Вместе с тем, несмотря на их полиэтиологичность, одной из врачебных стратегий с доказанной эффективностью является назначение препаратов микронизированного прогестерона целевой группе беременных.
Препарат «Праджисан» в качестве действующего вещества содержит натуральный микронизированный прогестерон. Используется для лечения различных акушерских и гинекологических патологий. Он был разработан в соответствии с требованиями Качественной производственной практики – GMP (good manufacturing practice) в 2000-е гг. И, если эффективность препарата была достаточно изучена [4–8], то комплаентность применения препарата «Праджисан» требует детализации.
Известно, что при вагинальном пути введения удается быстро создать необходимые концентрации прогестерона в матке. Ранее был описан механизм «первичного прохождения через матку», что объясняет преимущество таргетного воздействия при вагинальном применении, максимальной эффективности прогестерона при минимальных побочных эффектах [9, 10]. Оптимальный состав препарата «Праджисан» способствует максимальной биодоступности прогестерона при интравагинальном введении, а эффективная концентрация поддерживается более стабильно и продолжительно за счет оптимальной вязкости.
Поскольку переносимость препаратов при вагинальном применении зависит от связывания со слизистой влагалища за счет вспомогательных веществ, в первую очередь вида масла (арахисовое или растительное), то определенный интерес представляет исследование связывающих эффектов препаратов натурального прогестерона, в том числе и «Праджисана». Вязкость масла, а именно его способность к растеканию и «сцепленности» со слизистой влагалища, является важной характеристикой вагинальных препаратов. Существуют две связанные между собой величины, характеризующие вязкость жидкости, – это динамическая (абсолютная) и кинематическая вязкость (отношение динамической вязкости к плотности жидкости). Кинематическая вязкость может быть рассчитана как отношение динамической вязкости к плотности вещества (масла) и своим прохождением соответствует классическим методам измерения вязкости, таким как время вытекания заданного объема через калибровочное отверстие под действием силы тяжести.
Целью исследования явилось изучение «сцепленных свойств» препарата «Праджисан», а также его эффективности и профиля безопасности при различных акушерских состояниях.
Материалы и методы
Вязкость препаратов прогестерона измерялась с помощью капиллярного вискозиметра (Fungilab) при различных температурных режимах. Нами было проведено открытое проспективное когортное исследование по оценке эффективности и комплаентности препарата «Праджисан» у 49 беременных одним плодом. Показаниями к применению препарата являлись: лютеиновая поддержка в программе ЭКО (n=8), угрожающий выкидыш с кровяными выделениями (n=9), угрожающие преждевременные роды (n=10), ультразвуковые признаки укорочения шейки матки у «асимптомных» беременных (от 10 до 25 мм) в 18–236/7 недель беременности (n=7), преждевременные роды в анамнезе (n=8) или поздний выкидыш (n=7). Праджисан назначался ежедневно по 200–600 мг (в зависимости от клинической ситуации) с момента установления вышеперечисленных состояний. Длительность терапии определялась положительной клинической картиной или стабильной длиной «короткой» шейки матки у беременных с отягощенным акушерским анамнезом. Всем женщинам были проведены общеклинические и лабораторные исследования (ультразвуковое исследование, влагалищные и цервикальные мазки, клинические анализы крови, общий анализ мочи, определение глюкозы крови, аланинаминотрансферазы (АЛТ), аспартатаминотрансферазы (АСТ), термостабильной фракции щелочной фосфатазы (ТЩФ), общего и прямого билирубина, общего белка крови, параметров гемостаза (концентрация фибриногена, активированное частичное тромбопластиновое время, протромбиновое время по Квику, международное нормализованное отношение, D-димер). Лабораторные анализы повторялись регулярно через 2 недели. Также были оценены эффективность и комплаентность препарата «Праджисан», проанализированы исходы беременности.
Результаты
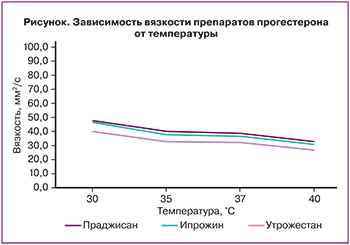 Показатели вязкости препаратов прогестерона представлены на рисунке.
Показатели вязкости препаратов прогестерона представлены на рисунке.
Согласно полученным данным (рисунок), прослеживается тенденция к снижению вязкости при температуре 36,6°С и ≤37,2°С (которая может иметь место в I триместре беременности за счет гипертермического эффекта прогестерона) у всех препаратов прогестерона: у «Праджисана» – 39 и 36,5 мм²/с, у «Ипрожина» – 37,8 и 35,5 мм²/с и у «Утрожестана» – 35,5 и 33,7 мм²/с соответственно. Как видно, показатели вязкости у перечисленных препаратов прогестерона были практически сопоставимыми.
Средний возраст беременных составил 30,71±4,14, основной – 33,26±5,24 года.
Анамнестические сведения о перенесенных соматических заболеваниях, характере становления и особенностях менструальной функции были практически однородными. Все беременные жили в единой климатогеографической зоне. Заслуживали внимания данные акушерского и репродуктивного анамнеза, а также особенности течения беременности, послужившие показанием к назначению препарата «Праджисан».
Длительность терапии у беременных составила 62,0±10,2 дня при влагалищном применении, 42,7±5,4 дня при комбинированном применении и 37,0 ± 3,9 дня при пероральном применении препарата «Праджисан».
36 (73,4%) беременных применяли препарат вагинально, 8 (16,4%) – комбинированно (вагинально и перорально), 5 (10,2%) – перорально.
Поддержка лютеиновой фазы [11] препаратом «Праджисан», начатая с момента переноса бластоцисты, продолжилась при положительном анализе на β-субъединицу хорионического гонадотропина человека (β-ХГЧ) и проведении расширенного комбинированного скрининга в 12–13 недель. При этом дозировки препарата варьировали от 600 мг в сутки на протяжении до 8 недель с постепенным снижением дозы до 200 мг в сутки к 12–13-й неделе беременности, затем препарат отменяли. Все беременные применяли препарат вагинально. Лабораторные анализы крови оставались в пределах нормативных значений. Женщины отмечали хорошую переносимость препарата.
У 9 беременных отмечался угрожающий выкидыш, в том числе у 6 беременных с ретрохориальной гематомой в 9–12 недель беременности с размерами до 4–5 см и нарушением микробиоценоза влагалища в виде дисбиотических процессов (отмечено меньшее видовое разнообразие лактобацилл и меньшая частота выделения отдельных представителей рода Lactobacillus: доминирование L. iners и редуцирование L. сrispatus (р=0,0440; ОШ 0,21; 95% ДИ 0,05–0,89), а также выявлены следующие статистически значимые виды условно-патогенных микроорганизмов (УПМ): Escherichia coli (E. coli), Staphylococcus epidermidis (S. epidermidis), Enterococcus faecalis (E. faecalis), Enterobacteriaceae spp., Peptostreptococcus spp. (p<0,05). То есть вагинальные микробные сообщества (community state type, CST) отличались значительным разнообразием (CST IV–V), в связи с чем были назначены местная терапия (санация влагалища) в течение 7–10 дней и перевод на пероральный прием препарата «Праджисан». В контрольных мазках отмечалось снижение титра УПМ и доминирование лактобацилл с преобладанием L. сrispatus. Эхографические признаки ретрохориальной гематомы свидетельствовали об уменьшении ее размеров и перехода в стадию организации. Клинически отмечалась регрессия симптомов угрожающего выкидыша с отсутствием кровяных выделений. В 3 случаях было продолжено вагинальное применение препарата «Праджисан» с повышением дозы до 600 мг в сутки. Динамика основных лабораторных показателей в зависимости от режима применения препарата «Праджисан» у беременных с угрожающим выкидышем и ретрохориальной гематомой выявила следующие особенности при пероральном приеме: тенденцию к повышению печеночных трансаминаз (АЛТ и АСТ) у 2 беременных (р<0,05); повышение концентрации Д-димера до 2000 нг/мл у 5 беременных с ретрохориальной гематомой больших размеров (5,0×4,0 см) в стадии организации, при отсутствии значимых изменений в плазменном звене гемостаза и количестве тромбоцитов.
36 (73,4%) беременных применяли препарат «Праджисан» вагинально по перечисленным ранее показаниям. Длительность применения составила 9–10 недель. Клинически у пациенток отмечались регрессия симптомов угрожающих преждевременных родов и/или стабильная длина эхографически «короткой» шейки матки, состояние психологического комфорта и уверенности в успешном завершении беременности.
В процессе динамического обследования 9/36 (25%) беременным потребовались переход на пероральный, а затем комбинированный прием препарата в связи с необходимостью местного лечения по поводу восстановления нормобиоценоза влагалища. Длительность этого режима терапии не превышала 6 недель. При этом в 2/9 случаев (22,2%) отмечалось статистически незначимое (p>0,05) повышение печеночных трансаминаз (АЛТ и АСТ), которые до лечения составили 23,56±7,5 и 28,44±5,4 МЕ/л, а после лечения соответственно 34,56±7,5 МЕ/л (р=0,09) и 36,76±4,5 МЕ/л (р=0,12). Явления холестатической желтухи с повышением концентрации прямого билирубина до верхненормальных значений (5,5 мкмоль/л) и ферментов АЛТ и АСТ до 90 и 102 МЕ/л имели место в 1 случае (11,1%).
Беременность завершилась благополучными своевременными родами у 47/ 49 (95,9%) пациенток. В 2 случаях (4,1%) имели место поздние преждевременные роды в 36 недель и 5 дней и преждевременное излитие околоплодных вод в 33 недели и 5 дней с последующим благополучным родоразрешением в обоих случаях. В доношенном сроке новорожденные имели нормальные массо-ростовые показатели. Оценка состояния по шкале Апгар составила 7–9 баллов. Неблагоприятных неонатальных исходов не отмечалось ни в одном случае.
Таким образом, большинство пациенток – 41/49 (83,7%) не отмечали каких-либо нежелательных явлений и хорошо переносили препарат «Праджисан». В 8/49 случаев (16,3%) применения препарата отмечены невыраженные нежелательные явления, которые не требовали отмены терапии: незначительное головокружение по утрам – 1 (2,0%), зуд/жжение – 1 (2,0%); сонливость (при пероральном приеме) – 1 (2,0%); быстрая смена настроения (при пероральном приеме) – 2 (4,1%); вагинальное кровотечение – 1 (2,0%); холестатическая желтуха (при пероральном и последующем комбинированном приеме) – 1 (2,0%); запор (при пероральном и вагинальном применении) – 2 (4,1%). Стоит отметить, что все перечисленные нежелательные явления были преходящими и отмечались в течение первой недели приема препарата. У единственной беременной с холестатической желтухой, диагностированной на 4-й неделе лечения, препарат был отменен.
В настоящее время в связи с углубленным изучением эффектов пренатального программирования, течения внутриутробного детства с точки зрения применения лекарственных препаратов и их воздействия на состояние здоровья взрослого человека безопасность терапии беременных выходит на авансцену лечебных стратегий. Препараты микронизированного прогестерона имеют обширную доказательную базу в отношении эффективности по ряду терапевтических направлений и благоприятный профиль безопасности, подтвержденный многолетней клинической практикой [12–16], что в полной мере относится и к препарату «Праджисан».
Заключение
Таким образом, препарат «Праджисан» является эффективным, безопасным, хорошо переносимым и патогенетически обоснованным средством поддержки лютеиновой фазы в циклах ЭКО, лечения беременных с угрозой прерывания на ранних и поздних сроках, а также медикаментозной профилактики преждевременных родов у беременных с отягощенным акушерским анамнезом.



