Воспалительные реакции являются ключевым звеном в реализации успешной репродуктивной функции, начиная с регуляции менструального цикла, овуляции, ранних этапов беременности (имплантации, плацентации) и до родового процесса при своевременных родах [1–7].
Описана роль избыточного воспаления при таких нарушениях репродуктивной функции, как бесплодие, обусловленное эндометриозом, привычный выкидыш, задержка роста плода, преэклампсия, преждевременные роды [8, 9]. По-видимому, это обусловлено тем, что одной из значимых причин нарушения инвазии трофобласта и ограниченной децидуализации является воспаление, как инфекционного, так и асептического характера [10]. Однако если воспаление инфекционного генеза и его последствия достаточно хорошо изучены, патогенез асептического воспаления остается неясным и представляет собой нерешенную проблему [11]. В качестве индукторов асептических воспалительных реакций исследователи описывают: внеклеточную фетальную ДНК, нуклеосомы, пурины, белки теплового шока, протеин S100, насыщенные жирные кислоты, антимикробные пептиды и другие факторы [1, 12–17].
Роль макромолекул, попадающих в периферический кровоток при повреждении клеток и тканей в генезе патологий, ассоциированных с окислительным стрессом, все чаще обсуждается в зарубежной литературе [18–20]. Особое внимание в последнее время уделяется так называемым МАСП (молекулам, ассоциированным с повреждением), называемым в иностранных источниках DAMP (Damage Associated Molecular Patterns) [21–23]. Среди последних, как наиболее активных в плане индукции воспаления, выделяют МАСП митохондриального происхождения, такие как митохондриальная ДНК (мтДНК), фактор инициации трансляции мтДНК белок TFAM, аденозинтрифосфат (АТФ), кислые липиды митохондриальных мембран и др. [24, 25]. Особую роль в каскаде реакций, приводящих к развитию воспаления неинфекционного генеза, играют протеолипиды и пептидные фрагменты митохондриальных мембранных белков, содержащие на N-конце остаток формилметионина, сходные по своей иммуностимулирующей активности с бактериальными белками [26, 27].
При этом прогностическая ценность определения митохондриальных МАСП (миМАСП) при беременности, осложненной на ранних сроках кровотечением, остается не исследованной [28]. В предыдущей публикации нами были изложены результаты изучения распределения одного из известных миМАСП (белка TFAM) и перспективного кандидата на эту роль – белка внешней мембраны митохондрий VDAC1 в плазме женщин в сроке 6 недель, беременность которых завершилась выкидышами (группа с привычным выкидышем) и своевременными родами (контрольная группа). Оказалось, что воспалительный ответ, вызываемый при участии этих белков, необходим для успешного пролонгирования беременности на ранних сроках [29]. Вероятно, жизнеспособное плодное яйцо способно индуцировать в эндометрии сбалансированный провоспалительный ответ, тогда как при угрожающем и привычном выкидыше имеет место нарушение данного взаимодействия, заканчивающееся гибелью плодного яйца. В то же время при пролонгировании беременности, осложненной кровотечением на ранних сроках, воспалительные реакции у данной группы женщин, возможно, отличаются от физиологических, что создает условия для развития поздних гестационных осложнений. Известно, что пациентки с угрожающим и привычным выкидышем являются группой риска по реализации плацентарной недостаточности, задержки роста плода, отслойкам плаценты и преждевременным родам.
Учитывая полученные ранее результаты, целью данного исследования стало определение уровней провоспалительных факторов митохондриального происхождения (миМАСП) в периферической крови пациенток с угрожающим и привычным выкидышем, беременность которых была пролонгирована при помощи лекарственной терапии в сравнении с аналогичными показателями женщин с физиологическим течением беременности и неразвивающейся беременностью в сроках 6–12 недель.
Материал и методы исследования
В исследование были включены 37 беременных, которые составили 3 группы. В I группу вошли 15 пациенток с привычным выкидышем, проходившие предгестационную подготовку и ведение беременности по принятому протоколу. Во II группу включены 10 пациенток с угрожающим выкидышем без отягощенного акушерского анамнеза. В состав III группы вошли 12 пациенток с физиологическим течением беременности. Из 15 женщин с привычным выкидышем в группу сравнения выделены 7 женщин, чья беременность остановилась в развитии в первом триместре, у 8 женщин беременность была пролонгирована до доношенного срока. Отбор образцов крови для исследования был проведен ретроспективно после того, как стал известен исход беременностей.
Пациентки трех групп были включены в исследование при сроке беременности 6–7 недель от первого дня последней менструации после подтверждения сердцебиения эмбриона по данным ультразвукового исследования. Критериями включения являлись: возраст женщин от 20 до 40 лет; самопроизвольное наступление беременностей (отсутствие бесплодия); отсутствие выраженных гормональных нарушений, регулярный менструальный цикл, отсутствие анатомических причин привычного выкидыша, подписанная пациенткой форма информированного согласия на проведение исследования.
Критерии включения в I группу: два и более самопроизвольных прерываний беременности от одного и того же партнера. Вне беременности проведено обследование для выявления причин привычного выкидыша, назначена терапия гестагенами с ранних сроков беременности.
У пациенток II группы на момент включения в исследование были проявления угрожающего выкидыша (кровяные выделения из половых путей, ретрохориальная гематома). Пациентки были госпитализированы в стационар, где проводилась терапия, направленная на пролонгирование беременности, включающая гестагенные (дидрогестерон 40 мг в сутки), спазмолитические и гемостатические препараты (антифибринолитик – транексамовая кислота).
Контрольную группу составили женщины с физиологическим течением беременности, неотягощенным акушерским анамнезом, подписавшие информированное согласие на проведение исследования.
Критериями исключения в трех группах были: многоплодная беременность, наличие онкологических, тяжелых экстрагенитальных, системных аутоиммунных заболеваний.
Как следует из представленных в таблице данных, женщины исследуемых групп не отличались по возрасту и индексу массы тела. Отличия в числе беременностей и родов обусловлены критериями включения в группы.
Забор крови для проведения исследования проведен в сроках 6, 9, 12 недель беременности.
Для определения уровня миМАСП белкового происхождения у пациенток исследуемых групп проводили фракционирование венозной крови, выделение микровезикул из полученной плазмы, и методом Вестерн-блот анализировали содержание митохондриальных белков. Забор крови проводился натощак из локтевой вены у беременных по стандартной методике в пробирку с ЭДТА. Плазму крови получали методом осаждения форменных элементов крови в течение 10 минут при 3000g, 4°С на центрифуге Eppendorf 5410R (США). Определение содержания белка в плазме крови проводили биуретовым методом. Микровезикулы плазмы крови получали при помощи дифференциального центрифугирования – 30 минут при 10000g, 4°С на центрифуге Beckman Airfuge (США). Осадок ресуспендировали в буфере нанесения для электрофореза.
Определение содержания белков VDAC1 и TFAM в осадке микровезикул плазмы крови проводили методом иммунодетекции после разделения белков электрофорезом в денатурирующих условиях по методу Лэммли в системе Biorad MiniProtean (США) и последующего переноса белков на нитроцеллюлозную мембрану методом Вестерн-блот. Первичные антитела, выработанные против изучаемых белков в мыши (Abcam, США) использовали в соответствии с рекомендациями производителя. Для проявления первичных антител использовали вторичные антитела к мышиным IgG, конъюгированные с пероксидазой хрена и биолюминесцентную систему визуализации ChemiDOC (Biorad, США). Количественное определение идентифицированных компонентов DAMP в полосе соответствующего молекулярного веса на нитроцеллюлозной мембране проводили при помощи системы Geldoc (США) с нормировкой на общий белок, нанесенный на дорожку. Статистическую обработку полученных данных проводили при помощи программы SPSS по методу ANOVA.
 Результаты исследования
Результаты исследования
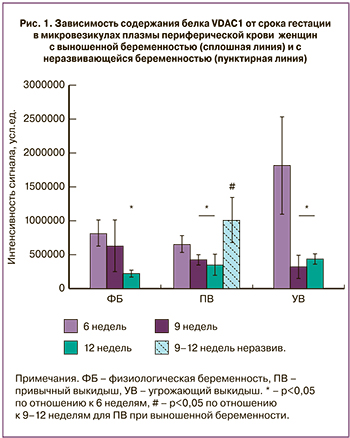
На рис. 1 представлены данные количественного анализа интенсивности сигнала, соответствующего белку VDAC1 на мембране после иммунодетекции для выделенных микровезикул плазмы крови групп физиологической беременности, угрожающего и привычного выкидыша.
Сравнительный анализ относительного содержания VDAC1 в микровезикулах плазмы периферической крови показал, что для женщин с выношенной беременностью, входивших в группы физиологической беременности и привычного выкидыша содержание VDAC1 прогрессивно падало с 6 по 12 неделю гестации (до 2,5 раза) (р<0,05), при этом между данными группами не наблюдалось статистических различий. В то же время, для группы угрожающего выкидыша наблюдалось аномально высокое значение содержания этого белка в первые 6 недель и нормализация его содержания к 9–12 неделям на фоне проводимой терапии до низких значений, статистически неотличимых от значений для групп физиологической беременности и привычного выкидыша. Однако, для случаев неразвивающейся беременности в группе привычного выкидыша наблюдалось резкое повышение уровня белка VDAC1 к сроку гестации 9–12 недель, достоверно отличающееся от случаев выношенной беременности (пунктирная линия на рис. 1, р<0,05).
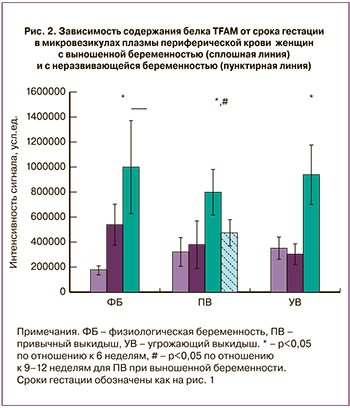 Анализ распределения содержания белка TFAM в микровезикулах плазмы периферической крови женщин c физиологической беременностью, привычным выкидышем и угрожающим выкидышем показал, что между данными группами нет достоверных различий для одинаковых сроков гестации, наблюдается сходная динамика изменения содержания этого белка (рис. 2). В то же время, в случае неразвивающейся беременности в группе с привычным выкидышем происходит достоверное падение содержание TFAM, сниженного для срока гестации 9–12 недель примерно в 2 раза, несмотря на проводимую терапию.
Анализ распределения содержания белка TFAM в микровезикулах плазмы периферической крови женщин c физиологической беременностью, привычным выкидышем и угрожающим выкидышем показал, что между данными группами нет достоверных различий для одинаковых сроков гестации, наблюдается сходная динамика изменения содержания этого белка (рис. 2). В то же время, в случае неразвивающейся беременности в группе с привычным выкидышем происходит достоверное падение содержание TFAM, сниженного для срока гестации 9–12 недель примерно в 2 раза, несмотря на проводимую терапию.
Обсуждение
На фоне проводимой успешной терапии при привычном и угрожающем выкидыше наблюдается синхронное падение уровня белка внешней мембраны митохондрий VDAC1, присутствующего в периферической крови в составе микровезикул при увеличении срока гестации. В то же время, у ряда пациенток, для которых применение терапии не привело к успешному вынашиваванию беременности при привычном выкидыше в анамнезе, наблюдалось резкое нарастание содержания данного белка в микровезикулах плазмы периферической крови. Следует отметить, что для VDAC1 ранее была показана ведущая роль в образовании мультибелковой структуры, ответственной в митохондриях за реализацию феномена неспецифической транзитной поры, приводящей к набуханию митохондрий, разрыву их внешней мембраны и выходу в цитозоль проапототических белков с последующей индукцией каскада реакций программированной клеточной гибели (апоптоза) [30].
В то же время, для данной группы пациенток наблюдается значимое падение TFAM – другого известного белка группы МАСП, провоспалительное действие которого было ранее показано, в том числе и в нашем исследовании [29]. Ранее было показано, что данный белок в составе микровезикул может проходить гематоэнцефалический барьер, что делает его вероятным кандидатом на триггер эндотелиальной дисфункции не только при поражении мозга, но и при плацентарных нарушениях и гибели трофобласта [31]. Интересно, что TFAM представляет собой нестабильный белок, быстро разрушающийся при освобождении из состава комплекса с митохондриальной ДНК [32], для которой известно выраженное провоспалительное действие, проявляющееся в активации нейтрофилов и индукции мощных провоспалительных реакций в отсутвие инфекционного агента [33]. Можно предположить, что падение содержания TFAM в микровезикулах плазмы крови опосредовано диссоциацией комплекса этого белка с митохондриальной ДНК, его разрушением и нарастанием уровня свободной митохондриальной ДНК с ее дальнейшим действием в качестве мощного МАСП, активирующего неспецифческий иммунный ответ и воспаление неинфекционного генеза, приводящее к завершению беременности.
Известно, что микровезикулы плацентарного происхождения в своих мембранах содержат белковые компоненты, обладающие адресностью в отношении клеток стенки сосудов, что делает возможным активацию эндотелия и последующие провоспалительные реакции, индуцированные погибающими апоптозом клетками сосудистой стенки [20, 34]. Вероятно, в основе таких реакций лежит доставка к эндотелию сосудов матери микровезикул, содержащих проапоптогенные комплексы митохондрий и митохондриальную ДНК.
Дальнейшие исследования состава микровезикул плазмы периферической крови беременных в норме и при патологии беременности, в том числе в отношении других митохондриальных белков, входящих в состав проапоптогенного комплекса, а также в отношении митохондриальной ДНК и кислых фосфолипидов, несомненно, позволят еще больше приблизиться к пониманию молекулярных механизмов неразвивающейся беременности. Так, несмотря на то, что известны работы по исследованию состава экзосом плацентарного происхождения при терапии невынашивания беременности низкомолекулярными гепаринами, на данный момент не был выяснен молекулярный механизм благотворного действия таких микровезикул и данное исследование может пролить свет на эту проблему [35, 36]. Кроме того, определение содержания миМАСП при невынашивании беременности позволит разработать диагностические системы нового поколения, позволяющие на ранних сроках гестации детектировать появление патологических проявлений и провести коррекцию терапевтического воздействия при лечении данной патологии.
Заключение
Таким образом, определение содержания миМАСП позволит на ранних сроках прогнозировать неблагоприятный исход беременности у женщин с привычным выкидышем и проводить оценку эффективности терапевтического воздействия при лечении данной патологии.



