HLA-G expression in patients with missed miscarriages and a normal embryonic karyotype
The HLA-G gene has central functions in the processing and presentation of antigen, inhibits the NK-cell receptor (KIR2DL4, killer cell immunoglobulin-like receptor-2 DL4), resulting in reduced immune response at the maternal-fetal interface and providing maternal tolerance of fetal tissues. Based on this, many experimental studies have investigated the expression of the HLA-G gene and its impact on the risk of pregnancy complications, such as early recurrent miscarriage, where immunological factors are believed to play a critical role. Objective: To investigate the expression of HLA-G and KIR2DL4 receptors in abortion material from women with missed miscarriages (with normal fetal karyotype and polyploidy) and women with normal ongoing pregnancy (with induced abortion). Materials and methods: The study analyzed 115 samples of abortion material in two study groups with missed miscarriages (group 1 with 36 samples with normal fetal karyotype, group 2 with 35 samples with fetal karyotype by polyploidy type) and a control group with induced abortion ( group 3 with 44 samples with normal fetal karyotype). The samples were analyzed using cytogenetic and morphological (histological, immunohistochemical, and morphometric) methods. Results: An immunohistochemical analysis verified the expression of HLA-G in extravillous trophoblast cells and the expression of KIR2DL4 in chorionic villi (in cytotrophoblast and syncytiotrophoblast), in the glands, stroma, and superficial epithelium of the gravid endometrium in samples from women with and without missed miscarriages. In samples from women with missed miscarriages, the expression of HLA-G was statistically significantly lower than in the control group [37.7 (9.9), 36.4 (8.4), and 67.1 (5.6) in groups 1, 2, and control groups, respectively, p <0.001]. Conclusion: Patients with missed miscarriages had significantly lower HLA-G expression in the chorionic villus structures than patients in the control group. These findings suggest a role for HLA-G in pregnancy progression.Bakleicheva M.O., Bespalova O.N., Ivashchenko T.E., Tral T.G., Tolibova G.Kh., Tikhonov A.V., Petrova L.I., Dudkina V.S.
Keywords
The etiology of spontaneous miscarriage is very diverse and multifactorial. Some risk factors are causal determinants of embryo abnormalities (genetic factors); others create adverse conditions for its normal development (immunological, endocrine disorders, infectious and inflammatory diseases, thrombophilia, and many others). Currently, there are more than 80 known immunological biomarkers associated with miscarriage at all stages of gestation [1–3].
First studies investigating alloimmune causes of miscarriage dating back to the early 1980s [4–8] identified the relationship between recurrent miscarriage and the individual characteristics of the HLA class I and II systems (human leukocyte antigen, major histocompatibility complex). They can cause impaired antibody production that blocks the reaction of mixed lymphocyte reaction blocking antibodies (MLR-Bf), anti-paternal cytotoxic antibodies (APCA), and anti-idiotypic antibodies (Ab2) required for successful implantation and pregnancy development [9, 10].
In pregnant women, it has been proven that protein products of the HLA-G gene – a non-classical HLA class I molecule – are mainly expressed on trophoblast cells. These protein products exert an immunomodulatory effect on the interactions of various immune cells (decidual natural killer (dNK) cells, T cells, macrophages) and regulate cell migration during placental development, ultimately determining pregnancy outcomes.
HLA-G production appears to be a critical factor in trophoblast differentiation and complete invasion. It is known that HLA-G expression is not strictly associated with protecting the embryo/fetus against the attack of maternal cells, but it is engaged with tissue remodeling. Expressed or secreted HLA-G molecules by extravillous trophoblast cells (EVT) regulate their decidual and endovascular invasion [11–13]. EVT cells progressively replace endothelial cells on the walls of uterine spiral arteries, increasing their diameter that ensures proper blood flow to the intervillous space for fetal nutrition. This process requires the presence of decidual natural killer (dNK) cells, the most numerous cell population at the maternal-fetal interface.
Besides, HLA-G mRNA was found in all EVT cell populations, including trophoblast cell culture: interstitial and endovascular trophoblast and placenta bed cells. HLA-G transcripts were found in cytotrophoblast and mesenchymal cells of chorionic villi. It has been shown that syncytiotrophoblast (ST) does not express HLA-G. In addition to EVT, protein products of HLA-G expression have been found in amnion cells and fetal liver cells. In the case of trophoblast invasion and on the cells of the basal lamina of the placenta, the heavy chains of HLA-G and HLA-C are presented in connection with 2β-microglobulin. Individual molecules of the same chains can be found, but not on every cell. In contrast, smooth chorionic cells express the HLA-G antigen exclusively, but not the HLA-A, B, or HLA-C antigens. This difference in the expression of classical and non-classical class I antigens indicates the presence of tissue-specific regulation.
Signaling from dNK endosomes stimulates the tolerogenic activity of NK cells while maintaining the ability to antiviral immunity at the maternal-fetal interface [14]. The universal HLA-G receptor, expressed in all studied NK cells, was described in the late 1990s: KIR2DL4 [15]. Thus, HLA-G can interact with its extracellular domains with leukocyte receptors, including CD8, LILRB1, and LILRB2, and with the immunoglobulin-like killer cell receptor KIR2DL4 [16]. It has been shown that EVT cells are significantly more active in the synthesis and assembly of HLA-G molecules than all other surrounding maternal and fetal cells. However, the expression of HLA-G molecules on the cell surface does not correlate with this increased activity. The constant flushing of HLA-G molecules from the surface of the trophoblast plays a central role in protecting the fetus from the mother's immune attack. HLA-G ensures the survival of the trophoblast in maternal tissues, being a protective molecule against the cytotoxicity of NK cells. Protection of target cells from cytolysis is shown only by the HLA-G antigen but not by the classical HLA class I antigens. The pregnancy recognition system includes peptides that specifically activate NK cell inhibitory receptors (KIR) or specifically activate NK cell activator receptors (KAR), including a cytotoxic response. Both types of receptors are present on the large granular lymphocytes of the uterus. These receptors belong to the immunoglobulin superfamily. HLA-G antigens are recognized by KIR receptors, and thus the cytotoxic response is turned off. KAR activation can lead to trophoblast rejection. Although HLA-G inhibits T cells' proliferation and cytotoxic activity, it activates dNK for cytokine secretion and proliferation [14].
There are no studies investigating the expression of HLA-G and the KIR2DL4 receptor in chorionic villi and the gravid endometrium during early gestation in patients with missed miscarriages of various etiology.
The present study aimed to investigate the expression of HLA-G and KIR2DL4 receptors in abortion material from women with missed miscarriages (with normal fetal karyotype and polyploidy) and women with normal ongoing pregnancy (with induced abortion).
Materials and methods
Study design
The material was collected at the D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, and the Center for Family Planning and Reproduction. The study was approved by the Ethics Committee of the D.O. Ott Research Institute of Obstetrics, Gynecology, and Reproductology. During the study, 115 samples of abortion material were obtained from patients with missed miscarriage (study group, n=71) and patients with ongoing pregnancy and induced abortion (control group, n=44). The samples were divided into three groups categorized by cytogenetic analysis results (karyotyping of abortion material). Group 1 included 36 samples from patients with missed miscarriage and normal fetal karyotype; group 2 included 35 samples from patients with missed miscarriage with abnormal fetal karyotype by polyploidy type. Group 3 had 44 samples from women with ongoing pregnancy and induced abortion of genetically normal embryos. Inclusion criteria were abortion material from 3 groups of women living in the North-West region with singleton pregnancy and missed miscarriage or induced abortion at the request of a woman at 6–12 weeks gestational age.
In this study, we used cytogenetic analysis (karyotyping of abortion material) and morphological (histological and immunohistochemical, morphometric) examination to assess the expression of HLA-G and KIR2DL4 in the chorionic villus and gravid endometrium.
Cytogenetic analysis
The cytogenetic analysis of chorionic villus cells was conducted by the standard method. Karyotyping included 115 chorionic samples from women with missed miscarriages and ongoing pregnancies terminated at the women’s request. Surgical specimens were represented by chorionic fragments in saline delivered within 2–4 hours after surgery.
Metaphase chromosome preparations were obtained from the cytotrophoblast cells of the villous chorion by an accelerated direct method. Chromosome staining was performed using Hoechst 33258 fluorochrome with actinomycin D contrast. The preparations were analyzed using a Zeiss Axio Imager.Z2 microscope equipped with a Cool Cube 1 camera and MetaSystems Ikaros V 5.9.1 CM software. When karyotyping, we complied with the requirements for cytogenetic testing in medical genetic services.
Histological and immunohistochemical examinations
Histological and immunohistochemical examinations were performed according to the standard technique. Histological examination evaluated endometrial transformation, the structure of the chorionic villi, the state of the vascular bed, and villus stroma [17]. The investigation was conducted on an Olympus CX31 microscope. The immunohistochemical analysis included quantitative and qualitative assessment of the expression of Anti-HLA-G (clone MEM-G/1, mouse monoclonal) in extravillous trophoblast using antibodies manufactured by Abcam (Great Britain) at a standard dilution of 1: 200 and Anti-KIR2DL1+KIR2DL3+KIR2DL4+KIR2DS4 (ab197927, rabbit polyclonal) produced by Abcam (Great Britain) at 1:30 dilution in chorionic villi.
Digital microscopy and morphometry
The quantitative assessment of the immunohistochemical reaction was carried out on 1150 micrographs obtained using a microscopic image fixation system comprising an Olympus BX46 microscope and CellSens 47 Entry software. The proportion of the occupied expression of the studied marker was calculated using the VideoTest-Morphology 5.2 software (VideoTest, Russia). The following parameters were assessed in each section in 5 fields of view: optical density of expression (the value was calculated automatically) and the relative area of expression (the ratio of the area of immunopositive cells to the total area of the preparation):
S (%) = (Spositive/Stotal) × 100.
After that, the average values of the studied indicators were calculated.
Statistical analysis
Statistical analysis was performed using the STATISTICA 10 software (StatSoft). The normality of the distribution was tested by the Shapiro–Wilk test for samples with 50 or fewer observations. Numerical variables were found to meet assumptions of normality and homogeneity of variance. Due to multiple pairwise comparisons, Bonferroni adjusted significance thresholds were calculated and used to declare significance for the hypothesis tests. The number of possible comparisons that can be made was determined by the formula m = n(n-1)/2, where n is the number of groups – 3. For this study, the critical value of the Student's t-test was determined for the required level of significance. So, when comparing the three groups, the differences between the two mean values were considered statistically significant at a new critical level of 0.05/3 = 0.017. The statistical significance of between-group differences for categorical variables, including pregnancy rates, reproductive loss, threatened miscarriage, and dilation and curettage, was compared by the χ2 test. Differences were considered significant at p<0.05.
Results and discussion
Patients of the three groups did not differ in age, body mass index, parity, and gestational age at the time of pregnancy termination. Patients from group 1 were 3.5 times more likely to have a history of reproductive losses than control subjects (58.3 and 15.9%, p=0.014, respectively). Every second woman in group 2 had symptoms of threatened abortion, absent in group 3 (48.5 and 0%, p=0.012, respectively) (Table 1).
Cytogenetic analysis
Cytogenetic analysis of 115 chorionic villi samples showed chromosomal abnormalities (polyploidy) of the trophoblast in every third pregnancy [35/115 (30.43%)] of the whole studied samples (n=115). Thus, abnormal karyotypes were subdivided into the following options: 18 cases with the 69XXY karyotype [18/35 (51.43%) of the total number of abnormal karyotypes], 8 cases with the 69XXX karyotype [8/35 (22.86%)], and also on one occasion of 70ХХУ (Х)+13 and 92ХХХУ (Х). In patients with missed miscarriages and normal fetal karyotype, 20 samples with the 46XX karyotype [20/36 (55.56%)] and 16 samples with the 46XY karyotype [16/36 (44.44%)] were identified. In the control group, there were 23 chorions with a female karyotype [23/44 (52.27%)] and 21 chorions with a male karyotype [21/44 (47.73%)].
Histological examination
In group 1, histological examination of 36 samples from the first trimester missed miscarriages showed a complete transformation of the compact and spongy layers of the endometrium in 23/36 (63.9%) cases. Inadequate transformation of the spongy layer was observed in 7/36 (19.4%) cases. Chorionic villi were characterized by hydropic changes in the stroma, uneven reduction of the vascular bed, and dystrophic changes in the chorionic syncytium. In addition, fibrinoid alteration of chorionic villi was noted in 2 cases.
In group 2, the complete transformation was observed in 22/35 (62.9%) cases. Inadequate transformation of the spongy layer was noted in 13/35 (37.1%) cases. Histological examination of the chorionic villi reveals a reduction in the vascular bed, from hypo-vascularization to the avascular stroma, their dystrophic and hydropic changes in the structure.
In the control group, a complete transformation was noted in all cases. The histological structure of the chorionic villi corresponded to the embryo-histological period.
Immunohistochemical analysis
An immunohistochemical analysis verified the expression of HLA-G in EVT cells and the KIR2DL4 in the stroma, glands, and surface epithelium of the endometrium in groups with missed miscarriage and during a healthy pregnancy.
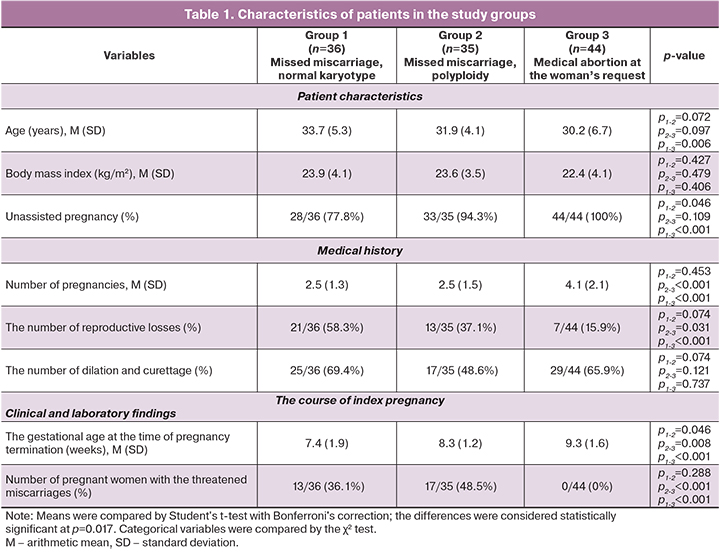
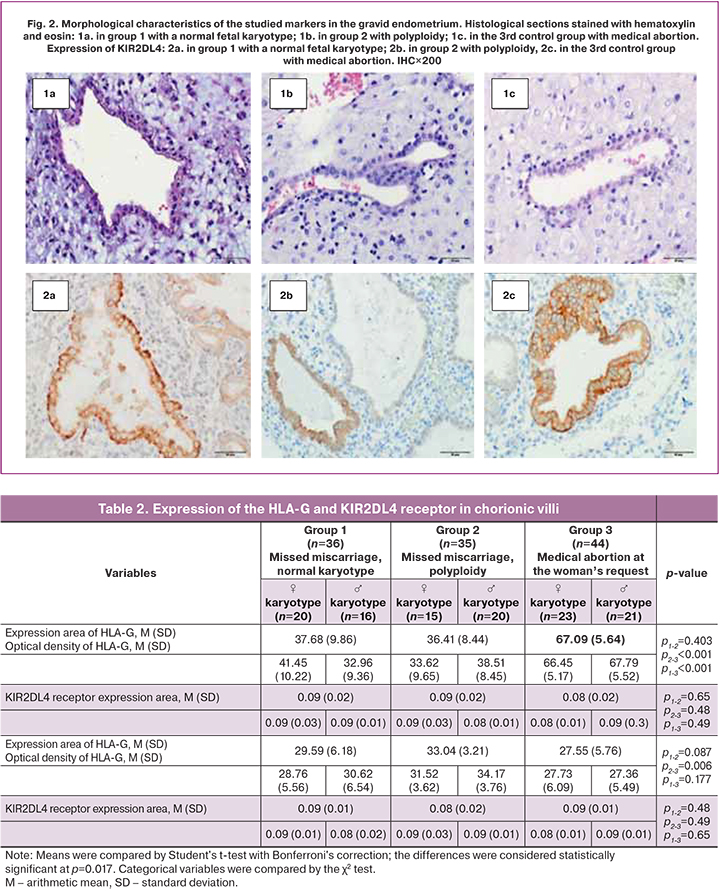
HLA-G has a membrane-bound expression (Fig. 1). The area of HLA-G expression in both groups with missed miscarriages did not differ significantly. In missed miscarriages, the area of HLA-G expression was significantly smaller than in the control group [in group 1 with a normal karyotype – 37.7 (9.9), in group 2 – 36.4 (8.4), in the control group – 67.09 (5.6), p<0.001]. There were no statistically significant differences in optical density [0.09 (0.02), 0.09 (0.02) and 0.08 (0.02), respectively]. Among patients with missed miscarriage and a normal karyotype of male fetuses, there was a tendency towards the lower expression of HLA-G molecules, compared with female fetuses [32.96 (10.22) and 41.45 (9.36), p=0.17, respectively], which was not observed in groups 2 and 3 (Table 2).
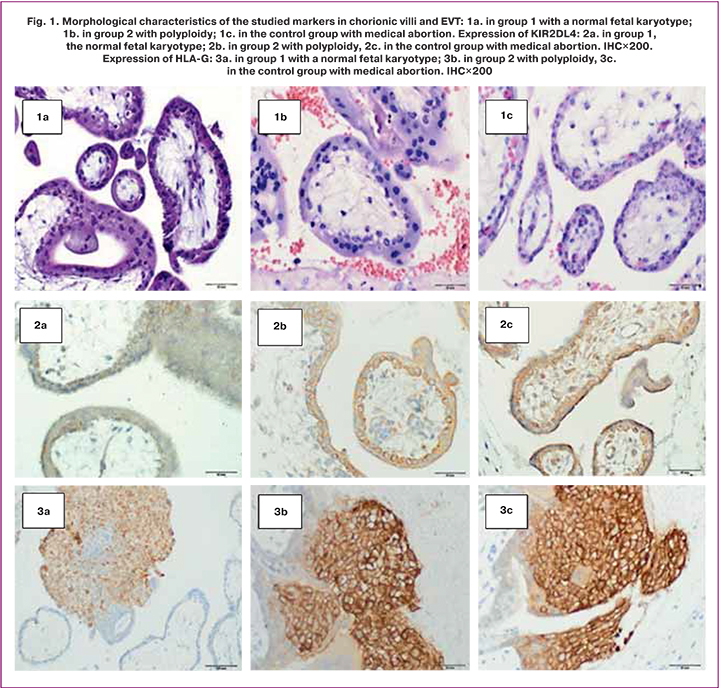
Expression of the KIR2DL4 receptor was verified in chorionic villi (cytotrophoblast and ST) (Fig. 1) and in the structures of the endometrium, including the stroma of the gland, and in the surface epithelium (Fig. 2), regardless of the karyotype. The KIR2DL4 receptor is characterized by a cell membrane-bound expression (Fig. 1). The area of expression of the KIR2DL4 receptor was not statistically different between the three groups [29.6 (6.18), 33.1 (3.21), and 27.55 (5.76), p=0.75]. Optical density also did not differ statistically significantly [0.09 (0.01), 0.08 (0.02) and 0.09 (0.01), respectively].
Semi-allogeneic trophoblast forms several interfaces with the maternal immune system depending on gestational age and anatomic location [18]. In the early stage of implantation, the blastocyst is surrounded by trophectoderm that will develop into both the definitive villous placenta, constituted by cytotrophoblast (CT) and syncytiotrophoblast cells (ST), as well as the invading extravillous trophoblast cells (EVT) that invade into the uterus to tap into the maternal blood supply.
Interface I (up to 8 weeks gestation) comprises the ST of the implanting embryo and the uterine decidua. This is the earliest stage of interaction between two organisms and the only time ST is at a tissue interface. The chorionic villi are washed directly by the maternal blood, thus providing histiotrophic nutrition [19, 20] until the establishment of intervillous circulation after eight weeks.
In the second trimester, three new models replace interface I: interface II between invading extravillous trophoblast (EVT) and maternal immune cells in the decidua; interface III between decidua parietalis and amniotic membranes; interface IV between the ST and maternal blood. In this case, ST represents the epithelial subtype surrounding chorionic villi and shows a large surface area directly in contact with the maternal blood.
It is worth noting that ST continuously shed extracellular vesicles (EVs) into intervillous space and, thus, into the maternal circulation. EVs are membrane-bound, cell-derived particles released as part of both normal physiological functions – such as hemostatic regulation and cell maintenance – and due to cellular stress (such as in pre-eclampsia). The term EV encompasses exosomes (~100 nm in diameter), microvesicles (0.1–1 µm) and apoptotic bodies (0.5–5 µm) [21]. ST-derived extracellular vesicles (STEVs) released into intervillous spaces are collected into venous blood in the uterine veins, go back into the heart, pass through the maternal pulmonary capillary bed, and out into the systemic circulation. In normal pregnancy, STEVs are released constitutively and are thought to have a role in inducing maternal adaptive changes, which include suppressed maternal cell-mediated immunity. Indeed, STEVs contain a complex cargo of RNAs, proteins, glycans, and lipids, traveling from the apical surface of ST to target cells of the maternal body. They are considered conveyors of biological massage between the mother and the fetus. It is commonly agreed that STEVs released into maternal circulation represent an extension of interface IV.
In the first half of pregnancy, interfaces I and II dominate, where the success of the pregnancy is determined early. Interface II diminishes after 16 weeks, while interface IV is activated with the onset of the uteroplacental circulation at 8–9 weeks [23] and enlarges with placental growth to become the dominant interface after 20 weeks [24]. Furthermore, STEVs shedding into the maternal circulation progressively increase from the first to the third trimester of pregnancy, contributing to the dominance of the interface IV in the second half of pregnancy.
In human pregnancy, tight control of human leukocyte antigens (HLA) class I and II expression in chorionic and EVT is essential for a successful outcome [25]. In humans, the villous ST does not express any HLA class I or class II molecules, so that T cells are theoretically unable to recognize and bind to the main placental barrier. This “antigens hiding” is a highly effective mechanism to protect the placenta from maternal immune rejection.
In contrast, EVT cells only express class I HLA molecules, not class II; thus, they do not act as antigen-presenting cells and cannot initiate direct allorecognition of maternal CD4+ T cells [25].
The lack of expression of HLA-A and HLA-B molecules and class II HLA on EVT cells precludes any exposure by maternal T cells to paternal HLA antigens. In addition, HLA-C and atypical class I (Ib) antigens such as the HLA-G and HLA-E molecules are thought to protect EVT from dNK and T cell destruction.
As EVT invades maternal uterine tissues during the implantation process, 80% of maternal leukocytes are represented by dNK, 10% by macrophages, and, for the remaining 10%, by T cells. However, these proportions vary greatly depending on the gestational age. At term pregnancy, T cells are the predominant lymphocyte population within the maternal decidua (40–70% of CD45+ leukocytes), although also dNK (20–50%) and decidual macrophages (10–15%) are present in relatively high amounts [26].
It is well known that decidual cells have the capacity to actively hinder invasion and outgrowth of abnormal human embryos that have breached the luminal epithelium, but what triggers this mechanism of genetically defective embryos rejection is still unclear. At the same time, it was shown that the pathomorphological pictures of missed miscarriage due to different reasons are similar. This question also concerns genetically normal embryos with low HLA-G molecules expression.
Conclusion
Regardless of fetal karyotype in missed miscarriage, the morphological picture had a lot in common, including hydropic changes in the stroma of the chorionic villi with an uneven reduction of the vascular bed and dystrophic changes in the chorionic syncytium. A significantly higher HLA-G expression in chorionic villi in ongoing pregnancies of genetically normal embryos, compared with groups with a missed miscarriage, is probably associated with an adequate maternal immune system response to a semi-allogeneic fetus.
The results of our study suggest that a decrease in the HLA-G expression is one of the immunological factors that determine early reproductive losses.
References
- Доброхотова Ю.Э., Ганковская Л.В., Бахарева И.В., Свитич О.А., Малушенко С.В., Магомедова А.М. Роль иммунных механизмов в патогенезе невынашивания беременности. Акушерство и гинекология. 2016; 7: 5-10. [Dobrokhotova Yu.E., Gankovskaya L.V., Bakhareva I.V., Svitich O.A., Malushenko S.V., Magomedova A.M. Role of immune mechanisms in the pathogenesis of miscarriage. Obstetrics and Gynecology. 2016; 7: 5-10. (in Russian)]. https://dx.doi.org/10.18565/aig.2016.7.5-10.
- Амян Т.С., Перминова С.Г., Кречетова Л.В., Вторушина В.В., Митюрина Е.В. Иммунологические аспекты повторных неудач имплантации в программе экстракорпорального оплодотворения. Акушерство и гинекология. 2017; 1: 5-12. [Amyan T.S., Perminova S.G., Krechetova L.V., Vtorushina V.V., Mityurina E.V. Repeated implantation failures in an IVF program: Immunological aspects. Obstetrics and Gynecology. 2017; 1: 5-12. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.1.5-12.
- Николаева М.А., Арефьева А.С., Степанова Е.О., Голубева Е.Л., Вторушина В.В., Тетруашвили Н.К., Кречетова Л.В. Сроки наступления беременности после предгестационной аллоиммунизации и цитокиновый профиль клеток периферической крови у женщин с привычным выкидышем в анамнезе. Акушерство и гинекология. 2021; 1: 79-87. [Nikolaeva M.A., Arefieva A.S., Stepanova E.O., Golubeva E.L., Vtorushina V.V., Tetruashvili N.K., Krechetova L.V Time to pregnancy after pre-gestational allogeneic immunotherapy and peripheral blood cell cytokine profile in women with a history of recurrent spontaneous abortion. Obstetrics and Gynecology. 2021; 1: 79-87. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.1.79-87.
- Orr H.T., Bach F.H., Ploegh H.L., Strominger J.L., Kavathas P., DeMars R. Use of HLA loss mutants to analyse the structure of the human major histocompatibility complex. Nature. 1982; 296(5856): 454-6. https://dx.doi.org/10.1038/296454a0.
- Redman C.W., McMichael A.J., Stirrat G.M., Sunderland C.A., Ting A. Class 1 major histocompatibility complex antigens on human extra-villous trophoblast. Immunology. 1984; 52(3): 457-68.
- Bodmer J.G., Marsh S.G., Albert E. Nomenclature for factors of the HLA system, 1989. Immunol. Today. 1990; 11(1): 3-10. https://dx.doi.org/10.1016/0167-5699(90)90003-r.
- Kovats S., Main E.K., Librach C., Stubblebine M., Fisher S.J., DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990; 248(4952): 220-3. https://dx.doi.org/10.1126/science.2326636.
- Chumbley G., King A., Robertson K., Holmes N., Loke Y.W. Resistance of HLA-G and HLA-A2 transfectants to lysis by decidual NK cells. Cell. Immunol. 1994; 155(2): 312-22. https://dx.doi.org/10.1006/cimm.1994.1125.
- Bespalova O., Bakleicheva M., Ivashchenko T., Tral T., Tolibova G., Kogan I. Expression of HLA-G and KIR2DL4 receptor in chorionic villous in missed abortion. Gynecol. Endocrinol. 2020; 36(Suppl. 1): 43-7. https://dx.doi.org/10.1080/09513590.2020.1816716.
- Хачатрян Н.А., Кречетова Л.В., Тетруашвили Н.К. Аллоиммунные механизмы привычного выкидыша. Акушерство и гинекология. 2014; 5: 3-8. [Khachatryan A.M., Krechetova L.V., Tetruashvili N.K. Alloimmune mechanisms of recurrent miscarriage. Obstetrics and Gynecology. 2014; 5: 3-8. (in Russian)].
- Dahl M., Djurisic S., Hviid T.V. The many faces of human leukocyte antigen-G: relevance to the fate of pregnancy. J. Immunol. Res. 2014; 2014: 591489. https://dx.doi.org/10.1155/2014/591489.
- Gregori S., Amodio G., Quattrone F., Panina-Bordignon P. HLA-G orchestrates the early interaction of human trophoblasts with the maternal niche. Front. Immunol. 2015; 6: 128. https://dx.doi.org/10.3389/fimmu.2015.00128.
- Nilsson L., Djurisic S., Hviid T.V. Controlling the Immunological Crosstalk during Conception and Pregnancy: HLA-G in Reproduction. Front. Immunol. 2014; 5: 198. https://dx.doi.org/10.3389/fimmu.2014.00198.
- Ferreira L.M.R., Meissner T.B., Tilburgs T., Strominger J.L. HLA-G: At the interface of maternal-fetal tolerance. Trends Immunol. 2017; 38(4): 272-86. https://dx.doi.org/10.1016/j.it.2017.01.009.
- Rajagopalan S., Long E.O. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 1999; 189(7): 1093-100. https://dx.doi.org/10.1084/jem.189.7.1093.
- Donadi E.A., Castelli E.C., Arnaiz-Villena A., Roger M., Rey D., Moreau P. Implications of the polymorphism of HLA-G on its function, regulation, evolution and disease association. Cell. Mol. Life Sci. 2011; 68(3): 369-95. https://dx.doi.org/10.1007/s00018-010-0580-7.
- Траль Т.Г., Толибова Г.Х. Морфологические и иммуногистохимические особенности неразвивающейся беременности I триместра. Журнал акушерства и женских болезней. 2014; 63(4): 60-8. [Tral T.G., Tolibova G.Kh. Morphological and immunohistochemical especially of stilled pregnancy of the first trimester. Journal of Obstetrics and Women's Diseases. 2014; 63(4): 60-8. (in Russian)].
- Brosens I., Puttemans P., Benagiano G. Placental bed research: I. The placental bed: from spiral arteries remodeling to the great obstetrical syndromes. Am. J. Obstet. Gynecol. 2019; 221(5): 437-56. https://dx.doi.org/10.1016/j.ajog.2019.05.044.
- Tersigni C., Meli F., Neri C., Iacoangeli A., Franco R., Lanzone A. et al. Role of Human leukocyte antigens at the feto-maternal interface in normal and pathological pregnancy: An Update. Int. J. Mol. Sci. 2020; 21(13): 4756. https://dx.doi.org/10.3390/ijms21134756.
- Moser G., Windsperger K., Pollheimer J., de Sousa Lopes S.C., Huppertz B. Human trophoblast invasion: New and unexpected routes and functions. Histochem. Cell Biol. 2018; 150(4): 361-70. https://dx.doi.org/10.1007/s00418-018-1699-0.
- Tannetta D., Collett G., Vatish M., Redman C., Sargent I. Syncytiotrophoblast extracellular vesicles – Circulating biopsies reflecting placental health. Placenta. 2017; 52: 134-8. https://dx.doi.org/10.1016/j.placenta.2016.11.008.
- Parham P., Norman P.J., Abi-Rached L., Hilton H.G., Guethlein L.A. Review: Immunogenetics of human placentation. Placenta. 2012; 33(Suppl.): S71-80. https://dx.doi.org/10.1016/j.placenta.2011.11.020.
- Ander S.E., Diamond M.S., Coyne C.B. Immune responses at the maternal-fetal interface. Sci. Immunol. 2019; 4(31): eaat6114. https://dx.doi.org/10.1126/sciimmunol.aat6114.
- Moett A., Chazara O., Colucci F. Maternal allo-recognition of the fetus. Fertil. Steril. 2017; 107(6): 1269-72. https://dx.doi.org/10.1016/j.fertnstert.2017.05.001.
- Burton G., Jauniaux E. What is the placenta? Am. J. Obstet. Gynecol. 2015; 213(4, Suppl.): S6-8. https://dx.doi.org/10.1016/j.ajog.2015.07.050.
- Papúchová H., Meissner T.B., Li, Q., Strominger J.L., Tilburgs T. The dual role of HLA-C in tolerance and immunity at the maternal-fetal Interface. Front. Immunol. 2019; 10: 2730. https://dx.doi.org/10.3389/fimmu.2019.02730.
Received 05.08.2021
Accepted 24.11.2021
About the Authors
Margarita O. Bakleicheva, Junior Researcher, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, +7(911)273-65-38, bakleicheva@gmail.com,https://orcid.org/0000-0002-0103-8583, 199034, Russia, Saint-Petersburg, Mendeleyevskaya Liniya, 3.
Olesya N. Bespalova, Dr. Med. Sci., Deputy Director for Research, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, shiggerra@mail.ru,
https://orcid.org/0000-0002-6542-5953, 199034, Russia, Saint-Petersburg, Mendeleyevskaya Liniya, 3.
Tatiana E. Ivashchenko, Dr. Bio. Sci., Professor, Leading Researcher of the Department of Genomic Medicine, D.O. Ott Research Institute of Obstetrics,
Gynecology and Reproductology, tivashchenko2011@mail.ru, https://orcid.org/0000-0002-8549-6505, 199034, Russia, Saint-Petersburg, Mendeleyevskaya Liniya, 3.
Tatiana G. Tral, MD, PhD, Pathologist, Senior Researcher at the Laboratory of Morphology, Head of the Department of Anatomic Pathology, D.O. Ott Research Institute
of Obstetrics, Gynecology and Reproductology, ttg.tral@yandex.ru, https://orcid.org/0000-0001-8948-4811, 199034, Russia, Saint-Petersburg, Mendeleyevskaya Liniya, 3.
Gulrukhsor Kh. Tolibova, Dr. Med. Sci., Leading Researcher, Head of the Laboratory of Immunohistochemistry and Morphology, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, gulyatolibova@yandex.ru, https://orcid.org/0000-0002-6216-6220, 199034, Russia, Saint-Petersburg, Mendeleyevskaya Liniya, 3.
Andrey V. Tikhonov, PhD, Researcher at the Laboratory of Cytogenetics and Cytogenomics of Reproduction, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology, tixonov5790@gmail.com, https://orcid.org/0000-0002-2557-6642, 199034, Russia, Saint-Petersburg, Mendeleyevskaya Liniya, 3.
Lyubov I. Petrova, Laboratory Assistant at the Medical Genetic Center, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
199034, Russia, Saint-Petersburg, Mendeleyevskaya Liniya, 3.
Verа S. Dudkina, Laboratory Assistant at the Medical Genetic Center, D.O. Ott Research Institute of Obstetrics, Gynecology and Reproductology,
199034, Russia, Saint-Petersburg, Mendeleyevskaya Liniya, 3.
Corresponding author: Margarita O. Bakleicheva, bakleicheva@gmail.com
Authors' contributions: Bespalova O.N., Ivaschenko T.E. – conception and design of the study; Bakleicheva M.O., Tral T.G., Tolibova G.Kh., Tikhonov A.V., Petrova L.I., Dudkina V.S. – data collection and analysis; Bakleicheva M.O. – statistical analysis; Bespalova O.N., Bakleicheva M.O., Tikhonov A.V. – manuscript drafting; Bespalova O.N., Ivaschenko T.E., Tolibova G.Kh., Tral T.G. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was conducted within the framework of the state order of the Ministry of Science and Higher Education of the Russian Federation No. AAAA-A20-120041390025-9 "Development of diagnostic criteria for predicting early reproductive losses based on the expression of antigens of the main histocompatibility complex class I, G, E, C" (Dr. Bio. Sci., Prof. Ivaschenko T.E.).
Patient Сonsent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Bakleicheva M.O., Bespalova O.N., Ivashchenko T.E., Tral T.G., Tolibova G.Kh., Tikhonov A.V., Petrova L.I., Dudkina V.S. HLA-G expression in patients with missed miscarriages and a normal embryonic karyotype.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 12: 77-86 (in Russian)
https://dx.doi.org/10.18565/aig.2021.12.77-86



