Role of preimplantation genetic testing of embryos for aneuploidy in assisted reproductive technology outcomes in different groups of patients
Objective: To investigate the effect of preimplantation genetic testing (PGT-A) on the outcomes of assisted reproductive technology (ART) in different patient groups.Perminova S.G., Savostina G.V., Ekimov A.N., Belova I.S.
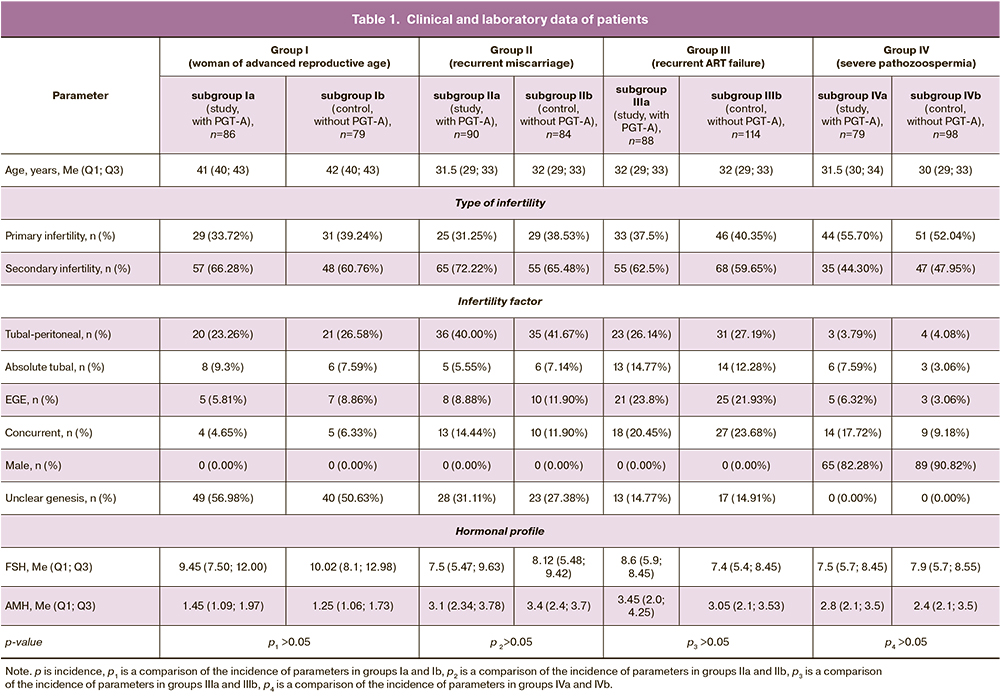
Materials and methods: This observational study analyzed 800 IVF cycles involving embryo cryopreservation followed by cryopreserved embryo transfer. The patients were divided into four groups based on their clinical and medical history. Group I included older reproductive age women, Group II included patients with recurrent miscarriages, Group III included women with repeat implantation failure, and Group IV consisted of married couples with severe pathozoospermia. Each of the four groups was divided into two subgroups: subgroup "a" included IVF programs with PGT-A and subgroup "b" (controls) included IVF programs without PGT-A.
Results: Clinical pregnancy rate in older reproductive age women was statistically significantly higher in subgroup Ia, compared with that in subgroup Ib (p=0.002; RR (95% CI) =3.18 (1.45; 6.97)). In women with recurrent miscarriages, there was no statistically significant difference in clinical pregnancy rates between subgroups IIa and IIb. However, there was a statistically significant reduction in the incidence of reproductive loss in subgroup IIa, compared with subgroup IIb (p=0.031; RR (95% CI)=0.39 (0.16; 0.96)). Patients with repeat implantation failures showed a statistically significant increase in clinical pregnancy rates in subgroup IIIa, compared with subgroup IIIb (p=0.008; RR (95% CI)=1.98 (1.19; 3.28)). The live birth and early reproductive loss rates were not significantly different between subgroups IIIa and IIIb. There were no statistically significant differences in the clinical pregnancy rates between subgroups IVa and IVb and in the rates of early reproductive loss and live birth in the group of patients with severe pathozoospermia.
Conclusion: This study demonstrated improved outcomes in older reproductive age women; an almost threefold reduction in the rate of early reproductive loss in women with recurrent miscarriages and an increase in the rate of clinical pregnancy in patients with repeat implantation failures. However, there were no statistically significant improvements in ART outcomes in the patients with severe pathozoospermia. These results suggest that PGT-A is feasible in selected patient groups. However, multicenter randomized controlled trials with a qualitative design will provide more comprehensive information on the role of PGT-A in ART outcomes.
Authors' contributions: Perminova S.G., Savostina G.V. – conception and design of the study; Savostina G.V. – data collection and analysis, manuscript drafting; Perminova S.G., Ekimov A.N., Belova I.S. – manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Perminova S.G., Savostina G.V., Ekimov A.N., Belova I.S.
Role of preimplantation genetic testing of embryos for aneuploidy
in assisted reproductive technology outcomes in different groups of patients.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (3): 73-82 (in Russian)
https://dx.doi.org/10.18565/aig.2022.288
Keywords
The selection of an embryo with optimal implantation potential is a major unresolved problem in the clinical practice of assisted reproductive technology (ART). None of the currently available non-invasive methods for assessing embryo quality can reliably determine implantation potential and exclude the presence of chromosomal abnormalities. It is known that even when a morphologically good embryo is transferred to an endometrium that is structurally appropriate for the phase of the menstrual cycle, pregnancy does not always occur; even when implantation is successful, it may be aborted at an early stage [1]. There is no doubt that chromosomal abnormalities of embryos make a significant contribution to the etiology of implantation failure and early reproductive pregnancy loss [2]. In addition, a significant proportion of morphologically normal embryos may exhibit aneuploidy [3]. The rate of aneuploidy in ART programs is quite high, varying between 54–84% depending on the age and history of the couple [3]. Therefore, in certain clinical cases, the traditional assessment of morphological and morphokinetic criteria is not sufficient for selective embryo transfer or for improving the results of ART programs.
The introduction of preimplantation genetic testing for aneuploidy (PGT-A) into clinical practice has opened new perspectives for the diagnosis of chromosomal abnormalities in embryos at the preimplantation stage and provides hope for improving the outcomes of ART programs in patients at a higher risk of producing aneuploid embryos. As PGT-A has evolved, it has undergone significant changes: embryo biopsy techniques have been improved, and new methods of molecular karyotype analysis have been developed, allowing the entire chromosome set of the embryo to be examined with high accuracy [4].
However, the role of PGT-A in ART outcomes has been actively debated in medical literature. Numerous studies have demonstrated controversial data regarding the effect of PGT-A on the effectiveness of ART in different patient groups [5–8].
According to the 2019 approved clinical guidelines for the diagnosis and treatment of infertility [9], PGT-A may be recommended in cases of older female reproductive age, recurrent miscarriage, recurrent implantation failure, and severe spermatogenesis disorders in men.
A key factor associated with aneuploid embryo production is the woman's age. Thus, while at the age of 26–30 years, the rate of aneuploidy in women is 20–27%, by the age of 45 years, it reaches 95.5% [10]. A strong positive correlation between maternal age and the incidence of embryonic aneuploidy is now beyond doubt [10]. According to the literature, PGT-A improves the outcomes of ART programs in patients of an advanced reproductive age. However, the results in other patient groups remain unclear [11].
The rate of early reproductive loss in ART programs is approximately 10–15% [12]. One of the leading causes of recurrent miscarriage is genetic; the shorter the gestational age, the higher the probability of chromosomal aneuploidy in the embryo [13]. For example, molecular karyotyping results show chromosomal abnormalities in 65–70% of spontaneous miscarriages before the 10th week of gestation [14]. According to published data, conducting PGT-A can reduce the risk of spontaneous abortion in ART programs [11].
Despite tremendous advances in ART, about 60% of women fail to become pregnant after their first In Vitro Fertilization (IVF) program, and 20% fail after their third IVF attempt [15]. Recurrent implantation failures are diagnosed in approximately one in three women using ART for infertility treatment. Their causes may be related to embryonic, uterine, oocyte, immunological factors, thrombophilic conditions, genetic disorders in parents, and severe partner pathozoospermia [16, 17]. The role of the embryo in recurrent implantation failure is thought to be 30–50%. A significant contribution seems to be caused by chromosomal abnormalities in the embryos [18]. There is evidence that the rate of aneuploid fetuses with chromosomes 13, 16, 18, 21, 22, X, or Y is doubled in women with a history of recurrent implantation failure compared to controls [19].
The appropriateness of PGT-A for severe spermatogenic abnormalities in men remains controversial. Patients with severe oligoasthenoteratozoospermia have a high incidence of chromosomal aberrations, including quantitative and structural abnormalities of the karyotype, which can lead to disruption of key steps in the first meiotic division, and consequently, to sperm and embryos with unbalanced chromosomal rearrangements [20, 21]. It has also been shown that the rate of euploid embryos is reduced in men with low morphologically normal spermatozoa and increased sperm DNA fragmentation [22–24]. However, several recently published studies have demonstrated no positive effect of PGT-A on the outcomes of ART programs in this group of patients [6–8].
This study aimed to investigate the effects of preimplantation genetic testing on ART outcomes in different patient groups.
Materials and methods
This observational study analyzed 800 IVF cycles involving embryo cryopreservation followed by cryopreserved embryo transfer at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology from 2018 to 2021.
Based on the history of the couples, four groups of patients were formed.
Group I consisted of 165 married couples with an older female reproductive age (≥ 38 years), who were divided into two subgroups: Ia (study group), which included 100 IVF/ICSI+PGT-A programs and 52 cryopreserved embryo transfers, and Ib (control group), which included 100 IVF/ICSI programs without PGT-A and 61 cryopreserved embryo transfers.
Group II consisted of 178 couples with a history of infertility and recurrent miscarriage (two or more spontaneous abortions at up to 22 weeks), who were divided into two subgroups: Group IIa (study group), which included 100 IVF/ICSI+PGT-A cycles and 90 cryopreserved embryo transfers, and Group IIb (control group), which included 100 IVF/ICSI cycles without PGT-A and 84 cryopreserved embryo transfers.
Group III consisted of 168 couples with recurrent implantation failures (three or more failed attempts of selective transfer of fresh or thawed embryos), who were divided into two subgroups: IIIa (study group), which included 100 IVF/ICSI+PGT-A cycles and 88 cryopreserved embryo transfers, and IIIb (control group), which included 100 IVF/ICSI cycles without PGT-A and 114 cryopreserved embryo transfers.
Group IV consisted of 164 married couples with severe spermatogenesis disorders in men (severe oligoasthenoteratozoospermia), which were divided into two subgroups: IVa (study group), including 100 IVF/ICSI+PGT-A cycles and 79 cryopreserved embryo transfers, and IVb (control), which included 100 IVF/ICSI cycles without PGT-A and 98 cryopreserved embryo transfers.
The inclusion criteria for the study groups were the absence of pregnancy for 1 year or more and a normal karyotype of the spouses. In Group I – a woman aged 38 or more; in Group II – two or more spontaneous terminations of pregnancy before 22 weeks in the history of pregnancy, a woman aged less than 35; in Group III – three or more unsuccessful attempts of selective transfer of fresh or thawed embryos, a woman aged less than 35; Group IV – spermatozoa concentration – less than 5 mln/ml, number of progressively mobile spermatozoa (a+b)–less than 29%, morphologically normal forms of spermatozoa – 2% or less in the spermogram, female age less than 35 years.
The non-inclusion criteria for the study groups were chronic endometritis verified by histological examination, endometrial thickness less than 7 mm, stage III-IV extragenital endometriosis, polycystic ovarian syndrome, antiphospholipid syndrome, high-risk thromboembolic complications, and all conditions that are contraindications to ART and pregnancy according to the Ministry of Health of the Russian Federation Order of 31 July 2020, No. 803n.
All married couples underwent a complete clinical and laboratory examination in accordance with the Ministry of Health of the Russian Federation Order No. 803n, dated July 31, 2020.
Ovarian stimulation was performed according to standard protocols with a gonadotropin-releasing hormone antagonist (GnRH antagonist) or gonadotropin-releasing hormone agonist (GnRH agonist). All embryos were cultured individually in droplets of culture in multigas incubators. Quality assessment of the obtained embryos was performed on day 5 of culture based on morphological criteria according to the Gardner grading system (ESHRE 2011 modified D. Gardner classification). In the study groups, all embryos of good and excellent quality (3BV and above) were biopsied for trophectoderm cells, followed by embryo cryopreservation. The trophectoderm cells were transferred to Eppendorf tubes containing lysis buffer for subsequent PGT-A analysis, which was performed by next-generation DNA sequencing (NGS). PGT-A-detected euploid embryos were transferred to the uterus as part of a cryopreserved embryo transfer on cyclic hormonal therapy or in the natural menstrual cycle when endometrial thickness was greater than 7 mm. In the control groups, embryos of good and excellent quality were cryopreserved for subsequent selective transfer into the uterine cavity by cryopreserved transfer. On day 10 after embryo transfer, a human chorionic hormone gonadotropin (hCG) β-subunit blood test was performed. A transvaginal ultrasound scan was performed 21 days after transfer to diagnose a clinical pregnancy with a positive β-hCG blood test result. Further follow-up and pregnancy management were individually performed by the treating physician.
Statistical analysis
Statistical analyses of ART outcomes with and without PGT-A in the different patient groups were performed. The data were analyzed using IBM SPS Statistics software. Categorical variables are reported as counts and proportions (%). A visual assessment of normality included the analysis of scatterplots. Continuous variables with asymmetric distribution are described as medians and interquartile ranges (Me (Q1; Q3)). Variables that did not meet normality assumptions were compared using a nonparametric Mann–Whitney test. The outcomes of ART programs in the study and control groups were compared using the chi-squared test (χ2). The significance threshold was set at p<0.05.
Results
The clinical and laboratory data of the patients are shown in Table 1.
Cryopreserved embryo transfer outcomes in patients with advanced reproductive age are shown in Table 2. A total of 86 couples, 100 cycles of IVF/ICSI+PGT-A (mean number of cycles per patient was 1.2), and 52 cryopreserved embryo transfers were analyzed in subgroup Ia (control). In the control Ib subgroup, there were 79 couples, 100 cycles of IVF/ICSI without PGT-A (mean number of cycles per patient was 1.3), and 61 cryopreserved transfers.
In the study subgroup (Ia), 211 embryos from 305 blastocysts on days 5 and 6 of culture were obtained and sent for PGT-A at a rate of 211/305 (69.2%). The proportion of euploid embryos was 58/211 (27.5%). Based on an analysis of the outcomes of 52 cryopreserved embryo transfers in the study group, the clinical pregnancy rate was 19/52 (36.5%) and no biochemical pregnancies were detected. The rate of early reproductive loss was 3/19 (15.8%). In addition, one patient (5.3%) had a preterm birth. The rate of live births was 16/19 (84.2%).
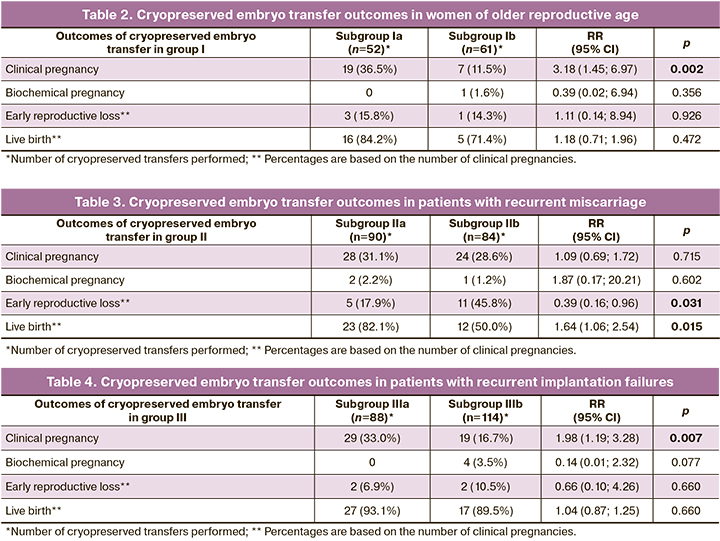
When analyzing the outcomes in the control subgroup (Ib), clinical pregnancy rate per transfer was 7/61 (11.5%). The proportion of biochemical pregnancies was 1/61 (1.6%). The early reproductive loss and live birth rates were 1/7 (14.3%) and 5/7 (71.4%), respectively. There was also one ectopic pregnancy, 1/7 (14.3%).
Thus, clinical pregnancy rate in subgroup Ia was statistically significantly higher than that in subgroup Ib (p=0.002; RR (95% CI) =3.18 (1.45; 6.97)). However, there was no statistically significant increase in live births in subgroup Ia compared with that in subgroup Ib. There were no statistically significant differences in the incidence of early reproductive loss.
The outcomes of cryopreserved embryo transfers in patients with recurrent miscarriages are summarized in Table 3. A total of 90 couples, 100 cycles of IVF/ICSI+PGT-A (mean number of cycles per patient was 1.1), and 90 cryopreserved embryo transfers were analyzed in study subgroup IIa. In the control subgroup (IIb), there were 88 couples, 100 IVF/ICSI cycles without PGT-A (mean number of cycles per patient was 1.1), and 84 cryopreserved embryo transfers.
In the IIa subgroup, a total of 341 embryos (58.5 %) from 583 blastocysts on days 5th and 6th day of cultivation were obtained and sent for PGT-A. The proportion of euploid embryos was 162/341 (47.5%). In the cryopreserved transfer group, 90/162 (55.6%) embryos were transferred. Clinical and biochemical pregnancy rates were 28/90 (31.1%) and 2/90 (2.2%), respectively. The rates of early reproductive loss, preterm birth, and live birth were 5/28 (17.9%), 2/28 (7.2%), and 23/28 (82.1%), respectively.
In an analysis of outcomes in subgroup IIb, the rate of clinical pregnancy was 24/84 (28.6%), the rate of biochemical pregnancy was 1/84 (1.2%); the rate of early reproductive loss was 11/24 (45.8%), the rate of late miscarriage was 1/24 (4.7%), the rate of preterm birth was 1/24 (4.7%), the rate of live birth was 12/24 (50.0%).
There was no statistically significant difference in the clinical pregnancy rates between subgroups IIa and IIb. The rate of live births in subgroup IIa was statistically significantly higher than in subgroup IIb (p=0.015; RR = 1.64 ((95% CI 1.06; 2.54)). There was a statistically significant reduction in the incidence of reproductive loss in subgroup IIa versus subgroup IIb (p=0.031; RR =0.39 (95% CI 0.16; 0.96)).
The outcomes of cryopreserved transfers in the patients with repeated unsuccessful implantation attempts are shown in Table 4. A total of 80 couples, 100 cycles of IVF/ICSI+PGT-A (mean number of cycles per patient was 1.1), and 88 cryopreserved transfers were analyzed in the study group. In the control group, there were 76 couples, 100 IVF/ICSI cycles without PGT-A (mean number of cycles per patient was 1.1), and 114 cryopreserved transfers.
In the IIIa subgroup, 277 embryos (65.33 %) from 424 blastocysts on days 5th and 6th day of culture were obtained and sent for PGT-A. The proportion of euploid embryos was 150/277 (54.2%). The clinical pregnancy rate in the study subgroup was 29/88 (33.0%), and no biochemical pregnancies were observed. The rates of early reproductive loss and live birth were 2/29 (6.9%) and 27/29 (93.1%), among which there was one case of birth with a congenital heart defect (interventricular septal defect).
In the analysis of outcomes in subgroup IIIb, clinical and biochemical pregnancy rates were 19/114 (16.7%) and 4/114 (3.5%), respectively. The rates of early reproductive loss and live birth were 2/19 (10.5%) and 17/19 (89.4%), respectively.
Thus, there was a statistically significant increase in clinical pregnancy rate in subgroup IIIa, compared with that in subgroup IIIb (p=0.007; RR =1.98 (95% CI 1.19; 3.28)). The rates of live births and early reproductive loss did not differ significantly between the study and control subgroups.
The outcomes of cryopreserved transfers in patients with severe pathozoospermia from their spouses are shown in Table 5. A total of 85 couples, 100 cycles of IVF/ICSI+PGT-A (mean of 1.1 cycles per patient), and 79 cryopreserved transfers were analyzed in the study subgroup. In the control subgroup, there were 92 couples, 100 IVF/ICSI cycles without PGT-A (mean number of cycles per patient was 1.1), and 98 cryopreserved transfers.
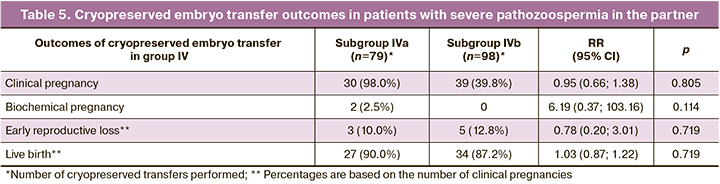
In total, in the IVa subgroup, 286 embryos (66.7 %) from 429 blastocysts on days 5 and 6 of culture were obtained and sent for PGT-A. The proportion of euploid embryos was 158/286 (55.2%). The frequencies of clinical and biochemical pregnancy rates were 30/79 (38.0%) and 2/79 (2.5%). The rate of early reproductive loss was 3/30 (10.0%) and one patient (3.3%) had late miscarriage. The rate of live births was 27/30 (90.0%).
In subgroup IVb, the pregnancy rate was 39/98 (39.8%). No biochemical pregnancy was observed. The rates of early reproductive loss, preterm births, and live birth were 5/39 (12.8%), 1/39 (2.6%), and 34/39 (87.2%), respectively.
There were no statistically significant differences in the rates of clinical pregnancy, early reproductive loss, or live birth between the IVa and IVb subgroups.
Discussion
In recent years, there has been an increasing trend towards the use of PGT-A in ART. For example, if the incidence of IVF/ICSI + PGT-A programs was 6% in 2016 and 8% in 2017 and 2018, it had already reached 19% by 2019 [2]. The use of PGT-A in couples at high risk of aneuploid embryos is thought to increase the rate of clinical pregnancy and live birth, and reduce the rate of early reproductive loss [11]. However, a growing number of studies have shown controversial data regarding the effect of PGT-A on ART outcomes in these patient groups [11]. To date, there is insufficient reliable evidence to support the positive effect of PGT-A on ART outcomes. Therefore, the feasibility of using PGT-A in different patient groups needs to be discussed.
A recent large observational study of 2,538 women of advanced reproductive age demonstrated the positive effect of PGT-A on ART outcomes. There was a significant increase in the rate of live births, a reduction in early reproductive loss, and multiple pregnancies in the study group compared with controls [25]. In our study, similar results were obtained; in patients aged 38 years and older, the rate of clinical pregnancy was significantly higher in the IVF/ICSI+PGT-A group than in controls. The probability of live births was higher in the PGT-A group than in the control group. These findings confirm that aneuploid embryo formation plays a major role in ART failure in patients of advanced reproductive age. PGT-A in this group of patients can offset the negative impact of age on the outcome of ART in euploid embryo transfer.
The largest study to date, including patients with idiopathic habitual miscarriage in different age groups, showed no reduction in early reproductive loss with PGT-A [5]. However, an increased rate of clinical pregnancies and live births was observed with PGT-A in women of all age groups, including those younger than 35 years. These results contradict the findings of another randomized trial in which clinical pregnancy rates were higher only in women aged 35–40 years, who are at a high risk of aneuploid embryos [26]. However, there were no significant differences in the miscarriage and live birth rates in any age group when PGT-A was used compared to controls. In our study, the incidence of early reproductive loss and live birth was statistically significantly lower in the group with PGT-A, compared to the control. The incidence of clinical pregnancy did not differ between the groups. These controversial results may be due to the different methods used for PGT-A. Retrospective analysis of patient data and inadequate screening of couples for other non-aneuploidy causes of infertility may also have influenced the results. PGT-A may be appropriate in patients with recurrent miscarriage if other causes of miscarriage are excluded.
According to Sato et al. (2019), in patients with recurrent implantation failure, no statistically significant differences in live birth and early reproductive loss rates were found with and without PGT-A [27]. However, the implantation rate was significantly higher in the PGT-A group than that in the control group. Similar results were reported in other studies [28, 29]. Pantou A. et al. (2022) in their study demonstrated a positive effect of PGT-A on both clinical pregnancy and live birth rates [30]. Our study also found an increased pregnancy rate with PGT-A compared to that in the controls. However, there were no significant differences in the rates of live births and early reproductive loss between the groups. It seems that the transfer of a quality healthy embryo plays a more important role in the outcomes of ART than the obstetric and gynecological history, including the endometrial condition. However, to date, few studies have assessed the effect of PGT-A in this group of patients. Although all of these studies confirmed an improvement in ART outcomes after PGT-A, multicenter randomized controlled trials with a larger sample of patients and a more personalized approach to the selection of candidates for participation are needed.
According to several studies, patients with male factor infertility have a significantly higher rate of mosaic embryos [31]. According to a study by Mazzilli R. et al. (2017), embryos obtained from patients with severe pathozoospermia are characterized by a reduced fertilization rate and slower embryonic development at the preimplantation stage [32]. However, severe male factors had no effect on the ploidy of the embryos or their implantation potential. A recent study by Asoglu M.R. et al. (2022), including patients with severe male factors, showed no positive effect of PGT-A on ART outcomes, and there was no statistically significant difference in either clinical pregnancy rates or live birth rates [33]. Our study also showed that PGT-A had no positive effects on ART outcomes in patients with severe pathozoospermia. Severe male infertility is not a critical factor in aneuploid embryo formation. This may be due to the reparative ability of oocytes to restore karyotype prior to zygotic genome activation or to the fact that paternal aneuploidy can lead to embryonic arrest at an earlier stage [11]. However, further studies are needed to assess the impact of PGT-A on ART outcomes in this group of patients.
It should be noted that the lack of positive effects of PGT-A on ART outcomes may be due to limitations of the technique. For example, NGS is the best method for PGT-A, and trophectodermal biopsy is the safest way to collect material in terms of invasiveness. Although NGS is accurate to 99%, false-positive results are not excluded [11], apparently due to the high frequency of mosaicism in human embryos and the proven ability of blastocysts to self-correct, that is, to activate apoptosis of aneuploid cells during embryonic development [11]. However, in PGT-A-detected mosaic embryos, the proportion of aneuploid cells in the biopsy material does not always reflect the true chromosomal status of the entire embryo. For example, Saifitdinova A.F. et al. (2020) showed that the proportion and type of mosaicism may differ in different areas of the trophectoderm and in the cells of the inner cell mass of the mosaic embryo [34]. According to the preliminary results, if mosaicism is detected in the blastocyst by PGT-A, the probability of the euploid chromosome set in other parts of the embryo is low. Therefore, transfer of mosaic embryos into the uterus should be considered with caution.
Conclusion
PGT-A has been the focus of studies that have investigated its efficacy, safety, and feasibility in clinical practice. To date, this is the only method that allows the ploidy of an embryo to be determined with high accuracy, improving the selection of an embryo with optimal implantation potential. Despite this, the role of PGT-A in improving ART outcomes remains debated. Our study demonstrated improved ART outcomes in women of advanced reproductive age, an almost three-fold reduction in the probability of early reproductive loss in women with recurrent miscarriage, and an increase in clinical pregnancy rates in patients with recurrent implantation failures. However, no statistically significant improvements in ART outcomes were observed in the couples with severe pathozoospermia. These results suggest that PGT-A should be used in selected patient groups. However, well-designed and adequately powered randomized clinical trials will provide better information on the role of PGT-A in ART outcomes.
References
- Российская Ассоциация Репродукции Человека. Отчет за 2018 год. Доступно по: http://www.rahr.ru/registr_otchet.php [Russian Association of Human Reproduction. Report for 2018. Available at: http://www.rahr.ru/registr_otchet.php (in Russian)].
- Кулакова Е.В., Калинина Е.А., Трофимов Д.Ю., Макарова Н.П., Хечумян Л.Р., Дударова А.Х. Вспомогательные репродуктивные технологии у супружеских пар с высоким риском генетических нарушений. Преимплантационный генетический скрининг. Акушерство и гинекология. 2017; 8: 21-7. [Kulakova E.V., Kalinina E.A., Trofimov D.Yu., Makarova N.P., Khechumyan L.R., Dudarova A.Kh. Assisted reproductive technologies in married couples at high risk for genetic disorders. Preimplantation genetic screening. Obstetrics and Gynecology. 2017; (8): 21-7. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.8.21-7.
- Fesahat F., Montazeri F., Hoseini S.M. Preimplantation genetic testing in assisted reproduction technology. J. Gynecol. Obstet. Hum. Reprod. 2020; 49(5): 101723. https://dx.doi.org/10.1016/j.jogoh.2020.101723.
- Киселева Ю.Ю., Кодылева Т.А., Кириллова А.О. Использование скрининговых тестов на выявление наиболее распространенных мутаций перед программой вспомогательных репродуктивных технологий. Проблемы репродукции. 2017; 23(2): 47-9. [Kiseleva Yu.Yu., Kodyleva T.A., Kirillova A.O. Use of genetic screening tests before ART. Russian Journal of Human Reproduction. 2017; 23(2): 47-9. (in Russian)].https://dx.doi.org/10.17116/repro201723247-49.
- Bhatt S.J., Marchetto N.M., Roy J., Morelli S.S., McGovern P.G. Pregnancy outcomes following in vitro fertilization frozen embryo transfer (IVF-FET) with or without preimplantation genetic testing for aneuploidy (PGT-A) in women with recurrent pregnancy loss (RPL, with recurrent pregnancy loss (RPL): a SART-CORS study. Hum. Reprod. 2021; 36(8): 2339-44.https://dx.doi.org/10.1093/humrep/deab117.
- Asoglu M.R., Celik C., Serefoglu E.C., Findikli N., Bahceci M. Preimplantation genetic testing for aneuploidy in severe male factor infertility. Reprod. Biomed. Online. 2020; 41(4): 595-603. https://dx.doi.org/10.1016/j.rbmo.2020.06.015.
- Rubio C., Bellver J., Rodrigo L., Castillón G., Guillén A., Vidal C. et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil. Steril. 2017; 107(5):1122-9. https://dx.doi.org/10.1016/j.fertnstert.2017.03.011.
- Munné S., Kaplan B., Frattarelli J.L., Child T., Nakhuda G., Shamma F.N. et al.; STAR Study Group. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil. Steril. 2019; 112(6): 1071-9.e7. https://dx.doi.org/10.1016/j.fertnstert.2019.07.1346.
- Министерство здравоохранения Российской Федерации. Женское бесплодие (современные подходы к диагностике и лечению). Клинические рекомендации. Протокол лечения. М.; 2019. [Ministry of Health of the Russian Federation. Female infertility (modern approaches to diagnosis and treatment). Clinical guidelines. Treatment protocol. Moscow; 2019.(in Russian)].
- Бейк Е.П., Коротченко О.Е., Гвоздева А.Д., Сыркашева А.Г., Долгушина Н.В. Роль преимплантационного генетического скрининга в повышении эффективности программ вспомогательных репродуктивных технологий у пациенток позднего репродуктивного возраста. Акушерство и гинекология. 2018; 4: 78-84. [Beik E.P., Korotchenko O.E., Gvozdeva A.D., Syrkasheva A.G., Dolgushina N.V. Role of preimplantation genetic screening in enhancing the effectiveness of assisted reproductive technology programs in late reproductive-aged patients. Obstetrics and Gynecology. 2018; (4): 78-84.(in Russian)]. https://dx.doi.org/10.18565/aig.2018.4.78-84.
- Greco E., Litwicka K., Minasi M.G., Cursio E., Greco P.F., Barillari P. Preimplantation genetic testing: where we are today. Int. J. Mol. Sci. 2020; 21(12): 4381. https://dx.doi.org/10.3390/ijms21124381.
- Dhillon R.K., Hillman S.C., Morris R.K., McMullan D., Williams D., Coomarasamy A. et al. Additional information from chromosomal microarray analysis (CMA) over conventional karyotyping when diagnosing chromosomal abnormalities, in miscarriage: a systematic review and meta-analysis. BJOG. 2014; 121(1): 11-21. https://dx.doi.org/10.1111/1471-0528.12382.
- Philipp T., Philipp K., Reiner A., Beer F., Kalousek D.K. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum. Reprod. 2003; 18(8): 1724-32. https://dx.doi.org/10.1093/humrep/deg309.
- McQueen D.B., Lathi R.B. Miscarriage chromosome testing: Indications, benefits and methodologies. Semin. Perinatol. 2019; 43(2): 101-4.https://dx.doi.org/10.1053/j.semperi.2018.12.007.
- Liu L., Zhou F., Lin X., Li T., Tong X., Zhu H., Zhang S. Recurrent IVF failure with elevated progesterone on the day of hCG administration. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013; 171(1): 78-83. https://dx.doi.org/10.1016/j.ejogrb.2013.08.025.
- Тетруашвили Н.К. Привычный выкидыш. Акушерство и гинекология: новости, мнения, обучение. 2017; 4: 70-87. [Tetruashvili N.K. Reccurent miscarriage. Obstetrics and Gynecology: News, Opinions, Training. 2017; (4): 70-87. (in Russian)].
- Bashiri A., Halper K.I., Orvieto R. Recurrent implantation failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 2018;.16(1): 121. https://dx.doi.org/10.1186/s12958-018-0414-2.
- Idelevich A., Vilella F. Mother and embryo cross-communication. Genes. 2020; 11(4): 376. https://dx.doi.org/10.3390/genes11040376.
- Tong J., Niu Y., Wan A., Zhang T. Next-Generation Sequencing (NGS)-based preimplantation genetic testing for aneuploidy (PGT-A) of trophectoderm biopsy for Recurrent Implantation Failure (RIF) patients: a retrospective study. Reprod. Sci. 2021; 28(7): 1923-9. https://dx.doi.org/10.1007/s43032-021-00519-0.
- Rodrigo L., Meseguer M., Mateu E., Mercader A., Peinado V., Bori L. et al. Sperm chromosomal abnormalities and their contribution to human embryo aneuploidy. Biol. Reprod. 2019; 101(6): 1091-101. https://dx.doi.org/10.1093/biolre/ioz125.
- Saei P., Bazrgar M., Gourabi H., Kariminejad R., Eftekhari-Yazdi P., Fakhri M. Frequency of sperm aneuploidy in oligoasthenoteratozoospermic (OAT) patients by comprehensive chromosome screening: a proof of concept. J. Reprod. Infertil. 2021; 22(1): 57-64. https://dx.doi.org/10.18502/jri.v22i1.4996.
- Киселева Ю.Ю., Азова М.М., Кодылева Т.А., Ушакова И.В., Кириллова А.О., Екимов А.Н., Ракитько А.С., Володяева Т.O., Мишиева Н.Г., Абубакиров A.Н. Результаты преимплатационного генетического скрининга эмбрионов у супружеских пар с фрагментацией ДНК сперматозоидов. Акушерство и гинекология. 2017; 8: 104-8. [Kiseleva Yu.Yu., Azova M.M., Kodyleva T.A., Ushakova I.V., Kirillova A.O., Ekimov A.N., Rakitko A.S., Volodyaeva T.O., Mishieva N.G., Abubakirov A.N. Results of preimplatation genetic screening of embryos in married couples with sperm DNA fragmentation. Obstetrics and Gynecology. 2017; (8): 104-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.8.104-8.
- Киселева Ю.Ю., Азова М.М., Кодылева Т.А., Кириллова А.О., Екимов А.Н., Ракитько А.С., Мишиева Н.Г., Абубакиров А.Н. Увеличение анеуплоидий эмбрионов ассоциировано с пониженной долей морфологически нормальных сперматозоидов. Генетика. 2017; 53(12): 1458-62. [Kiseleva Yu.Yu., Azova M.M., Kodyleva T.A., Kirillova A.O., Ekimov A.N., Rakitko A.S., Mishieva N.G., Abubakirov A.N. Increased aneuploidy embryos associated with a reduced proportion of morphologically normal sperm. Genetics. 2017; 53(12): 1458-62. (in Russian)]. https://dx.doi.org/10.7868/S0016675817120050.
- Макарова Н.П., Лобанова Н.Н., Кулакова Е.В., Непша О.С., Екимов А.Н., Калинина Е.А. Влияние преимплантационного генетического тестирования на результаты программ вспомогательных репродуктивных технологий у супружеских пар с мужским фактором бесплодия. Акушерство и гинекология. 2021; 11: 154-64. [Makarova N.P., Lobanova N.N., Kulakova E.V., Nepsha O.S., Ekimov A.N., Kalinina E.A. Impact of preimplantation genetic testing on assisted reproductive technology outcomes in couples with male factor infertility. Obstetrics and Gynecology. 2021; (11): 154-64. (in Russian)].https://dx.doi.org/10.18565/aig.2021.11.154-164.
- Sacchi L., Albani E., Cesana A., Smeraldi A., Parini V., Fabiani M. et al. Preimplantation genetic testing for aneuploidy improves clinical, gestational, and neonatal outcomes in advanced maternal, age patients without compromising cumulative live-birth rate. J. Assist. Reprod. Genet. 2019; 36(12): 2493-504. https://dx.doi.org/10.1007/s10815-019-01609-4.
- Sato T., Sugiura-Ogasawara M., Ozawa F., Yamamoto T., Kato T., Kurahashi H. et al. Preimplantation genetic testing for aneuploidy: A comparison of live birth rates in patients with, recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum. Reprod. 2019; 34(12): 2340-8.https://dx.doi.org/10.1093/humrep/dez229.
- Simopoulou M., Sfakianoudis K., Maziotis E., Tsioulou P., Grigoriadis S., Rapani A. et al. PGT-A: who and when? Α systematic review and network meta-analysis of RCTs. J. Assist. Reprod. Genet. 2021; 38(8): 1939-57.https://dx.doi.org/10.1007/s10815-021-02227-9.
- Greco E., Bono S., Ruberti A., Lobascio A.M., Greco P., Biricik A. et al. Comparative genomic hybridization selection of blastocysts for repeated implantation failure treatment: a pilot study. Biomed. Res. Int. 2014; 2014: 457913. https://dx.doi.org/10.1155/2014/457913.
- Sato T., Sugiura-Ogasawara M., Ozawa F., Yamamoto T., Kato T.,Kurahashi H. et al. Preimplantation genetic testing for aneuploidy: a comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum. Reprod. 2019; 34(12): 2340-8. https://dx.doi.org/10.1093/humrep/dez229. Erratum in: Hum Reprod. 2020; 35(1): 255.
- Pantou A., Mitrakos A., Kokkali G., Petroutsou K., Tounta G., Lazaros L. et al. The impact of preimplantation genetic testing for aneuploidies (PGT-A) on clinical outcomes in, high risk patients. J. Assist. Reprod. Genet. 2022; 39(6): 1341-9. https://dx.doi.org/10.1007/s10815-022-02461-9.
- Tarozzi N., Nadalini M., Lagalla C., Coticchio G., Zacà C., Borini A. Male factor infertility impacts the rate of mosaic blastocysts in cycles of preimplantation genetic testing for aneuploidy. J. Assist. Reprod. Genet. 2019; 36(10): 2047-55. https://dx.doi.org/10.1007/s10815-019-01584-w.
- Mazzilli R., Cimadomo D., Vaiarelli A., Capalbo A., Dovere L., Alviggi E. et al. Effect of the male factor on the clinical outcome of intracytoplasmic sperm injection combined with, preimplantation aneuploidy testing: observational longitudinal cohort study of 1,219 consecutive cycles. Fertil. Steril. 2017; 108(6): 961-72.e3. https://dx.doi.org/10.1016/j.fertnstert.2017.08.033.
- Lin X.H., Guo M.X., Wu D.D., Lu Y., Zhang J.L., Zhou C.L. et al. Preimplantation genetic testing for aneuploidy in severe male factor infertility: protocol for a multicenter randomised, controlled trial. BMJ Open. 2022; 12(7): e063030. https://dx.doi.org/10.1136/bmjopen-2022-063030.
- Saifitdinova A.F., Saifitdinova A.F., Glotov O.S., Polyakova I.V., Bichevaya N.K., Loginova Ju.A., Kuznetsova R.A. et al. Mosaicism in preimplantation human embryos. Integrative Physiology. 2020; 1(3): 225-30.https://dx.doi.org/10.33910/2687- 1270-2020-1-3-225-230.
Received 30.11.2022
Accepted 02.03.2023
About the Authors
Svetlana G. Perminova, Dr. Med. Sci., Leading Researcher at Reproductology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, perisvet@list.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.Guzel V. Savostina, PhD Student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, savostina2324@gmail.com, 117997, Russia, Moscow, Ac. Oparin str., 4.
Alexey N. Ekimov, Laboratory Geneticist at the Laboratory of Molecular Genetics, Academician V.I. Kulakov National Medical Research Center for Obstetrics,
Gynecology and Perinatology, Ministry of Health of Russia, a_ekimov@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Irina S. Belova, PhD Student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia,
irina-belova00@mail.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Corresponding author: Guzel V. Savostina, savostina2324@gmail.com



