Medical and social factors and pathogenetic mechanisms of early pregnancy loss in women with recurrent miscarriage
Aim. To identify medical and social risk factors and investigate the condition of the endometrium in women with the early loss of current pregnancy and a history of recurrent early miscarriages.Batrak N.V., Malyshkina A.I., Sotnikova N.Yu., Kroshkina N.V., Peretyatko L.P., Fateeva N.V.
Materials and methods. The study prospectively evaluated the course of pregnancy and perinatal outcomes of 184 women. The study group included 75 patients who experienced a current pregnancy loss in the first trimester and had a history of threatened and recurrent miscarriages of early pregnancy. The comparison group consisted of 73 women with a history of threatened and recurrent miscarriages of early pregnancy, but their current pregnancies progressed to 22 weeks.
Results. The histopathology reports of patients with recurrent miscarriage showed an abnormal villous differentiation, chromosomal disorders in the form of avascular chorionic villi, lymphocytic inflammation, edema of the endometrial stroma, and foci of necrosis. In patients with spontaneous abortion, histopathology findings included focal sclerosis, stromal fibrosis, delayed differentiation of fibroblast-like cells into pre-decidual cells, focal perivascular and peritubular lymphoplasmohistiocytic stromal infiltration, and sclerosis of the spiral artery walls.
Conclusion. Recurrent miscarriage is associated with impaired differentiation of chorionic villi accompanied by vascular abnormalities, which may be considered as a morphological confirmation of possible chromosomal disorders. Early pregnancy loss in the form of spontaneous abortion is caused by chronic endometrial inflammation. The study findings must be taken into account when planning antenatal care for these women.
Keywords
Appropriate formation of the maternal-fetal-placental unit in early pregnancy is a critical factor in determining the normal course of pregnancy. In recent years, literature has been abundant regarding the role of the maternal- fetal-placental unit, including embryo implantation and gestational changes in the endometrium [1, 2]. Numerous hormonal, immunological, genetic factors are involved in the regulation of these processes [3–5]. Nutrition, environmental factors, chronic intoxication, medications, gynecologic and somatic comorbidities impair formation of the maternal-fetal-placental unit [6–10]. The negative impact of these factors results in impairment of implantation and placentation leading to gestational complications, such as missed miscarriage, spontaneous miscarriage, hypertensive disorders, placental abruption, intrauterine growth restriction, and perinatal losses [11–13]. It is well known that the higher is the sensitivity of the fetus to damaging factors, the shorter is the gestation period. Human pregnancy is considered viable after three essential processes have occurred, fertilization, implantation, and placentation [14].
Implantation (nidation) occurs during the third week of pregnancy (day 20–24). Its initial stage is an apposition, i.e., the initial adhesion of the blastocyst to the endometrial surface. Apposition is followed by the adhesion stage when a stronger connection is established between the embryo and endometrium. Finally, in the invasion stage, trophoblastic cells invade the endometrium, followed by spiral artery remodeling. A significant role in blastocyst nidation is played by proteolytic enzymes that are controlled by matrix metalloproteinases; integrins, ensuring cytotrophoblast invasion into the endometrium [15, 16].
To date, the mechanisms of impaired invasion of vascular trophoblasts have not been sufficiently explored. Invasion of the vascular trophoblast occurs controlled by vascular endothelial growth factor (VEGF), and this process is also mediated by nitric oxide. Stromal trophoblast invasion is dependent on bone morphogenetic protein-2, and the intravascular distribution of trophoblast is regulated by CD31. Fibrosis and sclerosis in the endometrium and spiral arteries, resulting from chronic inflammation, slow down the full invasion of interstitial extravillous cytotrophoblast, endovascular trophoblast, and spiralization [17].
Previous studies reported that the impairment of the first wave of cytotrophoblast invasion manifested by early pregnancy loss, in particular anembryonic gestation, early spontaneous miscarriages, and missed miscarriages.
A morphological study of the endometrium during anembryonic pregnancy reveals minimal and superficial invasion of the interstitial cytotrophoblast, the absence of trophoblastic cell columns at the base of the anchor villi surrounding the chorionic sac. These morphological abnormalities are caused by chromosomal abnormalities in the trophoblastic membrane of the blastocyst, which is the source of extraembryonic provisional organs. An unfavorable cellular environment in the endometrium may also play a significant role.
A study of the material obtained by uterine curettage during early spontaneous miscarriages showed pronounced trophoblast hypoplasia at the base of the anchoring villi [17, 18].
Trophoblastic invasion in patients with missed miscarriage is characterized by several features. In patients with progesterone deficiency, impaired trophoblastic invasion is associated with retardation of the glandular apparatus and a decrease in the rate of endometrial stromal decidualization. In patients with antiphospholipid syndrome, circulating maternal antibodies negatively affect extravascular vascular trophoblast, reducing its proliferative potential, and limiting intravascular invasion [14].
A histological assessment of endometrial scrape samples from patients with different forms of early gestational losses allows an estimate of the endometrium during a miscarriage. It could aid decision-making regarding antenatal care of women with a history of reproductive disorders.
This study aimed to identify medical and social risk factors and investigate the condition of the endometrium in women with the early loss of current pregnancy and a history of recurrent early miscarriages.
Materials and methods
The study comprised 184 women who were managed at the Clinic of the V.N. Gorodkov Research Institute of Maternity and Childhood and antenatal clinics in Ivanovo City between 2013 and 2019. The study group included 75 patients who experienced a current pregnancy loss in the first trimester and had a history of threatened and recurrent miscarriages of early pregnancy. The comparison group consisted of 73 women with a history of threatened and recurrent miscarriages of early pregnancy, but their current pregnancies progressed to 22 weeks. Thirty-six women with a healthy pregnancy and an uncomplicated reproductive history made up the control group. Inclusion criteria were as follows: a singleton pregnancy threatened miscarriage and a history of recurrent miscarriage. Exclusion criteria were as follows: pregnancy resulting from the use of assisted reproductive technologies, and decompensated somatic comorbidities. Medical and social factors were assessed using the questionnaire, including social, medical, vocational, and living condition characteristics, obstetric and gynecological history, and non-gynecologic diseases. Serum levels of relative (percentage) number of CD178 + monocytes, intracellular synthesis of cytokines (interleukin (IL) -10, tumor necrosis factor (TNF) -α) by monocytes were measured using monoclonal antibodies with 2-color FACSCanto flow cytometer (Becton Dickinson, USA). The biological material obtained by uterine curettage was processed following the method by D.S. Sarkisova, Yu.L. Perova (1996).
Sections(4–5 μmthick) ofthegestationalendometrium and chorionic villous were examined histologically after hematoxylin and eosin staining. The pathomorphological examination included the following parameters: villous differentiation, chromosomal disorders in the form of avascular chorionic villi, remodeling of the endometrial segments of the spiral arteries, inflammatory infiltration and edema of the endometrial stroma, decidualization and blood flow disorders, viral cell transformation and focal necrosis. Additionally, signs of chronic endometritis were evaluated, including post-inflammatory fibrosis and periarterial sclerosis of the endometrial stroma, lymphoplasmocytic stromal infiltration, and delayed differentiation of stromal cells.
Statistical analysis
The normality of the distribution and equality of variance were tested by the Shapiro-Wilk and Levene's tests, respectively. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD) and compared using Student's t-test. Data with non-normal distribution were reported the median (Me) and the quartiles Q1 and Q3 and analyzed using nonparametric Wald-Wolfowitz, Kolmogorov- Smirnov, and Mann-Whitney tests. Qualitative variables were summarized as counts and percentages. Categorical variables were compared by a two-sided Fisher and a two-sided Pearson χ2 test. Comparing numerical data showing normal distribution and equality of variance between 3 groups was performed with analysis of variance (ANOVA) with Bonferroni correction as a post hoc test. The critical level of significance when testing statistical hypotheses was considered at p <0.05. Relative risk (RR) was calculated with a 95% confidence interval (95% CI). Statistical analysis was performed using Statistica for Windows 10.0, Microsoft Excel 2018, MedCalс, and OpenEpi.
Results and discussion
The age of women in the study and comparison groups was 34 (30; 38) and 30 (27; 34) years, respectively. The age of expectant fathers was 34 (30.3; 40) and 33 (29; 35) years, respectively. We identified a relationship between early pregnancy loss in women with a history of threat- ened and recurrent miscarriage and a lack of regular employment (RR 1.62; 95% CI 1.17–2.22). Women in the study group reported shorter time between the onset of sexual activity and the first pregnancy (2.5 (2.5); 4.2 (3.1) years), as well as earlier age at first pregnancy (20.3 (2.6); 22.1 (3.4) years)ю They also registered earlier for antenatal care (6.6 (0.7); 7.3 (2.1) weeks) than women in the comparison group. The results are presented in Table 1.
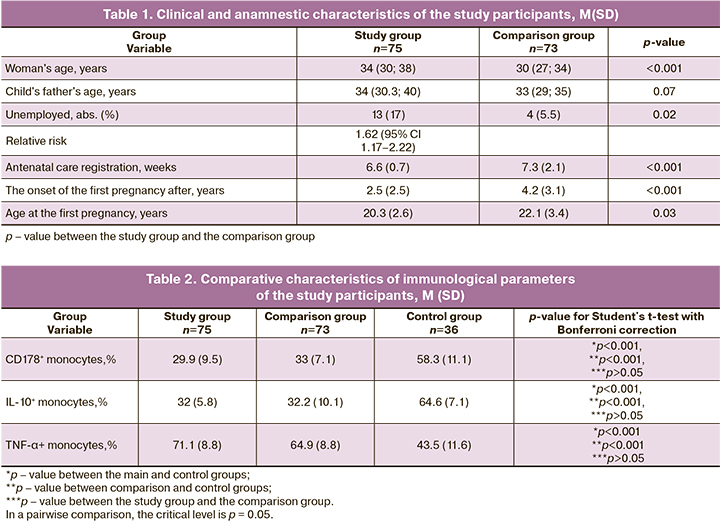
The immunological analysis showed that patients in both study and comparison groups had a decrease in the relative number of CD178 + monocytes and the level of IL-10 + monocytes against the background of an increase in the level of TNF-α + monocytes, compared with the control group. The results are shown in Table 2. In a pairwise comparison, the critical level is p = 0.05. Retrospectively, women who lost their pregnancies during the first trimester were divided into subgroup 1, including patients with early missed miscarriages and subgroup 2, including women with early spontaneous miscarriages.
The histopathology reports of patients with recurrent miscarriage showed higher rates of an abnormal chori- onic villi: differentiation (42.4% and 10%, p = 0.025), chromosomal disorders in the form of avascular chori- onic villi (74.3% and 0%, p <0.001), lymph-leukocytemacrophage inflammation (22.7% and 0%, p <0.001), edema of the endometrial stroma (48.5% and 0%, p <0.001), and foci of necrosis (22.7% and 0%, p = 0.005). In patients with spontaneous abortion, histopa- thology findings showed higher rates of focal sclerosis and stromal fibrosis (10.7% and 60%, p <0.001), delayed differentiation of fibroblast-like cells into pre-decidual cells (15.3% and 50%, p = 0.027), focal perivascular and peritubular lymphoplasmohistiocytic stromal infiltra- tion (7.7% and 40%, p = 0.016), and sclerosis of the spiral artery walls (12.3% and 50%, p = 0.013).
Therefore, among women with recurrent miscarriage, the gestational endometrium in the first trimester of pregnancy shows abnormal differentiation of chorionic villi with impairment of their vascularization. These changes constitute morphological confirmation of a possible chromosomal pathology impairing metabolic activity within the mother and the fetus that are neces- sary for the further development of the embryo. The cause of early reproductive losses in the form of spon- taneous miscarriage was chronic inflammation in the endometrium.
Recently, there has been growing interest in fetal- maternal immune interaction in patients with recurrent miscarriage and their relationship with the state of the endometrium in this pathology. Since early pregnancy loss and subsequent uterine curettage implies the devel- opment of chronic endometritis, a detailed analysis of the processes observed in the endometrium during inflammation suggests the presence of several factors that affect its condition during the fulfillment of repro- ductive function. For example, identification of patho- gens in the endometrium, an increase in the number of macrophages and granulocytes, morphological mark- ers of inflammation, the presence of plasma cells, an increase in the ratio of pro-and anti-inflammatory cyto- kines, impaired proliferation, apoptosis of fibroblast- like cells, a violation of angioarchitectonics with an increase in VEGF expression, an increase in endome- trial sclerosis [15, 18, 19] adversely affect the processes of implantation and continuation of a pregnancy.
Microorganisms, binding to toll-like receptors (TLRs), activate cells of the macrophage-monocytic series, which leads to the production of proinflamma- tory cytokines (interferons (IFN) α/β, TNF-α, IL-1, IL-6, IL-8) and to the development of inflammation to eliminate the pathogen [15, 16]. It has been established that in chronic endometritis, the levels of TNF-α, IL-1β, IL-6, IL-8, IL-10, IL-12α, transforming growth factor (TGF)-β1, TLR9, VEGF-A, and the leukemia inhibitory factor (LIF) increases. At the same time, in patients with chronic endometritis, combined with vascular wall sclerosis and endometrial stromal fibrosis, there is a significant increase in levels of several cyto- kines (TLR9, IL-6, IL-8, TNF-α, TGF-β1, IL-1β, IL-10) [16].
Proinflammatory cytokines play an essential role not only in endometritis, but also regulate many physi- ological processes including proliferation, implanta- tion, embryogenesis, but their excessive production causes microcirculation disorders, exudation and fibro- sis in the endometrium due to fibroblast proliferation, excessive synthesis of collagen and components of the extracellular matrix [20–24]. Vascular sclerosis and fibrosis of the endometrial stroma are considered one of the most significant markers of chronic endometritis, which leads to a cytokine storm [17]. Endometrial dys- function caused by inflammation is also accompanied by an impairment of the reparative mechanisms in the uterine cavity.
Pregnancy complications such as preterm labor and hypertensive disorders are known to be associated with an increase in the levels of pro-inflammatory cytokines (IFN-γ, TNF-α). In contrast, spontaneous abortions in the early stages of pregnancy are characterized by a decrease in the level of anti-inflammatory cytokines (IL-4, IL-10) [3, 5]. TNF-α is associated with the migration of leukocytes to the inflammation foci, caus- ing their degranulation and tissue damage. It also takes part in tissue regeneration, stimulates the formation of blood vessels and fibroblast proliferation, which, in turn, leads to the deposition of collagen and fibrosis in the placenta.
IL-10 is one of the critical anti-inflammatory cyto- kines. Several studies reported that pregnant women with hypertensive disorders had reduced expression of IL-10 by trophoblast villi with a simultaneous increase in TNF-α production [25].
In chronic endometritis, the differentiation of fibro- blast-like endometrial cells is impaired; their apoptosis increases. TNF-α, produced by macrophages, plays an essential role in this mechanism underlying chronic endometritis. Interaction with p55, one of the TNF-α receptors, results in activation of intracellular TRADD (TNFR1-associated death domain) proteins and their binding to CD95/Fas, inducing apoptosis. It is known that increased expression of CD178/FasL on macro- phages CD68+ and CD86+ in the decidua was observed in patients with spontaneous and recurrent miscarriage. FasL has been shown to mediate the induction of tro- phoblast apoptosis by macrophages in co-culture [26]. These results indicate that macrophage-induced FasL- mediated apoptosis may represent one of the causes of recurrent miscarriage.
The CD95/Fas-СD178/FasL system is one of the most important inducers of apoptosis, and changes in the production of one of these factors are sufficient to adversely affect the mechanisms of apoptosis in the trophoblast, disrupt the implantation, and impede the maintenance of pregnancy [27]. It has been established that apoptosis of endometrial stromal cells favorably affects the implantation of the ovum due to the interac- tion of CD95, expressed by trophoblast cells, and CD178 on monocytes and lymphocytes [26]. Also, mononucle- ar cells expressing CD178 stimulate apoptosis of smooth muscle and endothelial cells of spiral arteries expressing Fas. This ensures the invasion of extravillous trophoblast into the muscle layer, followed by the expansion of these vessels and the formation of adequate placental blood circulation, independent of the influence of vasocon- strictors [28, 29].
Therefore, the pathophysiology underlying endome- trial dysfunction in recurrent miscarriage coexisting with chronic endometritis, even with the elimination of the infectious agent, suggests the presence of an imbal- ance of pro- and anti-inflammatory cytokines, leading to a disruption in the formation of the fetal-placental unit, embryotoxicity, and apoptosis of invasive cytotro- phoblast.
Conclusion
The analysis of immunological parameters in women with a pregnancy loss and a history of recurrent miscarriage systemizes the data obtained during the study, eliminates some of the existing inconsistencies, and provides valuable insight into the role of factors playing a vital role in the formation of the fetal- placental unit. The study findings should be taken into consideration when planning individual antenatal care.
References
- Шумская Е.И., Якубовский Г.И., Кадыкаова А.И., Гвоздевская Т.О. Анализ цитогенетических факторов привычного невынашивания беременности в Рязанской области за период с 1986 по 2018 гг. Акушерство и гинекология. 2019; 4 (Приложение): 100-1. [Shumskaya E.I., Yakubovsky G.I.,Kadykaova A.I., Gvozdevskaya T.O. Analysis of cytogenetic factors of recurrent miscarriage in the Ryazan Region in the period from 1986 to 2018. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2019; 4 (Supplement): 100-1.(in Russian)].
- Фатеева Н.В., Перетятко Л.П. Морфо-функциональные критерии несостоятельности эндометрия при привычном невынашивании беременности на фоне хронического эндометрита. Медицина: теория и практика. 2019; 4 (Приложение): 564-5. [Fateeva N.V., Peretyatko L.P. Morphofunctional criteria for endometrial failure in recurrent miscarriage in the presence of chronic endometritis. Meditsina: Teoriya i Praktika (Medicine: Theory and Practice) 2019; 4 (S): 564-5. (in Russian)].
- Питиримова Л.Н., Загороднева Е.А., Гумилевский Б.Ю. Особенности аллельного полиморфизма генов интерлейкинов и цитокиновый баланс женщин с невынашиванием беременности. Акушерство и гинекология. 2014; 3: 33-8. [Pitirimova L.N., Zagorodneva E.A., Gumilevsky B.Yu. Features of allelic polymorphism of the interleukin genes and the cytokine balance of women with miscarriage. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2014; 3: 33-8. (in Russian)].
- Кометова В.В., Козырева Е.В., Давидян Л.Ю., Маланина Е.Н., Богдасаров А.Ю., Вознесенская Н.В. Особенности содержания плацентарного, тромбоцитарного и сосудистого эндотелиального факторов роста в сыворотке крови у женщин с бесплодием и невынашиванием беременности, ассоциированными с хроническим эндометритом. Акушерство и гинекология. 2017; 4: 74-80. [Kometova V.V., Kozyreva E.V., Davidyan L.Yu., Malanina E.N., Bogdasarov A.Yu., Voznesenskaya N.V. Features of the serum levels of placental, platelet-derived, and vascular endothelial growth factors in women with chronic endometritis-associated infertility and miscarriage. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2017; 4: 74-80. (in Russian)]. https://dx.doi.org/10.18565/0aig.2017.4.74-80.
- Сотникова Н.Ю., Малышкина А.И., Крошкина Н.В., Батрак Н.В. Особенности регуляции fas-зависимого апоптоза при привычном невынашивании беременности ранних сроков. Российский иммунологический журнал. 2017; 11(3): 510-2. [Sotnikova N.Yu., Malyshkina A.I., Kroshkina N.V., Batrak N.V. Features of the regulation of fas-dependent apoptosis in recurrent early miscarriage. Rossiyskiy Immunologicheskiy Zhurnal (Russian Immunological Journal). 2017; 11(3): 510-2. (in Russian)].
- Батрак Н.В., Малышкина А.И. Факторы риска привычного невынашивания беременности. Вестник Ивановской медицинской академии. 2016; 21(4): 37-41. [Batrak N.V., Malyshkina A.I. Risk factors for recurrent miscarriage. Vestnik Ivanoskoy Meditsinskoy Akademii (Bulletin of the Ivanovo Medical Academy). 2016; 21(4): 37-41. (in Russian)].
- Малышкина А.И., Назарова А.О., Жолобов Ю.Н., Батрак Н.В., Козырина А.А., Кулиева Е.Ю., Назаров С.Б. Социально-гигиеническая характеристика беременных, проживающих в центральной части европейской территории Российской Федерации. Российский вестник акушера-гинеколога. 2015; 15(5): 32-5. [Malyshkina A.I., Nazarova A.O., Zholobov Yu.N., Batrak N.V., Kozyrina A.A., Kulieva E.Yu. et al. Sociohygienic characteristics of pregnant women living in the central part of the European Russian Federation. Rossiyskiy Vestnik Akushera-Ginekologa (Russian Bulletin of the Obstetrician/Gynecologist). 2015; 15(5): 32-5. (in Russian)]. https://dx.doi.org/10.17116/rosakush201515432-35.
- Малышкина А.И., Назарова А.О., Батрак Н.В., Жолобов Ю.Н., Козырина А.А., Кулиева Е.Ю., Назаров С.Б. Особенности пищевого поведения беременных женщин. Российский вестник акушера-гинеколога. 2014; 14(3): 73-5. [Malyshkina A.I., Nazarova A.O., Batrak N.V., Zholobov Yu.N., Kozyrina A.A., Kulieva E.Yu. et al. Features of eating behavior in pregnant women. Rossiyskiy Vestnik Akushera-Ginekologa (Russian Bulletin of the Obstetrician/Gynecologist). 2014; 14(3): 73-5.(in Russian)].
- Малышкина А.И., Назарова А.О., Батрак Н.В., Жолобов Ю.Н., Козырина А.А., Кулиева Е.Ю., Назаров С.Б. Медико-социальная характеристика беременных женщин Иваново. Российский вестник акушера-гинеколога. 2014; 14(4): 9-12. [Malyshkina A.I., Nazarova A.O., Batrak N.V., Zholobov Yu.N., Kozyrina A.A., Kulieva E.Yu. et al. Sociomedical characteristics of Ivanovo pregnant women. Rossiyskiy Vestnik Akushera-Ginekologa (Russian Bulletin of the Obstetrician/Gynecologist). 2014; 14(4): 9-12. (in Russian)].
- Малышкина А.И., Назарова А.О., Батрак Н.В., Жолобов Ю.Н., Козырина А.А.,Кулиева Е.Ю., Назаров С.Б. Медико-социальная характеристика пациенток с привычным невынашиванием беременности. Российский вестник акушера-гинеколога. 2014; 14(6): 43-8. [Malyshkina A.I., Nazarova A.O., Batrak N.V., Zholobov Yu.N., Kozyrina A.A., Kulieva E.Yu. et al. Sociomedical characteristics of patients with miscarriage. Rossiyskiy Vestnik Akushera-Ginekologa (Russian Bulletin of the Obstetrician/Gynecologist). 2014; 14(6): 43-8. (in Russian)].
- Щеголев А.И., Серов В.Н. Клиническая значимость поражений плаценты. Акушерство и гинекология. 2019; 3: 54-62. [Shchegolev A.I., Serov V.N. The clinical significance of placental lesions. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2019; 3: 54-62. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.3.54-62.
- Батрак Н.В., Малышкина А.И., Сотникова Н.Ю., Крошкина Н.В. Клинико-иммунологические особенности беременных с привычным невынашиванием в анамнезе. Российский вестник акушера-гинеколога. 2015; 15(3): 35-9. [Batrak N.V., Malyshkina A.I., Sotnikova N.Yu., Kroshkina N.V. Clinical and immunological features of pregnant women with a history of recurrent miscarriage. Rossiyskiy Vestnik Akushera-Ginekologa (Russian Bulletin of the Obstetrician/Gynecologist). 2015; 15(3): 35-9. (in Russian)]. https://dx.doi.org/10.17116/rosakush201515335-39.
- Савельева Г.М., Аксененко В.А., Андреева М.Д., Базина М.И., Башмакова Н.В.,Боровкова Л.В. и др. Исходы второй половины беременности у пациенток с привычным выкидышем в анамнезе (результаты многоцентрового исследования ТРИСТАН-2). Акушерство и гинекология. 2018; 8: 111-21. [Savelyeva G.M., Aksenenko V.A., Andreeva M.D., Bazina M.I., Bashmakova N.V., Borovkova L.V. et al. Outcomes of the second half of pregnancy in patients with a history of recurrent miscarriage (results of the TRISTAN-2 multicenter trial). Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2018; 8: 111-21. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.8.111-121.
- Радзинский В.Е., Оразмурадов А.А., ред. Беременность ранних сроков. От прегравидарной подготовки к здоровой гестации. М.: Редакция журнала Status Praesens; 2018. 800 с. [Radzinsky V.E., Orazmuradova A.A., Ed. Early-stage pregnancy. From pregravid preparation to healthy gestation. M.: Redaktsiya Zhurnala Status Praesens (Editorial Office of the Status Praesens Journal); 2018. 800 p. (in Russian)].
- Базина М.И., Сыромятникова С.А., Егорова А.Т., Кириченко А.К., Хоржевский В.А. Прегестационная иммуноморфологическая оценка эндометрия и обоснование терапии у женщин с нарушением репродуктивной функции. Акушерство и гинекология. 2013; 10: 46-50. [Bazina M.I., Syromyatnikova S.A., Egorova A.T., Kirichenko A.K., Khorzhevsky V.A. Pregestational immunomorphological evaluation of the endometrium and a rationale for therapy in women with reproductive dysfunction. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2013; 10: 46-50.(in Russian)].
- Гомболевская Н.А., Бурменская О.В., Демура Т.А., Марченко Л.А., Коган Е.А., Трофимов Д.Ю., Сухих Г.Т. Оценка экспрессии мРНК генов цитокинов в эндометрии при хроническом эндометрите. Акушерство и гинекология. 2013; 11: 35-40. [Gombolevskaya N.A., Burmenskaya O.V., Demura T.A., Marchenko L.A., Kogan E.A., Trofimov D.Yu. et al. Evaluation of mRNA expression of cytokine genes in the endometrium in chronic endometritis. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2013; 11: 35-40.(in Russian)].
- Милованов А.П. Эмбриохориальная недостаточность: анатомо-физиологические предпосылки, обоснование, дефиниции и патогенетические механизмы. Архив патологии. 2014; 76(3): 4-8. [Milovanov A.P. Embryochorial insufficiency: anatomo-physiological prerequisites, a rationale, definitions and pathogenetic mechanisms. Arkhiv Patologii (Archives of Pathology). 2014; 76(3): 4-8. (in Russian)].
- Сухих Г.Т., Шуршалина А.В. Хронический эндометрит. Руководство. М.: ГЭОТАР-Медиа; 2010. 64 с. [Sukhikh G.T., Shurshalina A.V. Chronic endometritis: A guide. Moscow: GEOTAR-Media; 2010. 64 p. (in Russian)].
- Kitaya K., Yasuo T. Inter-observer and intra-observer variability in immunohistochemical detection of endometrial stromal plasmacytes in chronic endometritis. Exp. Ther. Med. 2013; 5(2): 485-8.
- Коган Е.А., Гомболевская Н.А., Демура Т.А., Марченко Л.А., Бурменская О.В., Файзуллина Н.М., Муравьева В.В. Роль toll-like рецепторов 2, 4, 9-го типов в патогенезе хронического эндометрита. Акушерство и гинекология. 2015; 12: 81-8. [Kogan E.A., Gombolevskaya N.A., Demura T.A., Marchenko L.A., Burmenskaya O.V., Faizullina N.M. et al. The role of toll-like receptors 2, 4, and 9 in the pathogenesis of chronic endometritis. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2015; 12: 81-8. (in Russian)].
- Хириева П.М., Мартынов С.А., Ежова Л.С., Адамян Л.В. Клинико-морфологические особенности эндометрия при внутриматочных синехиях: оценка экспрессии эстрогеновых и прогестероновых рецепторов. Акушерство и гинекология. 2018; 9: 48-54. [Khirieva P.M., Martynov S.A., Ezhova L.S., Adamyan L.V. Clinical and morphological features of the endometrium in intrauterine synechiae: assessment of the expression of estrogen and progesterone receptors. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2018; 9: 48-54. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.9.48-54.
- Кузнецова И.В., Землина Н.С., Рашидов Т.Н. Хронический эндометрит как исход инфекционного воспалительного заболевания матки. Гинекология. 2016; 18(2): 44-50. [Kuznetsova I.V., Zemlina N.S., Rashidov T.N. Chronic endometritis as the outcome of uterine infectious and inflammatory disease. Ginekologiya (Gynecology). 2016; 18(2): 44-50. (in Russian)].
- Маринкин И.О., Трунченко Н.В., Волчек А.В., Агеева Т.А., Никитенко Е.В., Макаров К.Ю., Кулешов В.М., Омигов В.В., Айдагулова С.В. Маркеры воспаления в нормальном и тонком эндометрии при хроническом эндометрите. Акушерство и гинекология. 2018; 2: 65-73. [Marinkin I.O., Trunchenko N.V., Volchek A.V., Ageeva T.A., Nikitenko E.V., Makarov K.Yu. et al. Inflammatory markers in the normal and thin endometrium in chronic endometritis. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2018; 2: 65-73. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.2.65-73.
- Доброхотова Ю.Э., Ганковская Л.В., Боровкова Е.И., Зайдиева З.С., Скальная В.С. Модулирование локальной экспрессии факторов врожденного иммунитета у пациенток с хроническим эндометритом и бесплодием. Акушерство и гинекология. 2019; 5: 125-32. [Dobrokhotova Yu.E., Gankovskaya L.V., Borovkova E.I., Zaidieva Z.S., Skalnaya V.S. Modulation of the local expression of innate immunity factors in patients with chronic endometritis and infertility. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2019; 5: 125-32. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.5.125-132.
- Низяева Н.В., Амирасланов Э.Ю., Ломова Н.А., Павлович С.В., Савельева Н.А., Наговицына М.Н., Сухачёва Т.В., Серов Р.А., Щеголев А.И., Kан Н.Е. Ультраструктурные и иммуногистохимические особенности плаценты при преэклампсии в сочетании с задержкой роста плода. Акушерство и гинекология. 2019; 11: 97-106. [Nizyaeva N.V.,Amiraslanov E.Yu., Lomova N.A., Pavlovich S.V., Savelyeva N.A., Nagovitsyna M.N. et al. Ultrastructural and immunohistochemical features of the placenta in preeclampsia concurrent with fetal growth restriction. Akusherstvo i Ginekologiya (Obstetrics and Gynecology). 2019; 11: 97-106. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.11.97-106.
- Ding J., Yin T., Yan N., Cheng Y., Yang J. FasL on decidual macrophages mediates trophoblast apoptosis: a potential cause of recurrent miscarriage. Int. J. Mol. Med. 2019; 43(6): 2376-86. https://dx.doi.org/10.3892/ijmm.2019.4146.
- Banzato P.C.A., Daher S., Traina E., Torloni M.R., GueuvoghlanianSilva B.Ya., Puccini R.F. et al. Fas and Fas-L genotype and expression in patients with recurrent pregnancy loss. Reprod. Sci. 2013; 20(9): 1111-5.https://dx.doi.org/10.1177/1933719113477488.
- Tao H., Liu X., Liu X., Liu W., Wu D., Wang R. et al. LncRNA MEG3 inhibits trophoblast invasion and trophoblast-mediated VSMC loss in uterine spiral artery remodeling. Mol. Reprod. Dev. 2019; 86(6): 686-95. https://dx.doi.org/10.1002/mrd.23147.
- Salomon C., Yee S., Scholz-Romero K., Kobayashi M., Vaswani K., Kvaskoff D. et al. Extravillous trophoblast cells-derived exosomes promote vascular smooth muscle cell migration. Front. Pharmacol. 2014; 5: 175. https://dx.doi.org/10.3389/fphar.2014.00175.
Received 18.02.2020
Accepted 09.06.2020
About the Authors
Nataliya V. Batrak, Ph.D., Associate Professor at the Department of Obstetrics and Gynecology and Medical Genetics, Ivanovo State Medical Academy.Tel.: +7(962)160-01-33. E-mail: batrakn@inbox.ru. https://orcid.org/0000-0002-5230-9961. 8 Sheremetevsky str., Ivanovo, 153012, Russian Federation.
Anna I. Malyshkina, Dr.Med.Sci., Professor, Director of the V.N. Gorodkov Research Institute of Maternity and Childhood of Minzdrav of Russia, Head of Department of Obstetrics and Gynecology and Medical Genetics, Ivanovo State Medical Academy. E-mail: ivniimid@inbox.ru.
20 Pobedy str., Ivanovo, 153045, Russian Federation; 8 Sheremetevsky str., Ivanovo, 153012, Russian Federation.
Natalya Yu. Sotnikova, Dr.Med.Sci., Professor, Merited Doctor of the Russian Federation, Head of the Laboratory of Clinical Immunology, V.N. Gorodkov Research Institute of Maternity and Childhood of Minzdrav of Russia, Department of Pathophysiology and Immunology, Ivanovo State Medical Academy. E-mail: ivniimid@inbox.ru.
20 Pobedy str., Ivanovo, 153045, Russian Federation; 8 Sheremetevsky str., Ivanovo, 153012, Russian Federation.
Natalya V. Kroshkina, Ph.D. (bio.sci.), Researcher at the V.N. Gorodkov Research Institute of Maternity and Childhood of Minzdrav of Russia.
Tel.: +7(980)693-18-22. E-mail: ivniimid@inbox.ru. 20 Pobedy str., Ivanovo, 153045, Russian Federation.
Lyubov P. Peretyatko, Dr.Med.Sci., Professor, Merited Doctor of the Russian Federation, Head of the Laboratory of Pathomorphology and Electron Microscopy,
V.N. Gorodkov Research Institute of Maternity and Childhood of Minzdrav of Russia. E-mail: ivniimid@inbox.ru. 20 Pobedy str., Ivanovo, 153045, Russian Federation.
Natalia V. Fateeva, Junior Researcher at the V.N. Gorodkov Research Institute of Maternity and Childhood of Minzdrav of Russia. E-mail: ivniimid@inbox.ru.
20 Pobedy str., Ivanovo, 153045, Russian Federation.
For citation: Batrak N.V., Malyshkina A.I., Sotnikova N.Yu., Kroshkina N.V., Peretyatko L.P., Fateeva N.V. Medical and social factors and pathogenetic mechanisms of early pregnancy loss in women with recurrent miscarriage.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2020; 7: 79-86 (in Russian)
https://dx.doi.org/10.18565/aig.2020.7.79-86



