Evaluating the accuracy of prenatal ultrasound diagnosis of heart defects in seven steps
Objective. To evaluate the accuracy of prenatal diagnosis of heart defects on the basis of comparison of pre- and postnatal diagnoses.Samsonova О.А., Malmberg О.L., Shekhovtsov D.B., Gaponenko Е.А., Klykova О.N., Tsiskarishvili Т.М.
Materials and methods. A total of 450 fetuses with heart defects were examined in seven steps at the Clinical Hospital of Mother and Child group of companies in Moscow, from January to December 2018. The fetuses were examined by Voluson E6, E8, and E10 ultrasound machines transabdominally and transvaginally using multi-frequency convex and intracavitary transducers. Pre- and postnatal diagnoses of heart defects were compared according to echocardiography (EchoCG) data.
Results. The study included 429 cases with confirmed diagnoses. Isolated ventricular septal defect (VSD) was diagnosed in 297 fetuses. Prenatal diagnosis of VSD was confirmed in 84% of cases postnatally. The rest of the heart defects were detected in 132 fetuses. The comparison of pre- and postnatal EchoCG data showed the highest percentage of coincidence in the diagnosis of 14 diseases and reached 100%. The most frequent pathologies were common atrioventricular canal (CAVC), double-outlet right/left ventricle with malposition of great arteries (DORV/DOLV with MGA), and hypoplastic left/right heart syndrome (HLHS/HRHS). The highest percentage (60%) of difference in diagnosis was observed in diagnosing Fallot’s tetrad (FT) and aortic coarctation (AC).
Conclusion. Prenatal evaluation of heart defects in 7 steps can help to achieve high accuracy in diagnosing and combine pre- and postnatal approaches to diagnosis formulation.
Keywords
The rate of heart defects detected during routine screening has recently increased from 45–48% to 77–95% [1–3]. One of the main criteria for the quality of diagnosis of heart defects is accuracy [4–7]. Prenatal detection is about 70% in the case of transposition of the great vessels, it is about 68% in Fallot’s tetrad (FT), and about 43% in aortic coarctation (AC) [8–10]. There was a difference in pre- and postnatal diagnoses of heart defects requiringurgentcare[3, 4]. Therefore, modernultrasound diagnostics should aim at increasing the accuracy of pre- and postnatal diagnosis of heart defects [5–7].
Methods and Materials
A total of 450 fetuses with heart defects were examined in seven steps at the Clinical Hospital of Mother and Child group of companies in Moscow, from January to December 2018. The study was performed using Voluson E6, E8, and E10 ultrasound machines transabdominally and transvaginally with 4D multi-frequency convex transducer 2–8 MHz, 4D multi-frequency intracavity transducer 4–9 MHz. We applied the technique called
«Seven Steps» [11], which is based on the segmental approach proposed by Van Pragh in 1960. It consists in a step-by-step segment assessment of the heart anatomy from the atria to the ventricles, from the ventricles to the outflow tracts, as well as the assessment of the atrioventricular and ventriculoarterial connections.
The description of the technique
Step 1. Determining situs. The abbreviation HASL (Heart Aorta Stomach Left) was proposed, which reflects the normal position of internal organs, situs solitus.
Step 2. The assessment of atrial anatomy and identification of the right and left atria, junction of the superior and inferior vena cava, pulmonary veins, and their number. The assessment of the primary atrial septum and the oval window.
Step 3. Determining the type of atrioventricular connection and valve patency.
Step 4. The assessment of ventricular morphology and the integrity of the interventricular septum.
Step 5. The visualization of the left ventricle outflow tract and the ascending aorta. The visualization of the right ventricle outflow tract, assessment of pulmonary valve patency and bifurcation of the pulmonary artery.
Step 6. The visualization of the section through the aortic arch, the assessment of the brachiocephalic vessels, and the assessment of the section through the ductus arteriosus.
Step 7. Section through three vessels and trachea.
Pre- and postnatal diagnoses of heart defects were compared. If prenatal diagnoses were not identical to postnatal ones, anomalies of particular anatomical structures of the heart were evaluated.
Results
The study included the findings of 429 patients whose were diagnosed with heart defect. Heart defect was confirmed in 429 cases (100%). Prenatal diagnoses were compared in 420 fetuses with the data of postnatal echocardiography and in 9 fetuses with the results of anatomic pathology report.
Isolated ventricular septal defect (VSD) was diagnosed in 297 patients with prenatally diagnosed heart defect out of 429 cases (Table 1). Fetuses whose ventricles of the heart functioned as one ventricle due to the severe VSD were referred to the group of patients with a functionally single ventricle. In our study, the defect in all these fetuses was more than 50% of the area of the ventricular septum.
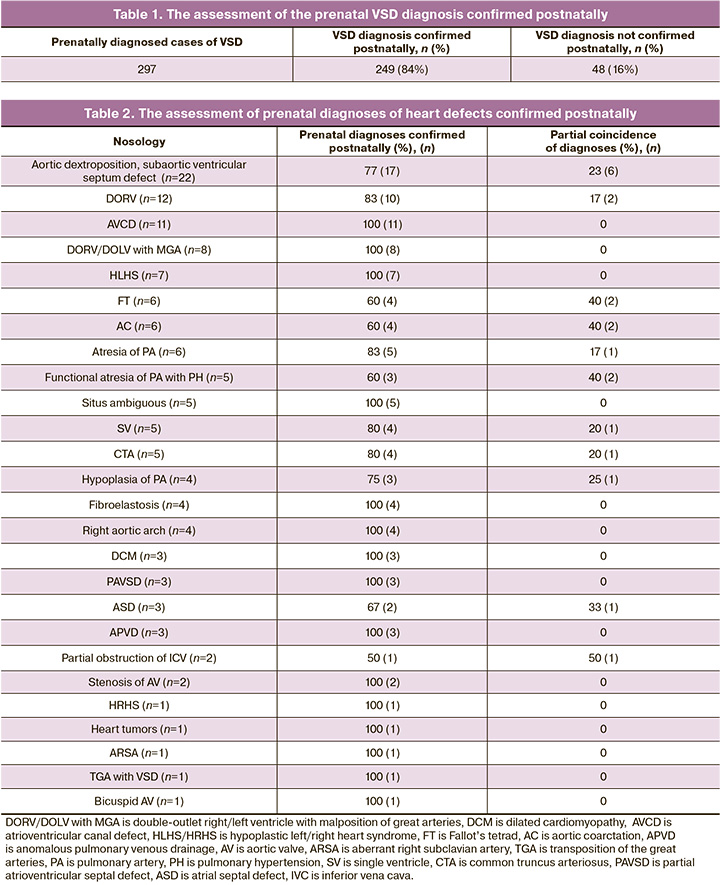
The rest heart defects were diagnosed prenatally in 132 fetuses. Heart defects were confirmed by postnatal echocardiography or by anatomic pathology report. Different descriptions of the diagnosis or different nosology of heart defects in case of their presence were regarded as partially identical diagnoses (Table 2).
The examined patients had a coincidence rate of pre- and postnatal diagnoses on average of 83.9%.
Discussion
The analysis of a group of patients with VSD demonstrated a large percentage of false positive results (16%) which may be due to spontaneous prenatal VSD correction and technical difficulties related to the specific position of the fetus. However, false positive diagnosis of isolated VSD does not cause a change in tactics.
In the group of patients with aortic dextroposition and subaortic VSD, a partial coincidence of results in 23% of cases might be associated with postnatal changes in hemodynamics and aortic displacement towards the right ventricle. At the same time, the final diagnosis was a double-outlet right ventricle. In 17% of cases, postnatal hemodynamic changes were associated with partial coincidence of diagnoses of double-outlet right ventricle, which were interpreted as aortic dextroposition and subaortic VSD by postnatal echocardiography. The analysis of these data and the difficulty in predicting aortic displacement with changes in hemodynamics allow us to consider these diagnoses as hemodynamically similar and provide a descriptive picture in prenatal echocardiography protocols. According to the literature, the coincidence of prenatal and postnatal diagnoses in these abnormalities reaches 92% if the hemodynamic similarity of the data on heart defects is considered [10].
Ahighpercentageofpartialcoincidenceof FTdiagnoses (40%) was associated with insufficient attention to the assessment of the intraventricular septum, which forms an obstruction of the right ventricular outflow tract, and results in the wrong FT diagnosis in a group of patients with valve stenosis and hypoplasia of the pulmonary artery trunk. According to the data in the literature, the coincidence of pre- and postnatal diagnoses in this abnormality is about 70% [12].
The prenatal AC diagnosis also causes difficulties. It is associated with the fact that some patients have manifestations of AC on the first day after birth due to the specific localization of the ductal endothelium, and heart defects can only be diagnosed postnatally in these patients. Our data are consistent with the literature data and constitute 60% and 53.8%, respectively [5].
In the group of patients with atresia of the pulmonary artery, a partial coincidence of pre- and postnatal echocardiography diagnoses was found in 17% of cases and was associated with the difficulty of visualizing the hypoplastic pulmonary artery. Diagnosis of common truncus arteriosus partially coincided in 25% of cases, which can be explained by the difficulties of differential diagnosis of various types of pulmonary atresia. At the same time, similar problems are noted in the postnatal diagnosis of these defects. In this case, the use of volumetric technologies to improve the accuracy of diagnostics may be promising.
Functional pulmonary atresia and pulmonary hypertension with total tricuspid regurgitation, reduced right ventricular contractile function, and lack of blood flow through the pulmonary valve were considered as pulmonary atresia in 40% of cases prenatally. It is associated with the difficulty in the visualization of the pulmonary artery flaps; therefore, fetal hemodynamics needs comprehensive assessment. Criteria for diagnosing pulmonary hypertension in the fetus differ from those in the newborn due to the presence of duplicate circulatory circles and blood shunting along the path of least resistance. When the pressure in the pulmonary artery increases, the final diastolic pressure rises in the right ventricle; as a result, a smaller volume of blood from the right atrium enters the right ventricle and part of the blood is shunted through the oval window to the left atrium. In this regard, it is impossible to assess the pressure in the pulmonary artery using the velocity characteristics of the flow on the tricuspid and pulmonary valves, and consequently one cannot estimate the degree of pulmonary hypertension accepted in postnatal practice. Our studies showed that the diastolic pressure in the pulmonary artery exceeded the systolic pressure in the right ventricle, and due to this, there was no blood flow through the pulmonary artery valve, which caused a partial coincidence of the results in 40% of cases. Identical diagnoses in patients of this group were achieved in 60% of cases owing to a comprehensive approach which included assessment of pulmonary valve cusps, ratio of heart chambers, right ventricle function, dynamic monitoring of the fetuses, and assessment of lung echogenicity. The diagnosis of functional pulmonary atresia with pulmonary hypertension does not exclude the diagnosis of pulmonary atresia and therefore it is necessary to perform echocardiography of the newborn’s heart in the first hours after birth.
Partial coincidence of the results in diagnosing the single ventricle (20%) was associated with an undervaluation of VSD size. We believe that it is most appropriate to provide a description of the presence of severe VSD in the second and early third trimesters, and the final diagnosis should be made at the end of the third trimester. In our opinion, this will increase the percentage of coincidence of pre- and postnatal diagnoses.
False positive diagnosis of partial narrowing of the inferior vena cava in 50% of cases does not allow us to speak about the reliability of prenatal diagnosis of this defect.
Difficulties in prenatal diagnosis of an isolated atrial septal defect are associated with the variability in the size of the functioning oval window.
The coincidence rate of prenatal and postnatal diagnoses of heart defects in 7 steps in our study with the results of echocardiography according to the literature is 83.9% and 82.1%, respectively [6].
Conclusion
Prenatal evaluation of heart defects in 7 steps can help to achieve high accuracy in diagnosing heart defects, when combining pre- and postnatal approaches and modifying postnatal care.
References
- Hoffman J.I.E., Kaplan S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002; 39(12): 1890-900. https://dx.doi.org/10.1016/s0735-1097(02)01886-7.
- Achiron R., Glaser J., Gelernter I., Hegesh J., Yagel S. Extended fetal echocardiographic examination for detecting cardiac malformations in low risk pregnancies. BMJ. 1992; 304(6828): 671-4. https://dx.doi.org/ 10.1136/bmj.304.6828.671.
- Xie D., Fang J., Liu Z., Wang H., Yang T., Sun Z. et al. Epidemiology and major subtypes of congenital heart defects in Hunan Province, China. Medicine (Baltimore). 2018; 97(31): e11770. https://dx.doi.org/ 10.1097/MD.0000000000011770.
- Khoshnood B., Lelong N., Houyel L., Bonnet D., Ballon M., Jouannic J.M. et al.; EPICARD Study group. Impact of prenatal diagnosis on survival of newborns with four congenital heart defects: a prospective, population-based cohort study in France (the EPICARD Study). BMJ Open. 2017; 7(11): e018285. https://dx.doi.org/10.1136/ bmjopen-2017-018285.
- Clur S.A., Van Brussel P.M., Ottenkamp J., Bilardo C.M. Prenatal diagnosis of cardiac defects: accuracy and benefit. Prenat. Diagn. 2012; 32(5): 450-5. https://dx.doi.org/10.1002/pd.3837.
- van Velzen C.L., Clur S.A. , Rijlaarsdam M.E.B., Pajkrt E., Bax C.J., Hruda J. et al. Prenatal diagnosis of congenital heart defects: accuracy and discrepancies in a multicenter cohort. Ultrasound Obstet. Gynecol. 2016; 47(5): 616-22. https://dx.doi.org/10.1002/uog.15742.
- Marek J., Tomek V., Skovránek J., Povysilová V., Samánek M. Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21-year experience. Heart. 2011; 97(2): 124-30. https://dx.doi.org/ 10.1136/hrt.2010.206623.
- Liu Y., Chen S., Zuhlke L., Black G.C., Choy M.K., Li N. et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019; 48(2): 455-63. https://dx.doi.org/10.1093/ije/dyz009.
- Bakker M.K., Bergman J.E.H., Krikov S., Amar E., Cocchi G., Cragan J. et al. Prenatal diagnosis and prevalence of critical congenital heart defects. BMJ Open. 2019; 9(7): e028139. https://dx.doi.org/10.1136/ bmjopen-2018-028139.
- Gottschalk I., Abel J.S., Menzel T., Herberg U., Breuer J., Gembruch U. et al. Prenatal diagnosis, associated findings and postnatal outcome of fetuses with double outlet right ventricle (DORV) in a single center. J. Perinat. Med. 2019; 47(3): 354-64. https://dx.doi.org/10.1515/jpm-2018-0316.
- Samsonova O., Shekhovtsov D., Malmberg O., Gaponenko E., Klikova O., Tsiskarishvili T. EP10.01: Standard versus segmental hemodynamic approach in prenatal ultrasound diagnostic of heart disease. Ultrasound Obstet. Gynecol. 2019; 54(Suppl. 1: Abstracts of the 29th World Congress on Ultrasound in Obstetrics and Gynecology, 12-16 October 2019, Berlin, Germany.): 291. https://dx.doi.org/10.1002/uog.21301.
- Zhao Y., Edington S., Fleenor J., Sinkovskaya E., Porche L., Abuhamad A. Fetal cardiac axis in tetralogy of Fallot: associations with prenatal findings, genetic anomalies and postnatal outcome. Ultrasound Obstet. Gynecol. 2017; 50(1): 58-62. https://dx.doi.org/10.1002/uog.15998.
Received 11.06.2020
Accepted 30.07.2020
About the Authors
Olga A. Samsonova, PhD, Professor assistant of the Department of ultrasound in the RMANPO, member of the ISUOG-international society of ultrasound doctors in obstetrics and gynecology, ultrasound specialist MD GROUP Clinical hospital. Tel.: +7(915)202-11-73. E-mail: usfox79@gmail.com.117209, Russia, Moscow, Sevastopol Ave., 24, build 1.
Olga L. Malmberg, M.D., PhD, associate Professor of the Department of ultrasound in the RMAPO, member of the ISUOG-international society of ultrasound doctors in obstetrics and gynecology, Head of the ultrasound diagnostics Group of Companies “Mother and Child” MD GROUP Clinical hospital. Tel.: +7(916)512-82-91. E-mail: malmberg.olga@gmail.com. 117209, Russia, Moscow, Sevastopol Ave., 24, build 1.
Dmitry B. Shekhovtsov, ultrasound specialist, Family Planning and Reproduction Center. Tel.: +7(495)718-20-88. 117209, Russia, Moscow, Sevastopol Ave., 24A.
Ekaterina A. Gaponenko, ultrasound specialist, MD GROUP Clinical hospital. Tel.: +7(495)332-80-12. 117209, Russia, Moscow, Sevastopol Ave., 24, build 1.
Oxana N. Klikova, ultrasound specialist, MD GROUP Clinical hospital. Tel.: +7(495)332-80-12. 117209, Russia, Moscow, Sevastopol Ave., 24, build 1.
Tarhan M. Tsiskarishvili, ultrasound specialist, Family Planning and Reproduction Center. Tel.: +7(495)718-20-88. 117209, Russia, Moscow, Sevastopol Ave., 24A.
For citation: Samsonova O.A., Malmberg O.L., Shekhovtsov D.B., Gaponenko E.A., Klykova O.N., Tsiskarishvili T.M. Evaluating the accuracy of prenatal ultrasound diagnosis of heart defects in seven steps.
Akusherstvo i Ginekologiya / Obstetrics and gynecology. 2020; 10: 94-98 (in Russian)
https://dx.doi.org/10.18565/aig.2020.10.94-98



