Quality of life in patients with premature ovarian insufficiency treated with hormone replacement therapy
Objective: To compare the quality of life (QoL) between patients with premature ovarian insufficiency (POI) treated with hormone replacement therapy (HRT) and women with preserved ovarian function. Materials and methods: This cross-sectional study of 374 patients was conducted at V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia. Group 1 included 223 patients with POI who received HRT, and group 2 included 151 patients with preserved ovarian function. Quality of life was assessed using the Health Status Survey (SF-36), which was validated for use in Russia. Results: Patients in group 1 had statistically significant differences in all eight domains of the SF-36 questionnaire compared with those in group 2 (p<0.0007, p<0.0001). The lowest scores in group 1 were on the VT (Vitality) scale at 43.3 (18.3), GH (General Health) at 40.1 (18.0), and RP (Role Physical) at 46.6 (34.8). POI was associated with an 18% (1.21 times) decrease in the mean score of the integral measures of the physical and mental health components of the patients compared to the control group. Patients in group 1 were 3.06 times less likely (95% CI 2.39–3.92) to have a score above the normal 50-point threshold for the physical component of health, and 4.43 times less likely (95% CI 2.96–6.63) to have a score above the mental component of health than those in group 2. The QoL of POI patients was directly related to the estradiol (E2) dose in HRT; an increase in the E2 dose was associated with higher scores on most SF-36 measures. Conclusion: POI has long-term adverse effects on all components of health and reduces QoL. HRT with standard doses of E2 in POI patients often fails to provide a QoL comparable to that of women of the same age with preserved ovarian function. Prescribing adequate doses of HRT, using an individualized approach and a multidisciplinary team to manage patients with POI, can significantly improve quality of life and outcomes. Authors' contributions: Yureneva S.V., Averkova V.G. – conception and design of the study; Averkova V.G., Yureneva S.V. – data collection and analysis, manuscript drafting; Averkova V.G. – statistical analysis; Yureneva S.V. – manuscript editing. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P. Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Averkova V.G., Yureneva S.V. Quality of life in patients with premature ovarian insufficiency treated with hormone replacement therapy. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (5): 50-58 (in Russian) https://dx.doi.org/10.18565/aig.2023.16Averkova V.G., Yureneva S.V.
Keywords
Spontaneous premature ovarian insufficiency (POI) is defined as the loss of normal ovarian function before the age of 40. POI affects approximately 1% of women worldwide and occurs in approximately 1 in 1,000 women aged under 30 years and 1 in 10,000 women aged under 20 years [1].
One of the earliest manifestations of POI is the loss of fertility and associated psycho-emotional disturbances, including stress, depression, and low self-esteem [2]. Patients with POI also experience a wide range of symptoms associated with estrogen deficiency (hot flashes, increased sweating, fatigue, anxiety, etc.), have sexual problems and may present with age-associated disease much earlier than their peers [2, 3]. In the absence of effective strategies to overcome infertility and restore ovarian function, estrogen replacement, prevention of age-related disease, and ensuring a high quality of life (QoL), mainly through long-term hormone replacement therapy (HRT) with sex steroids, are at the forefront of management strategies for women with POI [3, 4].
QoL is a complex characteristic of a person's physical, psychological, and social functioning, and is based on subjective perception [5]. In medical terms, QoL is always related to health, and in biomedical research, the most commonly used concept is "health-related quality of life,” which makes it possible to determine the impact of a particular disease and the effectiveness of ongoing treatment on the components of this indicator.
In recent years, patients’ QoL has received particular attention in the selection of therapeutic strategies. According to the UK National Institute for Health and Care Excellence (NICE), treatment interventions should aim to improve QoL [6]. This concept is of particular social and medical relevance in the treatment of young patients with POI, who need to remain active in family, social, and professional life.
To date, there has been a paucity of experience in the literature regarding the quality of life in patients with POI. In 2020. Li X.T. et al. published the first systematic review of 34 studies and a meta-analysis of 6 studies investigating health-related QoL in this cohort of patients [7]. Evidence suggests that POI has a negative impact on all components of QoL, including physical, psychological, and general health. Most of these studies recruited participants who either received or did not receive HRT, or did not emphasize treatment. As HRT for POI is designed to offset the effects of estrogen deficiency and provide a high QoL comparable to healthy female peers, this study aimed to analyze the QoL of POI patients receiving HRT and to compare it to the QoL of women of the same age with preserved ovarian function.
Materials and methods
This study was conducted at the V.I. Kulakov NMRC for OG&P to investigate the QoL of POI patients and healthy women with preserved ovarian function. This work is part of an analysis of treatment satisfaction and QoL in patients with POI; a detailed description of the study materials and methods has been previously published [8]. Briefly, the study included 165 patients with POI after a telephone interview (the data were taken from the medical database of the V.I. Kulakov NMRC for OG&P) and 60 patients with POI after a visit to a physician at the Gynecological Endocrinology Department of the Center. The control group for this phase of the study was comprised of 151 patients of comparable age with preserved ovarian function. The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P. All participants provided signed informed consent to participate in this study.
Inclusion criteria for patients with POI were age 18–45 years, diagnosis of POI at least 12 months ago, HRT at the time of the study, and for at least 3 months. The diagnostic criteria for POI were in accordance with those of the European Society of Human Reproduction and Embryology [1]. The inclusion criteria for the POI group were karyotype changes, iatrogenic POI, severe somatic pathology, thyroid and adrenal dysfunction, and contraindications for hormonal therapy. The control group included women aged 18–45 years with preserved ovarian functions. Patients with severe somatic pathology, thyroid disease with impaired thyroid function, taking combined oral contraceptives, and other hormonal drugs were not included in the control group.
QoL was assessed using the SF-36 (SF-36 Health Status Survey), which is widely used in international and domestic studies [9]. The Russian version of the SF-36 questionnaire was validated by St. Petersburg International QoL Research Centre [10]. The SF-36 reflects, in addition to a person's general well-being, his or her satisfaction with various aspects of life that are influenced by their health status. The SF-36 measures eight scales: physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). All scales form two distinct concepts measured by the SF-36: a physical dimension, represented by the Physical Component Summary (PCS), and a mental dimension, represented by the Mental Component Summary (MCS). Scores on each scale range from 0 to 100, with a higher score corresponding to a higher QoL score.
The HRT drugs administered to the study participants differed in the doses of the components and routes of administration. A detailed description of the type and route of administration has been previously provided [8]. The drugs administered to the study participants were divided according to the dose of the estrogenic component (E2), including HRT with low (1 mg E2 orally), standard (2 mg E2 orally, 1 g 0.1% gel transdermally, 1.5 mg 0.06% gel transdermally, 50 µg E2 daily transdermally), and high-dose estradiol (1.5 and 2.0 g 0.1% gel transdermally). Patients taking combined oral contraceptives were identified separately.
The study participants were asked to complete a questionnaire either by telephone, given in their medical records, or at a face-to-face appointment with their physician. Prior to counting, responses were recoded and summed according to the method presented by the authors of the questionnaire in the SF-36 v.2 manual [9] and the SF-36 questionnaire data-processing instructions (authored by Evidence).
Statistical analysis
Statistical analysis and data visualization were performed using the R 4.1.0 statistical computing environment (R Foundation for Statistical Computing, Vienna, Austria). The normality of the distribution was tested using the Kolmogorov–Smirnov test. Descriptive statistics included frequencies and proportions for categorical variables and the mean (standard deviation), median (Me), and quartiles (Q3; Q1) for continuous variables. Fisher's exact test was used to compare the categorical variables. For the comparison of continuous variables, the Mann–Whitney test (for two groups) and Kruskal–Wallis test (for more than two groups) with post-hoc analysis (Dunn's test for multiple comparisons) were used. Differences were considered statistically significant at p<0.05. Spearman's analysis was used in correlation analysis. The association was considered statistically significant at p<0.05.
Results
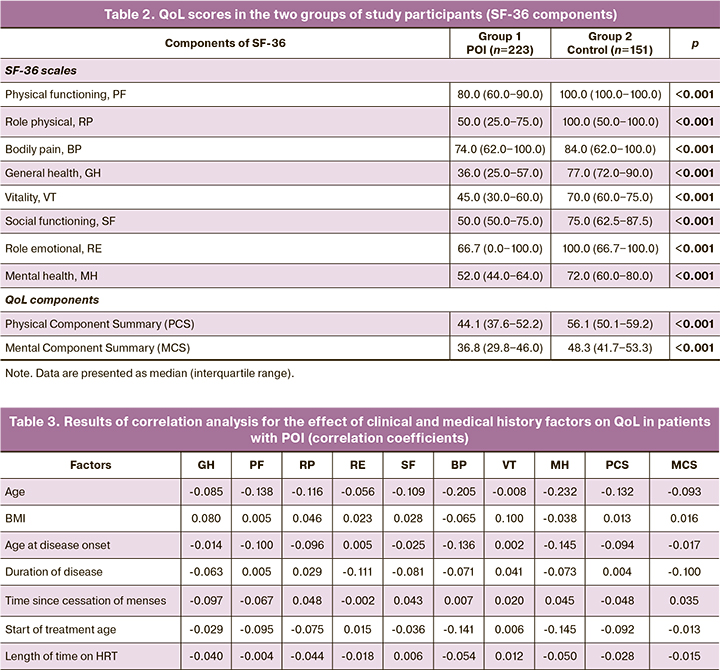
A total of 374 patients, 223 with POI (group 1) and 151 with normal ovarian function (group 2), were included in the study. The medical histories and anthropometric data of the study participants are presented in Table 1.
The age and body mass index (BMI) of the patients were comparable between the groups. Obstetric history also did not differ. The mean age at disease onset in patients with POI was 31.0 (6.7) years, the mean duration of disease at study inclusion was 5.2 (3.2) years, and the mean age at onset of HRT was 31.5 (6.5) years.
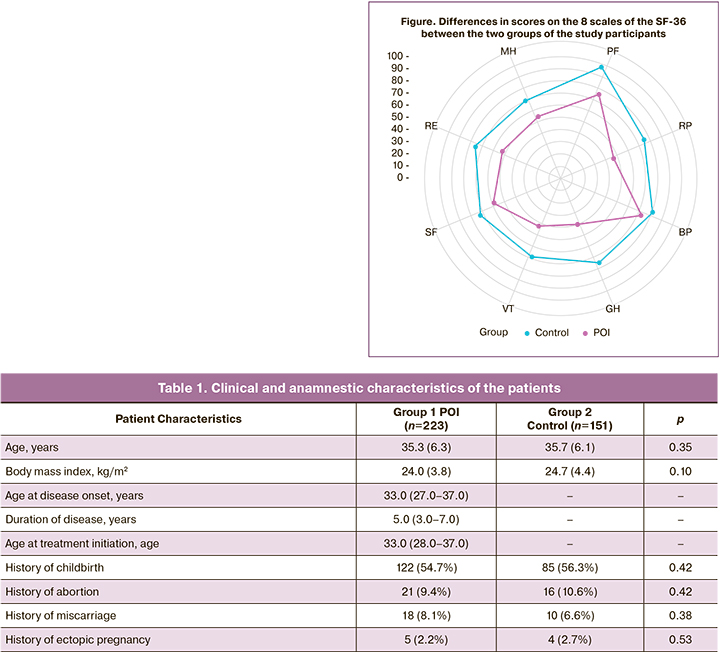
Table 2 and Figure show the scores on the eight scales of the SF-36 questionnaire in both study groups and the resulting integral measures.
The findings showed that women with POI had significant differences in all eight domains of the SF-36 questionnaire compared with the control group (p<0.001, p<0.001). The lowest scores in group 1 were on the VT (Vitality), GH (General Health), and RP (Role Physical) scales. Scores close to 50 in group 1 were MH (Mental Health) and RE (Role Emotional).
Based on the data (Table 2), the following characteristics were observed for patients with POI (group 1) compared to those without POI (group 2) (the data are shown as the difference in medians):
- 22% (1.28 times) decrease in role–physical score;
- 39% (1.64 times) decrease in physical functioning score;
- 10% (1.11-fold) decrease in bodily pain score
- 47% (1.89 times) decrease in general health score;
- 37% (1.59 times) decrease in vitality score;
- 19% (1.23 times) decrease in social functioning score;
- decrease in role-emotional by approximately 30% (1.43 times);
- decreased mental health by 23% (1.30 times) compared to the control group.
The following results were obtained when assessing the integral indicators in the two groups of study participants (Table 2). The mean scores for both physical and mental health components were significantly lower in group 1 than in group 2. Thus, POI (group 1) was associated with an 18% (1.21 times) decrease in the mean scores of the physical and mental health components compared to the control group (group 2). group 1 patients were also 3.06 times less likely (OR=3.06; 95% CI 2.39‒3.92) to have role-physical score above the normal 50-point threshold than group 2 patients. group 1 patients were 4.43 times less likely (OR=4.43; 95% CI 2.96‒6.63) to have a mental health score above the 50-point threshold than controls.
Correlation analysis was used to assess the effect of age, BMI, disease characteristics, and HRT duration on the QoL of patients with POI. There was an inverse correlation between age and pain intensity (r=-0.205, p=0.008) and mental health (r=-0.232, p=0.002). The QoL of patients with POI for these two (but not for the other six) components of the SF-36 questionnaire was significantly lower with increasing age. The QoL of patients with POI was not associated with BMI, age at disease onset and duration, or HRT duration (p>0.05) (Tables 3 and 4).
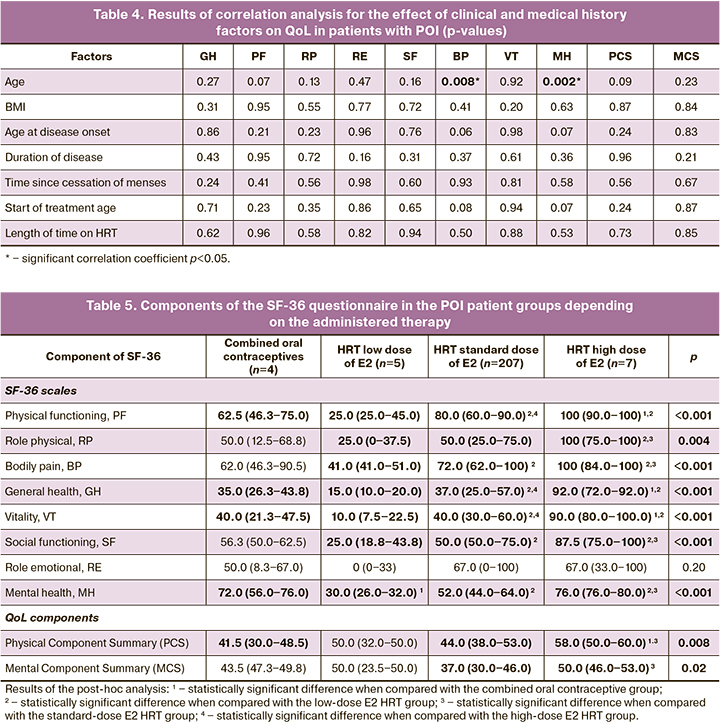
The final step of the study was to analyze the scores on the eight scales of the SF-36 questionnaire in patients with POI, depending on the dose of the estrogen component of HRT received at the time of the QoL assessment (Table 5). This analysis showed that the QoL of POI patients was directly related to the E2 HRT dose; an increase in the E2 dose resulted in an increase in most SF-36 scores. However, the QoL of patients on combined oral contraceptives was lower than that of patients on standard-dose HRT, and higher than that of patients on low-dose HRT for most indices. There was no statistically significant difference only in the role emotional (RE) measure, which may be due to the sample size and characteristics.
Discussion
Our results are largely consistent with those reported in a meta-analysis by Li X.T. et al. [7] and other previous studies investigating different aspects of QoL in patients with POI. In a study by Benetti-Pinto C.L. et al. involving 58 women with POI and 58 women with preserved ovarian function, the mean score in women with POI was 10 points lower on the physical health component and 5 points lower on the mental health component than in the control group. Patients with POI were also more than 2.5 times more likely to score below 70 on the physical and mental health component scales than their healthy female peers. Fifty-one (88%) female patients with POI in this study were taking HRT [11].
According to the Islam R. et al. study, the diagnosis of POI was associated with significant effects on seven of eight domains of the SF-36 questionnaire (p<0.001), and women with POI were more than twice as likely to report a low QoL, defined as scores below 50 on all SF-36 domains (OR 2.11, 95% CI 1.66‒2.68, p<0.001) [12].
In a British study on the psychosocial aspects of QoL in POI, the mean SF-36 scores were higher in all participants than in our study, but the difference in general health and mental health scores between patients with POI and controls was also significant and was greater than 10 points. Most patients (69%) in the study received HRT [13].
Considering the findings on the individual components of integral health indicators, the following conclusions can be drawn: the scores on the scales comprising the Physical Component Summary (PCS) differed significantly in the POI group.
The Physical Component Summary includes indicators such as physical functioning (PF), role-physical (RP), bodily pain (BP), and general health (GH). group 1 scored 75 on the BP (Bodily pain) and PF (Physical Functioning) scales. The BP scale reflects the intensity of bodily pain and its impact on the ability to engage in daily activities. Typical complaints of pain in POI as part of the manifestations of estrogen deficiency are joint and muscle pain as well as headaches [14]. Although there was a statistically significant difference between the two groups in the BP domain, the difference in absolute mean scores was 7 points, and the women with POI in our study differed less from the control group than in the other domains on body pain scores. However, the mean scores on the BP scale in group 1 were consistent with norms for middle-aged women 40‒60 years (75.15 (24.31)), but not for young women of reproductive age 25‒34 years (81.7 (21.1)) according to population-based studies [15, 16].
The mean PF scale (75.5 (20.5)), which describes the extent to which physical activity (carrying heavy weights, climbing stairs, etc.) is limited by health status, was significantly lower in the POI group than in both controls (p<0.001) and norms in two other studies in young (90.19 (15.42) and middle-aged women (85.08 (18.66) and 81.81 (18.50)) [5, 17]. The role-physical (RP) score in group 1 was more than 1.5 times lower than the PF scale. This means that POI patients perceive most acutely, not the limitation of their physical activity but their daily activities at work and household chores. Based on the PF scores alone, physical activity-related role functioning of POI patients was comparable to that of young and middle-aged patients with psoriatic arthritis (18‒34 years old, 46.4 (36.6); 35‒44 years old, 47.1 (24.8)), and significantly different from reference values for healthy subjects of the same age (p<0.001) [18].
The lowest integral physical component in group 1 was 40.1 (18.0) on the General Health (GH) scale. This scale reflects patients’ assessment of their current health status, treatment prospects, and future health status. Patients with POI experience emotional distress when they learn about their diagnosis and continue to do so for many years [3]. Women with this pathology unwittingly compare themselves with their peers in terms of their physical and psychological well-being [13]. A lack of sufficient social support, both in the community and in communication with the doctor [19], a lack of information about the disease and treatment perspectives and goals [13, 20], and fear of premature aging [21] may explain why the POI patients in our study demonstrated a health status score almost twice as low as women of the same age with preserved ovarian function, despite HRT. These findings are consistent with those of several studies included in the meta-analysis [7]: women with POI have significantly worse physical health outcomes (p<0.001), "feel less healthy" than their peers, feel "worthless and hopeless about their physical health" [11].
Overall scores on PH (Physical Component Summary) were slightly higher for patients with POI in the current study than on Mental Component Summary (44.6 (8.9) versus 37.9 (9.8)). The mental health component of the SF-36 questionnaire included the following scales: vitality (VT), Social Functioning (SF), role-emotional (RE), and Mental Health (MH). The lowest scores in group 1 were on the VT (vitality) scale. VT refers to feeling full of energy and vigor, not being tired, or, conversely, feeling exhausted. Our previous study showed that fatigue was the most common symptom in patients with POI and persisted despite HRT (unpublished data). The patients complained of feelings of exhaustion, tiredness in the morning, and fatigue inconsistent with physical and emotional exertion. The VT scores of patients with POI in the current study were similar to those of women with depressive disorder (51.3 (40.6‒60.8)) and multiple sclerosis (47.6 (36.7‒62.5)) in a population-based study of over 15 000 participants [22]. The results of the Mann E. et al. study comparing QoL scores of women with POI and women with physiological menopause were completely consistent with our findings on VT assessment: among 136 patients with POI, 94 (70%) of whom regularly took HRT, the mean VT score was 41.3 (21.3). The scores obtained were significantly lower than those of the comparison group (59.4 (22.4)) (p<0.001) [23].
The mental health (MH) scores of the patients in group 1 in our study were close to those of women with anxiety and depressive disorders (59.1 (48.4‒67.7)) [22]. The MH scale describes the presence or absence of anxiety and depression and is a general indicator of positive emotions. According to our data, anxiety and depression continue to bother over 40% of women with POI receiving HRT and increase irritability and nervousness by over 80% (data not published). The emotional state of group 1 participants was perceived to have a significant negative impact on daily activities (more time spent on work, less work, lower quality of work, etc.) and social activities (communication with relatives, friends, colleagues), as reflected by low scores on the RE (Role emotional) (52.7 (44.0)) and SF (Social Functioning) scales (58.7 (18.7)). Lower scores on all psychosocial aspects of QoL in women with POI compared with healthy female peers have been documented by Liao K.L. et al., and the study group (POI) was significantly more likely to report depression, high levels of stress, lower overall life satisfaction, low self-esteem, and sexual problems [24]. It is also important to note that 84% of the participants in this study were treated with HRT. Significantly lower scores on all the mental health component scales (RE, SF, VT, MH) were also demonstrated by POI patients compared with postmenopausal women (p<0.001) in the previously mentioned work of Mann E. et al. [23].
According to the literature, women have a negative perception of menopause, and its onset is associated with adverse psychological consequences [25]. However, the above findings suggest that premature rather than timely physiological menopause causes more emotional stress. Women with natural menopause perceive their health according to age, and do not have inflated expectations. Patients with POI are likely to have higher expectations of their health, leading to emotional distress. In addition to the above, it is important to add that QoL correlates with the severity of the condition and the manifestations of the disease as judged by the patient, and does not always coincide with the assessment of the severity of the disease as judged by the physician. Many researchers have confirmed these facts in their studies. For example, Coffey S. et al. associated polycystic ovarian syndrome with greater adverse effects on the psychological component of QoL than in many other chronic illnesses, such as asthma, epilepsy, diabetes, heart disease, and arthritis, which are medically perceived as much more serious illnesses with severe health consequences [26].
A QoL analysis of POI patients in our study showed that the total score for the mental component of health, as with the physical component, was dependent on the dose of estrogen with HRT. Only those patients taking high-dose HRT and not the most frequently prescribed standard dose of E2 were closer to the normal 50-point threshold (49.0 (5.9)) (p=0.019). This improvement in QoL with increasing doses of E2 in HRT was consistent with the results of a treatment satisfaction analysis of women with POI; only patients on high-dose HRT achieved a 100% satisfaction rating with their treatment, whereas users of the more frequently prescribed HRT with standard-dose E2 were only 55% satisfied with their treatment [8].
Conclusion
The results of our study showed that POI have a lasting adverse impact on physical and, to a greater extent, mental health. Low QoL, comparable to that of a number of serious chronic diseases, is seen in patients with POI despite regular HRT. Clinicians should use QoL measurement as an integral part of their overall health assessment. The main strategy to improve the QoL of women with POI should be to adequately replace estrogen deficiencies by prescribing adequate doses of HRT. The introduction of a multidisciplinary counselling model and psychosocial support for patients should be considered to improve outcomes.
References
1. Webber L., Davies M., Anderson R., Bartlett J., Braat D., Cartwright B. et al.; European Society for Human Reproduction and Embryology (ESHRE). ESHRE Guideline: management of women with premature ovarian insufficiency. Hum. Reprod. 2016; 31(5): 926‑37. https://dx.doi.org/10.1093/humrep/dew027.
2. Chon S.J., Umair Z., Yoon M.S. Premature ovarian insufficiency: past, present, and future. Front. Cell Dev. Biol. 2021; 9: 672890. https://dx.doi.org/10.3389/ fcell.2021.672890.
3. Panay N., Anderson R.A., Nappi R.E., Vincent A.J., Vujovic S., Webber L., Wolfman W. Premature ovarian insufficiency: an International Menopause Society White Paper. Climacteric. 2020; 23(5): 426‑6. https://dx.doi.org/ 10.1080/13697137.2020.1804547.
4. Torrealday S., Kodaman P., Pal L. Premature ovarian insufficiency ‑ an update on recent advances in understanding and management. Version 1. F1000Res. 2017; 6: 2069. https://dx.doi.org/10.12688/f1000research.11948.1.
5. Swift B., Naci H., Taneri B., Becker C.M., Zondervan K.T., Rahmioglu N. The Cyprus Women's Health Research (COHERE) initiative: normative data from the SF‑36v2 questionnaire for reproductive aged women from the Eastern Mediterranean. Qual. Life Res. 2022; 31(7): 2011‑22. https://dx.doi.org/10.1007/s11136‑022‑03100‑7.
6. Heavy menstrual bleeding: assessment and management. London: National Institute for Health and Care Excellence (NICE); Clinical Guidelines. May 24 2021.
7. Li X.T., Li P.Y., Liu Y., Yang H.S., He L.Y., Fang Y.G. et al. Health‑ related quality‑of‑life among patients with premature ovarian insufficiency: a systematic review and meta‑analysis. Qual. Life Res. 2020; 29(1): 19‑36. https://dx.doi.org/10.1007/s11136‑019‑02326‑2.
8. Аверкова В.Г., Юренева С.В. Анализ удовлетворенности лечением пациенток с преждевременной недостаточностью яичников. Акушерство и гинекология. 2022; 10: 83‑92. [Averkova V.G., Yureneva S.V. Analysis of treatment satisfaction in patients with premature ovarian failure. Obstetrics and Gynecology. 2022; (10): 83‑92. (in Russian)]. https://dx.doi.org/10.18565/aig.2022.10.83‑92.
9. Ware J.E., Snow K.K., Kosinski M., Gandek B. SF‑36 Health survey. Manual and interpretation guide. The Health Institute, New England Medical Center. Boston: Mass; 1993.
10. Новик А.А., Ионова Т.И. Руководство по исследованию качества жизни в медицине. Шевченко Ю.Л., ред. .2‑е изд. М.: ОЛМА Медиа Групп; 2007. 320с. [Novik A.A., Ionova T.I. Guidelines for the study of quality of life in medicine. Shevchenko Y.L., ed. Moscow: OLMA; 2007. 320p. (in Russian)].
11. Benetti-Pinto C.L., de Almeida D.M., Makuch M.Y. Quality of life in women with premature ovarian failure. Gynecol. Endocrinol. 2011; 27(9): 645‑9. https://dx.doi.org/10.3109/09513590.2010.520374.
12. Islam R., Cartwright R., Zhen X., Qiao J., Li R., Kawachiya S. et al. Selected oral communication session, session 69: Endocrinology and POF Wednesday 6 July 2011 14:00‑15:45. Hum Reprod. 2011; 26 (Suppl. 1): i108‑i110. https://dx.doi.org/10.1093/humrep/26.s1.69.
13. Singer D., Mann E., Hunter M.S., Pitkin J., Panay N. The silent grief: psychosocial aspects of premature ovarian failure. Climacteric. 2011; 14(4): 428‑37. https://dx.doi.org/10.3109/13697137.2011.571320.
14. Allshouse A.A., Semple A.L., Santoro N.F. Evidence for prolonged and unique amenorrhea‑related symptoms in women with premature ovarian failure/primary ovarian insufficiency. Menopause. 2015; 22(2): 166‑74. https://dx.doi.org/10.1097/GME.0000000000000286.
15. Anderson D.J., Yoshizawa T. Cross‑cultural comparisons of health‑related quality of life in Australian and Japanese midlife women: the Australian and Japanese Midlife Women's Health Study. Menopause. 2007; 14(4): 697‑707. https://dx.doi.org/10.1097/gme.0b013e3180421738.
16. Jenkinson C., Coulter A., Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ. 1993; 306(6890): 1437‑40. https://dx.doi.org/10.1136/bmj.306.6890.1437.
17. Mishra G., Schofield M.J. Norms for the physical and mental health component summary scores of the SF‑36 for young, middle‑aged and older Australian women. Qual. Life Res. 1998; 7(3): 215‑20. https://dx.doi.org/10.1023/a:1024917510063.
18. Salaffi F., Carotti M., Gasparini S., Intorcia M., Grassi W. The health‑related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a comparison with a selected sample of healthy people. Health Qual. Life Outcomes. 2009; 7: 25. https://dx.doi.org/10.1186/1477‑7525‑7‑25.
19. Orshan S.A., Ventura J.L., Covington S.N., Vanderhoof V.H., Troendle J.F., Nelson L.M. Women with spontaneous 46,XX primary ovarian insufficiency (hypergonadotropic hypogonadism) have lower per‑ ceived social support than control women. Fertil. Steril. 2009; 92(2): 688‑93. https://dx.doi.org/10.1016/ j.fertnstert.2008.07.1718.
20. Groff A.A., Covington S.N., Halverson L.R., Fitzgerald O.R., Vanderhoof V., Calis K., Nelson L.M. Assessing the emotional needs of women with spontaneous premature ovarian failure. Fertil. Steril. 2005; 83(6): 1734‑41. https://dx.doi.org/10.1016/j.fertnstert.2004.11.067.
21. Graziottin A. Menopause and sexuality: key issues in premature menopause and beyond. Ann. N. Y. Acad. Sci. 2010; 1205: 254‑61. https://dx.doi.org/10.1111/ j.1749‑6632.2010.05680.x.
22. Sprangers M.A., de Regt E.B., Andries F., van Agt H.M., Bijl R.V., de Boer J.B. et al. Which chronic conditions are associated with better or poorer quality of life? J. Clin. Epidemiol. 2000; 53(9): 895‑907. https://dx.doi.org/10.1016/ s0895‑4356(00)00204‑3.
23. Mann E., Singer D., Pitkin J., Panay N., Hunter M.S. Psychosocial adjustment in women with premature menopause: a cross‑sectional survey. Climacteric. 2012; 15(5): 481‑9. https://dx.doi.org/10.3109/13697137.2011.647841.
24. Liao K.L., Wood N., Conway G.S. Premature menopause and psychological well‑being. J. Psychosom. Obstet. Gynaecol. 2000; 21(3): 167‑74. https://dx.doi.org/10.3109/01674820009075624.
25. Clayton A.H., Ninan P.T. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim Care Companion J. Clin. Psychiatry. 2010; 12(1): PCC.08r00747. https://dx.doi.org/10.4088/PCC.08r00747blu.
26. Coffey S., Bano G., Mason H.D. Health‑related quality of life in women with polycystic ovary syndrome: a comparison with the general population using the Polycystic Ovary Syndrome Questionnaire (PCOSQ) and the Short Form‑36 (SF‑36). Gynecol. Endocrinol. 2006; 22(2): 80‑6. https://dx.doi.org/10.1080/09513590600604541.
Received 25.01.2023
Accepted 10.04.2023
About the Authors
Victoria G. Averkova, Ph.D. student of Gynecological Endocrinology Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, buch1202@mail.ru, https://orcid.org/0000-0002-8584-5517, 117997, Russia, Moscow, Oparin str., 4.Svetlana V. Yureneva, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology of the Department of Vocational Education; Deputy Director for Science of the Institute of Oncogynecology and Mammology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, syureneva@gmail.com, https://orcid.org/0000-0003-2864-066X, 117997, Russia, Moscow, Oparin str., 4.
Corresponding author: Victoria G. Averkova, buch1202@mail.ru



