В настоящее время осторожное отношение к гормональной терапии эстрогенами со стороны врачебного сообщества отчасти обусловлено неуверенностью специалистов в вопросах корректного назначения эстрогенов, включающих подбор оптимальной дозы и ее титрование, пути введения, возраст начала и длительность лечения, ожидаемые преимущества и риски применения эстрогенов в различных возрастных группах, а также назначение женщинам с неблагоприятным преморбидным фоном, коморбидностью. В этой связи целью настоящего обзора является обобщение рекомендаций международных протоколов и научных исследований, посвященных практическим вопросам применения гормональной терапии эстрогенами.
Для более точного определения целей и задач гормональной терапии эстрогенами важно разделять два понятия: «заместительная гормональная терапия» (ЗГТ), под которой подразумевают восполнение дефицита эстрогенов у женщин в репродуктивном возрасте, и термин «менопаузальная гормональная терапия» (МГТ), применяемый при назначении эстрогенов женщинам в пре- и постменопаузе [1–5]. Врачу-клиницисту необходимо избегать экстраполяции данных о рисках, связанных с МГТ, полученных в исследованиях с участием женщин в постменопаузе, на молодых пациенток. Сегодня целью гормональной терапии эстрогенами является не «сохранение женской красоты и вечной молодости», как это считалось в 60–70-х годах прошлого столетия, а лечение и профилактика серьезных заболеваний и патологических состояний, связанных с эстрогенодефицитом. ЗГТ необходима для нивелирования вазомоторных расстройств и влагалищной атрофии у молодых женщин с преждевременной недостаточностью яичников (ПНЯ), а также для предотвращения таких последствий дефицита эстрогенов, как остеопороз, ишемическая болезнь сердца (ИБС), инсульт, деменция и когнитивные нарушения, сексуальная дисфункция [4, 5]. Задачи МГТ несколько уже и включают коррекцию менопаузальных расстройств, существенно снижающих качество жизни [1–3].
 Хотя общее число участниц исследований, посвященных изучению эффективности и безопасности МГТ, уже превысило несколько миллионов, важнейшим принципом назначения МГТ является индивидуальный подход с учетом персонального риска и пользы [6], которые определяются временем начала, режимом и длительностью терапии, дозой и типом эстрогена/прогестина, путем доставки, наличием базовых рисков венозных тромбоэмболических осложнений (ВТЭО), сердечно-сосудистых заболеваний (ССЗ) и рака молочной железы (РМЖ). Под МГТ сегодня понимают применение эстрогенов женщинами с гистерэктомией и комбинированной терапии эстрогенами и прогестинами женщинами с интактной маткой. Если единственным менопаузальным нарушением является вульвовагинальная атрофия, то вполне достаточным является топическое применение эстрогенов [7]. Несмотря на то что дефицит эстрогенов влияет на функционирование всего женского организма, основными «мишенями» для МГТ являются лишь некоторые из возникающих нарушений (рисунок). Самым частым показанием для назначения МГТ являются приливы, которые встречаются почти у 80% женщин. Кроме того, прием препаратов для МГТ существенно уменьшает выраженность целого ряда менопаузальных расстройств, таких как нарушение сна, психоэмоциональная лабильность/депрессия и, в ряде случаев, суставные боли. МГТ сегодня не рекомендована для профилактики ССЗ или деменции у женщин в постменопаузе. МГТ считается эффективной мерой для предотвращения постменопаузального остеопороза, однако в настоящее время может быть рекомендована только в качестве второй линии при наличии противопоказаний к бисфосфонатам или деносумабу [1, 8, 9].
Хотя общее число участниц исследований, посвященных изучению эффективности и безопасности МГТ, уже превысило несколько миллионов, важнейшим принципом назначения МГТ является индивидуальный подход с учетом персонального риска и пользы [6], которые определяются временем начала, режимом и длительностью терапии, дозой и типом эстрогена/прогестина, путем доставки, наличием базовых рисков венозных тромбоэмболических осложнений (ВТЭО), сердечно-сосудистых заболеваний (ССЗ) и рака молочной железы (РМЖ). Под МГТ сегодня понимают применение эстрогенов женщинами с гистерэктомией и комбинированной терапии эстрогенами и прогестинами женщинами с интактной маткой. Если единственным менопаузальным нарушением является вульвовагинальная атрофия, то вполне достаточным является топическое применение эстрогенов [7]. Несмотря на то что дефицит эстрогенов влияет на функционирование всего женского организма, основными «мишенями» для МГТ являются лишь некоторые из возникающих нарушений (рисунок). Самым частым показанием для назначения МГТ являются приливы, которые встречаются почти у 80% женщин. Кроме того, прием препаратов для МГТ существенно уменьшает выраженность целого ряда менопаузальных расстройств, таких как нарушение сна, психоэмоциональная лабильность/депрессия и, в ряде случаев, суставные боли. МГТ сегодня не рекомендована для профилактики ССЗ или деменции у женщин в постменопаузе. МГТ считается эффективной мерой для предотвращения постменопаузального остеопороза, однако в настоящее время может быть рекомендована только в качестве второй линии при наличии противопоказаний к бисфосфонатам или деносумабу [1, 8, 9].
Все препараты для МГТ эффективно уменьшают частоту приливов в среднем на 75%. Применение МГТ оправдано в случае, если приливы существенно нарушают ежедневную активность, в то время как легкие приливы нивелируются иными способами, основанными преимущественно на модификации образа жизни [10, 11]. Около 32–53% женщин в перименопаузе могут страдать расстройствами сна в виде спонтанного пробуждения, бессонницы, обструктивного апноэ или «синдрома беспокойных ног», что влияет на психоэмоциональное состояние в дневное время [12]. Доля женщин с тревожно-депрессивными расстройствами в перименопаузе достигает 30–60%, при этом частота депрессии в 2,5 раза выше в перименопаузе по сравнению с репродуктивным периодом [13]. В исследовании WHI было отмечено уменьшение суставных болей на фоне МГТ [14]. В случаях отсутствия облегчения проявлений урогенитальной атрофии, которая регистрируется у 47% женщин в постменопаузе, дополнительно к системным назначаются топические эстрогены [15].
При назначении МГТ рекомендуется придерживаться временных границ в пределах «окна терапевтического воздействия», когда польза от фармакологического вмешательства будет преобладать над потенциальными рисками. Последнее означает, что либо возраст начала МГТ не должен превышать 60 лет, либо старт МГТ должен приходиться на первые 10 лет менопаузы [3]. Для женщин в возрасте 50–59 лет были доказаны преимущества МГТ продолжительностью не более 5 лет. Последние включали снижение рисков колоректального рака и смертности, а при применении монотерапии эстрогенами – дополнительное сокращение частоты инсульта и ИБС [17], а также уменьшение числа остеопоротических переломов на 4,9 и 5,9 на 1000 женщин за 5 лет использования комбинированных эстроген-гестагенных препаратов и на фоне монотерапии эстрогенами соответственно [18].
Если ранее считалось, что МГТ должна начинаться с приема стандартных доз эстрогенов, то в настоящее время рекомендуется использование минимальных доз эстрогенов с постепенным титрованием до оптимальной, обеспечивающей достижение эффекта [1, 3, 9, 19]. Важно отметить, что под низкой дозой эстрогена подразумевают 0,5 мг перорального эстрадиола, стандартной – 1 мг и высокой – 2 мг соответственно [1]. При исчезновении менопаузальных симптомов и отсутствии нежелательных явлений выбранный режим МГТ продолжается в течение нескольких лет. Через 3 года (между 3-м и 5-м годами) МГТ можно пересмотреть дозу в сторону уменьшения для постепенной отмены. При наличии у женщины тяжелых менопаузальных симптомов возможно стартовать с более высоких доз эстрогенов с постепенным титрованием в сторону уменьшения [9, 16, 17, 20].
Рекомендуется начинать МГТ с применения трансдермальных форм эстрогенов во избежание эффекта первичного прохождения через печень, что значительно снижает риски венозных тромбоэмболических осложнений (ВТЭО), инсульта, желчнокаменной болезни, холецистита [9, 16, 17, 20]. Терапия менопаузальных расстройств, как правило, начинается с назначения 17β-эстрадиола в ежедневной дозе 0,5 мг/сут – для 0,1% трансдермального геля в саше (Дивигель), 0,05 мг/сут – для трансдермального пластыря, 1 мг/сут – для пероральных эстрогенов, что для большей части женщин является достаточным для устранения приливов [11]. Пероральные высокодозные конские (эквинные) эстрогены (КЭЭ) в стандартной дозе 0,625 мг/день, которая соответствует 1,0 мг 17β-эстрадиола, назначаются сегодня гораздо реже. Трансдермальные эстрогены выпускаются в виде пластырей, гелей с различной системой доставки (насос (помпа), алюминиевые саше). Гели с эстрадиолом для трансдермального использования доступны в различных дозировках, содержат различные наполнители, наносятся на различную по площади поверхность кожи, что не позволяет проводить прямые сравнения эквивалентности доз только по содержанию эстрадиола в единице объема препарата. По этой причине в метаанализе, сравнивающем эффективность применения в течение 12 недель различных трансдермальных гелей с эстрадиолом по скорости купирования вазомоторных симптомов, использовалась методика «непрямых сравнений» с группой плацебо. Результаты исследования продемонстрировали, что показатели эффективности препарата 17β-эстрадиола в виде 0,1% геля в дозе 0,5 мг аналогичны таковым для 17β-эстрадиола в виде 0,06% геля в дозах 0,75 и 1,5 мг: применение 0,1% геля 17β-эстрадиола приводило к статистически значимому уменьшению частоты и интенсивности приливов ко 2–5-й неделе, а 0,06% геля – только через 6–9 недель. В дозировке 1,0 мг 0,1% гель 17β-эстрадиола статистически значимо превосходил обе дозы 0,06% геля 17β-эстрадиола по эффективности, но связан с несколько повышенным риском нежелательных реакций, частота которых не превышала статистически значимо риск нежелательных явлений в группе плацебо [21]. При переходе с перорального использования препаратов эстрогенов на трансдермальный путь введения необходимо руководствоваться данными об эквивалентности доз эстрогенов в зависимости от пути введения, и в ряде случаев для подбора оптимальной дозы трансдермальных форм эстрогенов необходимо титровать по клинической эффективности.
В качестве прогестинового компонента большинство экспертов сегодня предпочитают использовать микронизированный прогестерон вместо медроксипрогестерона ацетата (МПА), прием которого связан с повышенным риском ССЗ и РМЖ. Обычная доза микронизированного прогестерона составляет 200 мг/сут (дидрогестерона 10 мг/сут) в течение последних 12 дней месяца на фоне приема эстрогенов – при циклическом режиме МГТ или 100 мг/сут (10 мг/сут) ежедневно – при непрерывном приеме соответственно [22]. Поскольку отдельные метаболиты прогестерона обладают выраженным седативным эффектом, некоторые авторы придерживаются мнения, что прием прогестерона лучше перенести на вечернее время. У женщин с менопаузальными расстройствами, помимо прочего заинтересованных в надежной контрацепции, возможно использование левоноргестрел-содержащего внутриматочного средства.
Задачей врача-клинициста в рамках консультирования по вопросам безопасности МГТ является предоставление пациентам достоверной и доступной информации о преимуществах и рисках терапии с использованием, в частности, абсолютных цифр, а не относительных рисков [23]: например, лучше указать на 1 дополнительный случай неблагоприятного события на 1000 женщин, использующих МГТ 5 лет, чтобы позволить женщине оценить назначения врача без излишних волнений.
При этом самым острым вопросом безопасности МГТ является взаимосвязь использования терапии эстрогенами с онкорисками, а в первую очередь – рисками РМЖ. В исследовании WHI использование МГТ ассоциировалось с увеличением абсолютного числа случаев заболеваний РМЖ на фоне комбинированных препаратов с эстрогенами и прогестином в отличие от монотерапии эстрогенами [24]. Указанное согласуется с результатами метаанализа, включившего данные 58 исследований, проведенных с 1992 по 2018 гг. с участием 108 647 женщин в постменопаузе с РМЖ, из которых 55 575 (51%) использовали МГТ. Выводы метаанализа обнаружили несколько большие риски развития РМЖ на фоне МГТ как комбинированными препаратами, так и в виде монотерапии эстрогенами, чем считалось ранее, что чуть было не привело к возникновению «второй волны» недоверия к МГТ. Авторы исследования рассчитали, что для женщин с нормальным индексом массы тела использование МГТ в течение 5 лет, начиная с возраста 50 лет, увеличивает 20-летний риск РМЖ (в возрасте от 50 до 69 лет) примерно на один дополнительный случай на 50 женщин – при использовании монофазного режима МГТ, на 70 женщин – на фоне циклической МГТ, на 200 женщин – при монотерапии эстрогенами [25]. Однако выводы о серьезных негативных последствиях МГТ, связанных с повышением риска РМЖ, были вновь подвергнуты критическому переосмыслению, и эксперты ведущих профессиональных организаций, в сфере научно-практических интересов которых находится МГТ, опубликовали в открытом доступе официальные заявления о качественных недостатках метаанализа, что ограничило безоговорочное принятие его выводов врачами-клиницистами.
Во-первых, метаанализ 2019 г. объединил только наблюдательные исследования. Во-вторых, большинство женщин в наблюдательных исследованиях принимали ККЭ и МПА, в то время как современные варианты МГТ обычно включают более низкие дозы эстрогена в виде 17β-эстрадиола, вводимого безопасным трансдермальным путем, с микронизированным прогестероном, что, по мнению экспертов, может быть связано с более низким риском РМЖ [1, 3, 16]. В другом наблюдении в течение 8,1 года за 80 377 женщинами в постменопаузе, у 2354 из которых развился РМЖ, было установлено, что МГТ с микронизированным прогестероном и дидрогестероном не увеличивала риски РМЖ, в то же время синтетические прогестины ассоциировались с повышением риска РМЖ на 70% [26]. В-третьих, в метаанализе 2019 г. не принимались во внимание положительные эффекты МГТ, связанные с лечением приливов, повышением качества жизни, профилактикой остеопороза, что не позволяет сформировать целостное представление о влиянии МГТ на все аспекты здоровья. Важно отметить, что эпидемиологические исследования показали, что РМЖ у женщин, принимающих МГТ, имеет относительно благоприятный прогноз по сравнению с РМЖ, возникшим у женщин без МГТ [20, 27].
Иные, помимо молочной железы, локализации рака органов репродукции, потенциально связанные с приемом МГТ, либо эффективно предотвращаются добавлением прогестинов (рак эндометрия), либо имеют крайне низкую вероятность возникновения (рак яичников). Показано, что вне зависимости от дозы монотерапия эстрогенами от 1 до 3 лет ассоциируется с повышенным риском гиперплазии эндометрия [16] и раком эндометрия [28] у женщин с интактной маткой, который, по аналогии с РМЖ на фоне МГТ, протекает менее агрессивно. Циклическое (не менее 12 последних дней использования эстрогенов) и непрерывное применение низких доз прогестинов одинаково эффективно в профилактике рака эндометрия. Предполагается, что более короткий курс прогестинов в циклическом режиме продолжительностью менее 10 дней не обеспечивает канцеропротективный эффект для эндометрия [16]. Поскольку комбинированная МГТ сопряжена с повышенным риском РМЖ по сравнению с монотерапией эстрогенами [25], женщины с гистерэктомией в анамнезе не нуждаются в добавлении прогестинов, задача которых при МГТ – нивелировать пролиферативные эффекты эстрогенов на эндометрий.
Поскольку риск рака яичников крайне незначительно выше у тех, кто когда-либо принимал МГТ, по сравнению с женщинами, никогда не использующими МГТ (1,14; 95% ДИ 1,10–1,19) [6], данный факт практически не оказывает влияния на принятие решения врачом о необходимости МГТ.
Наличие у женщины с менопаузальными расстройствами доброкачественных пролиферативных заболеваний органов репродукции зачастую становится поводом для отказа от МГТ из-за предполагаемого стимулирующего воздействия МГТ на эндометриоидные гетеротопии, которые обнаруживаются у 2,2% женщин в постменопаузе, или миоматозные узлы. Метаанализ обнаружил противоречивые данные о безопасности применения МГТ у женщин с миомой матки в постменопаузе: несмотря на возможное влияние МГТ на увеличение размеров узлов, наличие миомы матки не является абсолютным противопоказанием к МГТ. Некоторые рандомизированные клинические исследования (РКИ) демонстрировали увеличение у отдельных женщин размеров лейомиомы матки в течение 12 месяцев использования МГТ на 30% с преимущественным ростом в первые 6 месяцев и последующей стабилизацией роста. В одной из работ был показан чуть более интенсивный, но клинически незначимый рост миоматозных узлов при применении трансдермальных форм эстрадиола с МПА, что может быть связано с более высокой биодоступностью трансдермальных форм или пролиферативными эффектами МПА. В целом женщин с лейомиомой матки, получающих МГТ, следует периодически обследовать и искать альтернативные пути лечения менопаузальных расстройств в случае быстрого увеличения лейомиомы в размерах. Авторы рекомендуют использовать для МГТ минимальную эффективную дозу прогестина [29].
Некоторые данные свидетельствуют о том, что женщинам не следует отказывать в МГТ только из-за наличия в анамнезе эндометриоза. Основываясь на доказательствах низкого качества по литературным данным, авторы рекомендуют отдавать предпочтение комбинированной МГТ, избегая монотерапии эстрогенами [30].
Недостаточная осведомленность специалистов относительно реальных рисков ССЗ на фоне МГТ и способов их минимизации порой подрывает авторитет всего направления в глазах пациенток и лишает их возможности получения эффективной помощи при возникновении менопаузальных симптомов. Как известно, в 80–90-е гг., в «золотую эру» МГТ, к показаниям для назначения эстрогенов в перименопаузе относили профилактику ССЗ. Последнее не нашло полного подтверждения при анализе данных, полученных в РКИ WHI и последующих крупных работах, что объясняется влиянием целого ряда факторов: от вида эстрогенов/прогестинов до способа введения препаратов. Так, было показано, что частота ИБС увеличивалась на 8 случаев на 10 000 женщин в год на фоне комбинированной МГТ (ККЭ/МПА), в то время как монотерапия ККЭ, вероятно, не ассоциировалась с повышением риска ИБС [31]. Риск инсульта у женщин на фоне МГТ имел клинически незначимую тенденцию к росту в возрастной группе от 50 до 59 лет [31]. В исследовании по типу случай-контроль, в котором было задействовано около 16 000 женщин с инсультом и 60 000 здоровых, проиллюстрировано, что трансдермальная МГТ по сравнению с пероральной не увеличивает риски инсульта [32].
Принимая решение о назначении МГТ, выбирая препарат, акушеры-гинекологи нередко высказывают опасения относительно рисков ВТЭО на фоне терапии эстрогенами, поскольку известно, что МГТ в целом, без деления на возрастные группы, повышает риск ВТЭО у женщин в постменопаузе [33]. Частота ВТЭО на фоне комбинированной МГТ в сравнении с плацебо составляла 3,5 и 1,7 случая на 1000 женщин в год соответственно. К дополнительным факторам, увеличивающим риски ВТЭО на фоне МГТ, относятся пожилой возраст, ожирение, наличие мутации в V факторе Лейдена [33]. Частота ВТЭО при монотерапии эстрогенами по сравнению с группой плацебо составляла 3,0 и 2,2 случая на 1000 женщин в год соответственно [34].
В исследовании случай-контроль было установлено, что использование КЭЭ ассоциировалось с повышенным риском ВТЭО, в отличие от этерифицированных эстрогенов в составе МГТ [35].
В метаанализе было продемонстрировано, что трансдермальные эстрогены не увеличивают риск ВТЭО даже у женщин с мутациями V Лейдена и избыточным весом [36]. Эти данные перекликаются с выводами другого исследования, в котором подтверждается, что риск ВТЭО не увеличивался при использовании трансдермальных эстрогенов при монотерапии и при их комбинации с прогестинами. Напротив, риск ВТЭО был дозозависимым и возрастал почти на 50% при монотерапии пероральными эстрогенами. Риски были самыми высокими в течение первого года и исчезли через 4 месяца после прекращения МГТ [37]. В одном из последних метаанализов обнаружено увеличение рисков ВТЭО на 66% при использовании пероральной МГТ по сравнению с трансдермальной [38]. Вопрос прогнозирования рисков ВТЭО у женщин, получающих МГТ, во время пандемии COVID-19 имеет важнейшее клиническое значение. Некоторые профессиональные сообщества рекомендовали женщинам, использующим МГТ, при коронавирусной инфекции, патогенетическим звеном которой считают коагулопатию, пересматривать пользу/риск МГТ: либо отказываться от МГТ, либо переходить на трансдермальные формы препаратов на фоне инфекции [39].
Врачу необходимо принимать во внимание некоторые различия фармакокинетики эстрогенов в различных клинических ситуациях (табл. 1). Так, пероральный прием эстрогенов индуцирует повышение продукции тироксинсвязывающего глобулина, что снижает уровень биодоступного тироксина. Вероятно, женщинам, получающим заместительную терапию тироксином, потребуется увеличение дозы тироксина. Также известно, что пероральный прием эстрогенов может увеличивать синтез глобулина, связывающего половые гормоны, что приводит к снижению в сыворотке биологически активного тестостерона, что гипотетически может усугублять сексуальную дисфункцию, хотя исследования, подтверждающие последнее, отсутствуют. Особенности фармакокинетики пероральных эстрогенов приводят к клинически значимому повышению уровня триглицеридов. С другой стороны, пероральные эстрогены оказывает благоприятное влияние на липидный профиль, способствуя повышению липопротеинов высокой плотности и снижению липопротеинов низкой плотности, но доказательства того, что эти изменения имеют клиническое значение, отсутствуют [40, 41]. Рекомендуется избегать одновременного перорального приема эстрогенов с противосудорожными препаратами, которые увеличивают печеночный клиренс эстрогенов [42]. Частое и избыточное употребление алкоголя приводит к трехкратному увеличению концентрации эстрадиола в сыворотке при пероральном приеме препаратов для МГТ, что обусловлено замедлением метаболизма эстрадиола в печени [43]. У женщин с терминальной почечной недостаточностью концентрация эстрадиола в плазме после перорального приема эстрогенов выше, чем у здоровых женщин [44]. Кроме того, были установлены перенасыщение желчи холестерином и усиление нуклеации под воздействием пероральных эстрогенов в отличие от трансдермальных [40, 45].

Очевиден тот факт, что в подавляющем большинстве случаев симптомы менопаузы, требующие назначения МГТ, манифестируют в возрасте от 40 до 50 лет, когда женщина относительно здорова и имеет невысокие базовые риски ССЗ и РМЖ, что определяет максимальную безопасность МГТ в позднем репродуктивном, переходном и раннем менопаузальном периодах. В этой связи при отсутствии противопоказаний для МГТ, к которым относят РМЖ, ИБС, ВТЭО, инсульт в анамнезе или острое заболевание гепатобилиарной системы, глобальная польза от данного вмешательства превышает возможные риски. Кроме того, серьезные риски увеличиваются при использовании МГТ свыше 5 лет, в то время как данные наблюдательных исследований показывают, что 40–50% женщин, начинающих МГТ, завершают ее в течение одного года, а 65–75% – в течение 2 лет [7]. Поскольку у 10–20% женщин симптомы менопаузы могут длиться намного дольше 10 лет после последней самостоятельной менструации, возможно рассмотреть более длительное назначение МГТ с минимально возможной дозой эстрогенов, информируя пациентку о соответствующих рисках [1, 19].
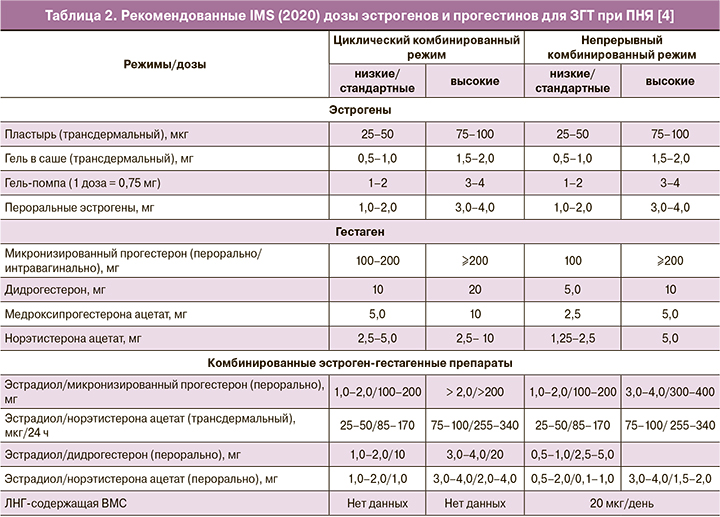
В сентябре 2020 г. экспертами Международного общества по менопаузе (IMS) были опубликованы обновленные подходы к ЗГТ пациенток с ПНЯ [4]. Наряду с необходимостью продолжения ЗГТ у женщин с ПНЯ до среднего возраста менопаузы (51 год), другим важнейшим принципом ЗГТ является использование трансдермальных эстрогенов в качестве первой линии терапии для более физиологичного восполнения дефицита эстрогенов, а также для снижения риска ВТЭО и заболеваний желчного пузыря [46]. Данные о преимуществах трансдермальных форм эстрогенов, связанных с безопасностью для сердечно-сосудистой системы, в меньшей степени изучены на женщинах репродуктивного возраста [46]. Установлено, что при ПНЯ развивается выраженная, но обратимая эндотелиальная дисфункция, которая почти полностью исчезает на фоне ЗГТ [47, 48]. В исследовании с участием 301 438 женщин установлено, что риск ССЗ у женщин с ПНЯ был выше по сравнению с женщинами, у которых наступила менопауза в возрасте 50–51 лет (1,55; 95% ДИ 1,38–1,73). Каждый год уменьшения возраста наступления менопаузы ассоциируется с повышением риска ССЗ на 3% [49]. Третий базовый принцип назначения ЗГТ заключается в использовании более высоких доз эстрогенов у женщин репродуктивного возраста по сравнению с дозами для МГТ [3–5]. В рекомендациях IMS эффективные дозы (табл. 2), назначаемые женщинам с ПНЯ, оказались выше, чем предлагалось ранее. Если раньше максимальная суточная доза эстрогенов для трансдермального использования у женщин с ПНЯ, например, для 0,1% геля 17β-эстрадиола ограничивалась 1,5 мг (эквивалентно 3 мг перорального эстрогена), то в соответствии с новыми рекомендациями IMS [4] при необходимости суточную дозу можно увеличивать до 2 мг (эквивалентно 4 мг перорального эстрогена). Для защиты эндометрия при использовании высоких доз эстрогенов рекомендованы следующие дозы гестагенов: микронизированный прогестерон 200 мг/дидрогестерон 20 мг/сут – для циклической ЗГТ и микронизированный прогестерон 200 мг/дидрогестерон 10 мг/сут – для непрерывной ЗГТ [4]. Женщины с ПНЯ могут перейти на непрерывный режим ЗГТ через несколько лет или, по своему желанию, начинать с непрерывного режима при условии, что длительность аменореи составляет уже более года. Непрерывный режим ЗГТ связан с большей протекцией эндометрия, но циклическая ЗГТ может быть сопряжена с более низким риском РМЖ [4].
Заключение
Таким образом, принимая решение о необходимости гормональной терапии с эстрогенами, акушер-гинеколог должен взвешивать индивидуальные риски и преимущества, связанные как с назначением, так и с неназначением терапии для конкретной пациентки с учетом возраста, возраста наступления менопаузы, выраженности симптомов эстрогенодефицита, базовых рисков ССЗ и онкорисков, придерживаясь принципа «окна терапевтического воздействия», отдавая приоритет более безопасным трансдермальным формам эстрогенов.



