New perspectives in searching diagnostic markers for ovarian endometriosis
Levakov S.A., Gromova T.A., Mamedova A.E., Antipova N.V.
Objective: Determination of expression levels of molecular genetic markers to improve the diagnosis of ovarian endometriosis (OE) and define prognostic criteria for recurrence and possible malignant transformation.
Materials and methods: Expression levels of lncRNAs in 30 and 25 cases of ovarian endometriotic cysts and ovarian adenocarcinomas, respectively, as well as in 25 cases in the control group, were studied using molecular genetic testing.
Results: Persistent increase in lncRNA MALAT1 expression level was found with the disease progression, and consistent increase and reduction of Linc-ROR expression level in the control group and in the group of patients with adenocarcinoma. For differential diagnosis of normal condition and OE, OE and adenocarcinoma, ROC curves for lncRNA expression levels were constructed, and important rule was derived to determine the histological status of the surgical material.
Conclusion: According to the results of expression levels of lncRNA markers MALAT1 and Linc-ROR in the groups of patients with endometriosis, adenocarcinoma and in the control group, the assessment of their potential possibility for predicting these conditions was suggested. Further targeted research in this area is necessary to develop new prognostic markers for endometriosis-associated ovarian cancer and search for additional therapeutic targets.
Authors' contributions: Levakov S.A. – the concept and desigh of the study; Antipova N.V., Mamedova A.E. – material collection and processing, statistical data processing; Gromova T.A., Mamedova A.E. – article writing; Gromova T.A. – article editing.
Conflicts of interest: The authors declare that they have no conflict of interest to declare.
Funding: The study was conducted in the frames of the Grant No. 22-14-00234 of the Russian Scientific Fund (RSF).
Ethical Approval: This study was approved by the local Ethics Committee of I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University).
Patient Consent for Publication: The patients signed informed consent for publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Levakov S.A., Gromova T.A., Mamedova A.E., Antipova N.V. New perspectives in searching diagnostic markers for ovarian endometriosis.
Akusherstvo i Gynecologia/Obstetrics and Gynecology. 2024; (1): 110-116 (in Russian)
https://dx.doi.org/10.18565/aig.2023.91
Keywords
Endometriosis is a multisystem chronic inflammatory disease that occurs in 10–15% of female patients of reproductive age (190,000,000 women all over the world [1].
Currently, the relevance of endometriosis in the structure of gynecological morbidity is increasing with increased risk of subsequent diagnosis of malignant neoplasms, autoimmune diseases, early natural menopause, cerebrovascular and cardiovascular diseases, as well as concomitant chronic diseases (migraine, rheumatoid arthritis, fibromyalgia, psoriatic arthritis, osteoarthritis) [2–9]. A number of recent studies found that relative risk for developing ovarian cancer (clear cell carcinoma and endometrial adenocarcinoma) increased by 2 times among the patients with endometriosis up to 2.5%, and is by 1.2% higher of the absolute risk for patients. The incidence of malignancies is 1–13% [1, 10].
Almost 60% of patients consult three or more specialists before the final diagnosis is made and treatment is prescribed, that on average takes about 7 years. Therefore, at present, the relevance of searching sensitive specific diagnostic markers of ovarian endometriosis (OE) is increasing due to the lack of such markers today [1, 11].
Recently, expression of long non-coding RNAs (lncRNAs) – a large group of non-coding RNAs greater than 200 nucleotides in length – is of particular interest due to their crucial role in molecular functions including RNA processing, transcriptional and post-transcriptional regulation of gene expression. LncRNAs are involved in many important biological processes, such as cell proliferation, survival, differentiation, organogenesis, dosage compensation, genomic imprinting and chromatin remodeling [12]. However, research results also show their important role in carcinogenesis through Wnt, Hedgehog, Notch and PI3K/AKT/mTOR signaling pathways [13]. Dysregulation of several lncRNAs can influence the major characteristics of cancer cells, including the control of oncogene expression associated with their suppressive and oncogenic functions. Therefore, circulating lncRNAs have a number of advantages compared to traditional biomarkers (proteins) and can be competing biomarkers in cancer, including endometriosis-associated ovarian cancer, and also determine pathological depth of invasion into healthy tissues [14–17].
Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) is a multi-functional RNA, which forms molecular frameworks for ribonucleoprotein complexes that regulate cell proliferation and migration [18, 19]. MALAT1 is one of primarily identified lncRNAs that are associated with cancerogenesis. In several studies it was considered to be as biomarker of oncological process [20–22]. This RNA may act as a transcriptional regulator of multiple genes involved in cancer metastasis and cell migration, as well as may influence cell cycle regulation. A number of studies found that MALAT1 is one of the primarily identified lncRNAs involved in regulation of alternative splicing. Disrupted alternative splicing is a key factor that favors development of the oncological process. This fact provides a new insight into the mechanisms of MALAT1 in gene expression regulation [23]. A number of studies reported upregulation of MALAT1 expression levels in ovarian, breast, cervical and endometrial cancers [18, 19]. MALAT1 showed significantly high expression levels in ectopic endometrial tissue compared to eutopic tissue. This lncRNA promotes apoptosis of endometrial cells and has a regulatory effect on expression of matrix metalloproteinase-9 through the NF-kB/iNOS pathway, thus mediating the pathogenesis of endometriosis [16, 24, 25].
Long intergenic non-coding RNA, regulator of reprogramming (Linc-ROR) is a key regulatory factor that influences the occurrence and development of human tumors, including colorectal, breast, pancreatic cancers, hepatocellular carcinoma, etc. [26]. Recent studies related to Linc-ROR and tumorigenesis showed that overregulation of this lncRNA positively correlates with clinicopathological characteristics and tumor progression. Their growth and metastasis are stimulated by Linc-ROR through activation of epithelial-mesenchymal transition. One of the studies found increased Linc-ROR expression in ectopic endometrium compared to eutopic and normal endometrium, that may promote the proliferative activity of endometrial cells by activating the PI3K-Akt pathway [27]. The association between Linc-ROR expression, the type and severity of dysmenorrhea in adenomyosis was found [24, 27]. Since Linc-ROR can regulate cell proliferation, apoptosis, migration and invasion, it can be used as a potential biomarker for oncologic patients and has potential clinical significance as a therapeutic target [28–30].
In view of the above, identification of previously unexplored molecular genetic markers for ovarian endometriotic lesions and endometriosis-associated ovarian cancer will help to improve understanding of the etiology, depth of invasion and spread of the neoplastic process, occurrence of relapses, as well as can open potential prospects for the development of new biomarkers (with further prediction of treatment outcomes and management of patients).
The purpose of the study was determination of expression levels of molecular genetic markers MALAT1 and Linc-ROR to improve the diagnosis of ovarian endometriosis and define prognostic criteria for recurrence and possible malignant transformation.
Materials and methods
The prospective study included 80 patients with unilateral or bilateral ovarian masses, who were selected for elective surgical treatment. The study participants were divided into three groups: with ovarian endometriotic cysts (group 1, n=30), with ovarian adenocarcinomas (group 2, n=25) and the patients, who underwent ovarian biopsy for medical indications (group 3, the control group, n=25). Group 1 was divided into 2 subgroups: with primarily diagnosed OE (n=15) and recurrent OE (n=15).
Inclusion criteria for group 1 and two: reproductive age of patients 18–49 years; histologically confirmed OE and ovarian adenocarcinoma, respectively; absence of acute gynecological pathology and significant pathomorphological disorders of the reproductive system.
Inclusion criteria for group 3: reproductive age of patients 18–49 years; strict indications for ovarian biopsy: the presence of borderline ovarian tumors detected by histological examination, and due to this fact performance of biopsy of the contralateral ovary (serous and mucinous borderline tumors); the absence of acute gynecological pathology and significant pathomorphological disorders of the reproductive system.
Exclusion criteria: pregnancy and lactation; the presence of acute inflammatory diseases, severe extragenital pathology at the decompensation stage; any type of oncological disease in anamnesis and at the time of the study (for groups 1 and 3); the presence of other oncological diseases of any location in anamnesis or at the time of the study, except for ovarian adenocarcinoma (for group 2); the presence of any structural pathology of ovary (for the control group).
After surgical treatment (enucleation of endometriotic cysts, oophorectomy for adenocarcinoma and biopsy of the contralateral ovary), a small area of the obtained material (0.3–0.5 cm) was separated and immersed in the sterile tube. To inhibit RNase activity, 1 ml of TRIzol solution was added to each sample and placed in the freezer for 1 hour.
Subsequently, the surgical material was transported for further examination to M.M. Shemyakin and Yu.A. Ovchinnikov Institute of Bioorganic Chemistry (IBCh), the Russian Academy of Sciences (RAS). In the laboratory, total mRNA was isolated using ExtractRNA reagent BC032 (Evrogen) according to the manufacturer’s method, and for the first strand cDNA synthesis, MMLV RT SK021 kit was used according to the protocol of Evrogen. Expression levels of long non-coding RNAs Linc-ROR and MALAT1 were determined by real-time reverse transcription–polymerase chain reaction (RT-PCR) assay using LightCycler 96 Real-Time PCR System (Roche) and specific primers of 18S, Linc-ROR and MALAT1. PCR was carried out with preincubation for 150 s at 95°C, 3-step amplification for 20 s at 95°C, 20 s at 60°C, 20 s at 72°C; the reaction included 45 cycles to detect the melting point of the products.
Normalization of complementary DNA samples was carried out according to the control gene 18S ribosomal RNA. Relative gene expression levels of Linc-ROR and MALAT1 were calculated by the 2-ΔΔCT method. The absence of by-products in PCR was determined by melting curve analysis. For each primer pair in all samples, identical melting peaks in PCR were observed with 3 replicates of each sample. The obtained Ct s (cycle threshold) values for each sample did not exceed the value of 35.
Statistical analysis
Statistical data analysis was performed with MS Excel 2016, Jamovi v.2.0 software package, as well as IBM SPSS Statistics software program, version 22.0. Quantitative variables are presented in the form of tables, that showed the mean, median (Me), standard deviation, interquartile range (Q1; Q3), minimum (Min) and maximum (Max). The hypothesis that distribution of continuous random variables was in accordance with normal distribution was tested using the Shapiro–Wilk test. To analyze n>2 independent groups, a nonparametric analog of the one-way analysis of variance, the Kruskal–Wallis test was used. The results were considered to be statistically significant at p≤0.05. Visualization of quantitative variables was done using raincloud plots. ROC analysis was used to assess the prognostic value of lncRNAs. The results were presented by the values of sensitivity, specificity, PPV, NPV, the Youden index and AUC, and were visualized using ROC curves, as well as plot graphs for cut-off. Selection of threshold values was based on the Youden index.
Results
The analysis of lncRNA expression showed that there was statistically significant difference between the groups in MALAT1 and Linc-ROR expression levels. However, the trends were not universal (Table). The analysis of Linc-ROR expression found that with disease progression from normal endometrium (the control group) to adenocarcinoma, there was consistent increase and decrease in the expression levels of this marker (Fig.1). All findings in the study were statistically significant (p<0.001).
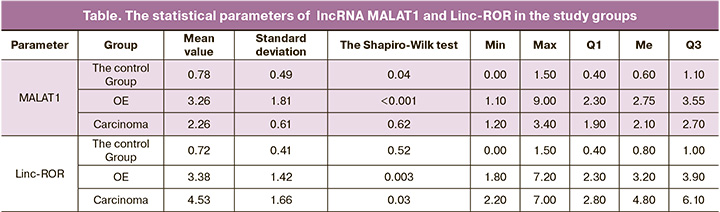
Consistent increase in lncRNA MALAT1 expression level was found with the disease progression (Fig.2)
With purpose of differential diagnosis between normal endometrium and ovarian endometriosis, normal endometrium and carcinoma ROC curves were constructed to evaluate expression of the markers under study – lncRNA MALAT1 (the area under the curve is 98%) and Linc-ROR (the area under the curve is 100%) (Fig. 3, 4).
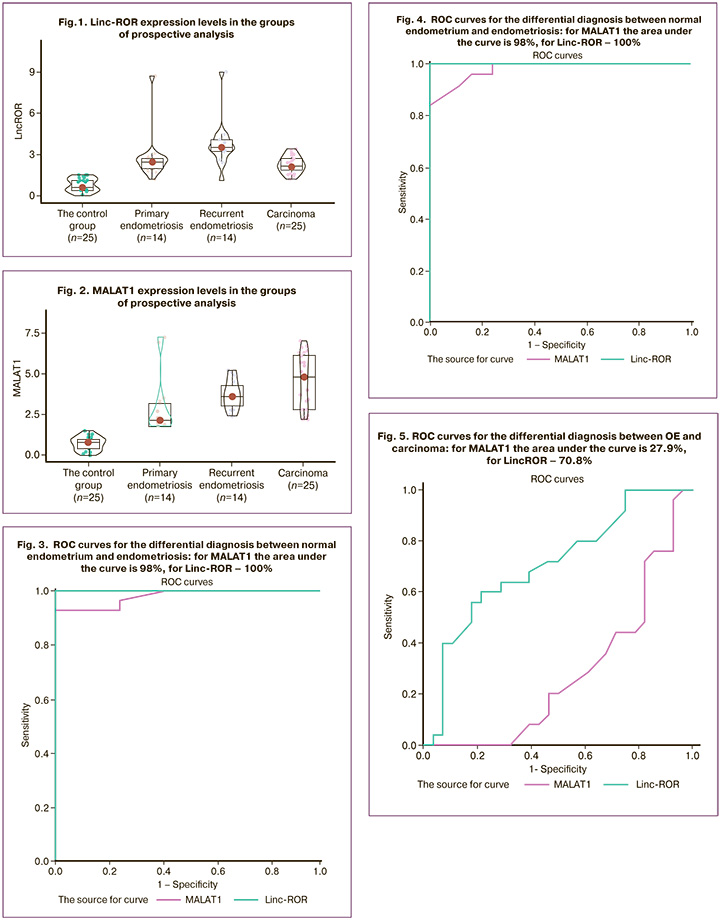
Also, ROC curves were constructed for differential diagnosis between OE and carcinoma: for MALAT1 the area under the curve is 27.9%, for Linc-ROR 70.8% (Fig. 5).
According to the obtained results of constructed ROC curves, as well as to the results of complex statistical analysis of 3 study groups, based on the expression levels of lncRNA Linc-ROR and MALAT1 the following rules for determining the histological status of surgical material were suggested:
- Differential diagnosis between OE and normal endometrium according to MALAT1 expression level: ≤1 equals to norm; level ≥1.6 indicates OE. The interval of 1.1–1.5 indicates the ”grey zone” (there were 12 study patients: 10 from the control group and 2 patients with endometriosis – 15% of the total number of patients);
- Differential diagnosis between OE and ovarian carcinoma according to MALA1 expression level: ≤1.1 equals to norm; ≥1.6 indicates carcinoma. The interval 1.2–1.5 indicates the “grey zone” (there were 10 study patients: 6 in the control group and 4 patients with carcinomas – 12.5% of total number of patients);
- Differential diagnosis between OE and normal endometrium in accordance with Linc-ROR expression level: ≤1.5 equals to norm, >1.5 indicates OE;
- Differential diagnosis between OE and carcinoma in accordance with Linc-ROR expression level: ≤1.5 equals to norm, ≥ 2.2 indicates carcinoma.
Thus, any values of Linc-ROR and MALAT1 expression level are sufficient for differentiation between normal endometrium and the presence of pathology, but for differential diagnosis between carcinoma and OE, it is necessary to build a multi-factor model.
For prognostic purposes, logistic regression was used to analyze a possibility of using the factors, that characterized the group of prospective analysis and showed significant differences in comparison between the subgroups in the control group, the group with OE and ovarian adenocarcinoma (Fig. 6).
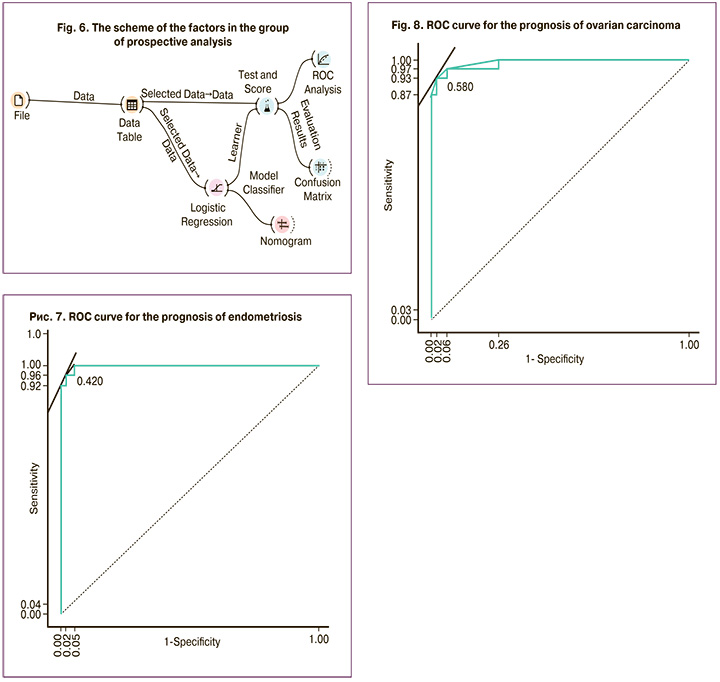
The results of analysis showed that the predictive ability of the logistic regression model is high and close to absolute AUC=0.995 (Fig. 7, 8).
Conclusion
Our study determined expression levels of lncRNA markers MALAT1 and Linc-ROR in the groups of patients with endometriosis, adenocarcinoma and in the control group and suggested their potential possibility for predicting these conditions. It was found that there was statistically significant difference in expression levels of these lncRNAs in the study groups. The study showed that MALAT1 expression level tended to gradual increase in the control group with conditionally intact ovarian tissue versus the group of patients with OE. It should be noted that the identified changes were more pronounced in patients with a combination of OE with internal endometriosis and uterine fibroids, the occurrence of which closely correlates with epithelial-mesenchymal transition. The highest expression level was observed in the group of patients with ovarian adenocarcinoma. Also, a consistent increase in expression level of Linc-ROR was found in the control group and in the group with recurrent forms of OE, respectively. In these groups the levels of expression were higher compared to the group of patients with ovarian adenocarcinomas, where reduction of expression level was observed.
Further targeted researches in this area are necessary to develop new prognostic markers for endometriosis-associated ovarian cancer and search for additional therapeutic targets.
References
- Andrew W.H., Stacey A.M. Pathophysiology, diagnosis, and management of endometriosis. BMJ. 2022; 379: e070750. https//dx.doi.org/10.1136/bmj-2022-070750.
- Coloma J.L., Martínez-Zamora M.A., Collado A., Gràcia M., Rius М., Quintas L. et al. Prevalence of fibromyalgia among women with deep infiltrating endometriosis. Int. J. Gynaecol. Obstet. 2019; 146: 157-63.https//dx.doi.org/10.1002/ijgo.12822.
- Адамян Л.В., Протасова А.Э., Асатурова А.В., Раскин Г.А. Эндометриоз-ассоциированные заболевания, эндометриоз и рак:что общего? Проблемы репродукции. 2022; 28(1): 65-74. [Adamyan L.V., Protasova A.E., Asaturova A.V., Raskin G.A. Endometriosis-associated diseases, endometriosis and cancer: what is in common? Russian Journal of Human Reproduction. 2022; 28(1): 65-74. (in Russian).] https//dx.doi.org/10.17116/repro20222801165.
- Shafrir A.L., Palmor M.C., Fourquet J., DiVasta A.D., Farland L.V., Vitonis A.F. et al. Co-occurrence of immune-mediated conditions and endometriosis among adolescents and adult women. Am. J. Reprod. Immunol. 2021; 86: e13404. https//dx.doi.org/10.1111/aji.13404 pmid:33583078.
- Enabi E., Khazaei S. Endometriosis and migraine headache risk: a meta-analysis. Women Health. 2020; 60: 939-45. https//dx.doi.org/10.1080/03630242.2020.1779905.
- Harris H.R., Korkes KM.N., Li T., Kvaskoff М., Cho Е., Carvalho L.F. et al. Endometriosis, psoriasis, and psoriatic arthritis: a prospective cohort study. Am. J. Epidemiol. 2022; 191(6): 1050-60. https//dx.doi.org/10.1093/aje/kwac009.
- Kvaskoff M., Mahamat-Saleh Y., Farland L.V., Shigesi N., Terry K.L., Harris H.R. et al. Endometriosis and cancer: a systematic review and meta-analysis. Hum. Reprod. Update. 2021; 27(2): 393-420. https//dx.doi.org/10.1093/humupd/dmaa045.
- Давыдов А.И., Михалева Л.М., Пацап О.И. К вопросу о маркерах ранней детекции эдометриоз-ассоциированных опухолей яичника. Вопросы гинекологии, акушерства и перинатологии. 2019; 18(4): 133-7. [Davydov A.I., Mihalyova L.M., Pacap O.I. On markers of early detection of endometriosis-associated ovarian tumors. Gynecology, Obstetrics and Perinatology. 2019; 18(4): 133-7. (in Russian).] https//dx.doi.org/10.20953/1726-1678-2019-4-133-137.
- Farland L.V., Degnan W.J.3rd., Bell M.L., Kasner S.E., Liberman A.L., Shah D.K. et al. Laparoscopically confirmed endometriosis and risk of incident stroke: a prospective cohort study. Stroke. 2022; 53(10): 3116-22.https//dx.doi.org/10.1161/STROKEAHA.122.039250 pmid:35861076.
- Жорданиа К.И., Паяниди Ю.Г., Сонова М.М., Савостикова М.В., Баринов В.В., Калиничева Е.В. Эндометриоз и рак яичников. Продолжение темы. Онкогинекология. 2015: 2: 16-24. [Zhordania K.I., Payanidi Yu.G., Sonova M.M., Savostikova M.V. Endometriosis and ovarian cancer. Continuing the theme. Oncogynecology. 2015; (2): 16-24.]
- Shafrir A.L., Farland L.V., Shah D.K., Harris H.R., Kvaskoff M., Zondervan K. et al. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 51: 1-15. https//dx.doi.org/10.1016/j.bpobgyn.2018.06.001.
- Kanduri C. Long noncoding RNAs: Lessons from genomic imprinting. Biochim. Biophys. Acta. 2016; 1859(1): 102-11. https//dx.doi.org/10.1016/j.bbagrm.2015.05.006.
- Mapanga W., Girdler-Brown B., Singh E. Knowledge, attitudes and practices of young people in Zimbabwe on cervical cancer and HPV, current screening methods and vaccination. BMC Cancer. 2019; 19(1): 845. https//dx.doi.org/10.1186/s12885-019-6060-z.
- Tan Y.T., Lin J.F., Li T., Li J.J., Xu R.H., Ju H.Q. LncRNA-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Communications. 2021; 41(2): 109-20. https//dx.doi.org/10.1002/cac2.12108.
- Kuang Y., Shen W., Zhu H., Huang H., Zhou Q., Yin W. The role of lncRNA just proximal to XIST (JPX) in human disease phenotypes and RNA methylation: The novel biomarker and therapeutic target potential. Biomed. Pharmacother. 2022; 155:113753. https//dx.doi.org/10.1016/j.biopha.2022.113753.
- Yu J., Chen L.H., Zhang B., Zheng Q.M. The modulation of endometriosis by lncRNA MALAT1 via NF-κB/iNOS. Eur. Rev. Med. Pharmacol. Sci. 2019; 23(10): 4073-80. https//dx.doi.org/10.26355/eurrev_201905_17908.
- Mai H., Wei Y., Yin Y., Huang S., Lin H., Liao Y. et al. LINC01541 overexpression attenuates the 17β-Estradiol-induced migration and invasion capabilities of endometrial stromal cell. Syst. Biol. Reprod. Med. 2019; 65(3): 214-22. https//dx.doi.org/10.1080/19396368.2018.1549290.
- Jiang M.C., Ni J.J., Cui W.Y., Wang B.Y., Zhuo W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am. J.Ccancer Res. 2019; 9(7):1354-66.
- Stelzle D., Tanaka L.F., Lee K.K., Khalil A.I., Baussano I., Shah A.S.V. et. al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health. 2021; 9(2): e161-9. https//dx.doi.org/10.1016/S2214-109X(20)30459-9.
- Wilusz J.E. Long noncoding RNAs: Re-writing dogmas of RNA processing and stability. Biochim. Biophys. Acta. 2016; 1859(1): 128-38. https//dx.doi.org/10.1016/j.bbagrm.2015.06.003.
- Yoshimoto R., Mayeda A., Yoshida M., Nakagawa S. MALAT1 long non-coding RNA in cancer. Biochim. Biophys. Acta. 2016; 1859(1): 192-9.https//dx.doi.org/10.1016/j.bbagrm.2015.09.012.
- Liu J., Peng W.X., Mo Y.Y., Luo D. MALAT1-mediated tumorigenesis. Front. Biosci. (Landmark Ed). 2017; 22(1): 66-80. https//dx.doi.org/10.2741/4472.
- Miao H., Wu F., Li Y., Qin C., Zhao Y., Xie M. et al. MALAT1 modulates alternative splicing by cooperating with the splicing factors PTBP1 and PSF. Sci. Adv. 2022; 23; 8(51): eabq7289. https//dx.doi.org/10.1126/sciadv.abq7289.
- Li Y., Liu Y.D., Chen S.L., Chen X., Ye D.S., Zhou X.Y. et.al. Downregulation of long non-coding RNA MALAT1 inhibits granulosa cell proliferation in endometriosis by up-regulating P21 via activation of the ERK/MAPK pathway. Mol. Hum. Reprod. 2019; 25(1): 17-29. https//dx.doi.org/10.1093/molehr/gay045.
- Liang Z., Chen Y., Zhao Y., Xu C., Zhang A., Zhang Q. et. al. MiR-200c suppresses endometriosis by targeting MALAT1 in vitro and in vivo. Stem. Cell. Res. Ther. 2017; 8(1): 251. https//dx.doi.org/10.1186/s13287-017-0706-z.
- Chiu H.S., Somvanshi S., Chen T.W., Sumazin P. Illuminating lncRNA function through target prediction. Methods Mol. Biol. 2021; 2372: 263-95. https//dx.doi.org/10.1007/978-1-0716-1697-0_22.
- Xu X.Y., Zhang J., Qi Y.H., Kong M., Liu S.A., Hu J.J. Linc-ROR promotes endometrial cell proliferation by activating the PI3K-Akt pathway. Eur. Rev. Med. Pharmacol. Sci. 2018; 22(8): 2218-25. https//dx.doi.org/10.26355/eurrev_201804_14807.
- Cho B.J., Choi Y.J., Lee M.J., Kim J.H., Son G.H., Park S.H. et. al. Classification of cervical neoplasms on colposcopic photography using deep learning. Sci. Rep. 2020; 10(1): 13652. https//dx.doi.org/10.1038/s41598-020-70490-4.
- Gatta L.A., Kuller J.A., Rhee E.H.J. Pregnancy outcomes following cervical conization or loop electrosurgical excision procedures. Obstet. Gynecol. Surv. 2017; 72(8): 494-9. https//dx.doi.org/10.1097/OGX.0000000000000468.
- Zeng J., Ma Y.X., Liu Z.H., Zeng Y.L. LncRNA SNHG7 contributes to cell proliferation, invasion and prognosis of cervical cancer. Eur. Rev. Med. Pharmacol. Sci. 2019; 23(21): 9277-85. https//dx.doi.org/10.26355/eurrev_201911_19420.
Received 27.11.2023
Accepted 15.01.2024
About the Authors
Sergey A. Levakov, Dr. Med. Sci., Professor, Head of the Department of Obstetrics and Gynecology, ICM named after N.V. Sklifosovsky, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), +7(495)609-14-00, levakoff@yandex.ru, 119991, Russia, Moscow, Trubetskaya str., 8, bld. 2,https://orcid.org/0000-0002-4591-838X
Tatyana A. Gromova, PhD, Assistant of the Department of Obstetrics and Gynecology, ICM named after N.V. Sklifosovsky, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), +7(495)609-14-00, tgromova928@yandex.ru, 119991, Russia, Moscow, Trubetskaya str., 8, bld. 2,
https://orcid.org/0000-0001-6104-9842
Aynur E. Mamеdova, post-graduate student of the Department of Obstetrics and Gynecology, ICM named after N.V. Sklifosovsky, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), +7(495)609-14-00, 119991, Russia, Moscow, Trubetskaya str., 8, bld. 2, https://orcid.org/0000-0002-9642-4523
Nadezhda V. Antipova, PhD, Senior Researcher at the Laboratory of Membrane and Bioenergetic Systems, Academicians M.M. Shemyakin and Yu.A. Ovchinnikov Institute of Bioorganic Chemistry Russian Academy of Sciences, +7(495)335-01-00, 117997, Russia, Moscow, GSP-7, Miklukho-Maklaya str., 16/10, https://orcid.org/0000-0002-5799-7767
Corresponding author: Tatyana A. Gromova, tgromova928@yandex.ru



