Optimizing management strategy in infertile patients with ovarian endometriosis
Aim. To develop a personalized approach to managing infertile patients with stage III–IV ovarian endometriosis based on the clinical picture, the state of the endometrium, and ovarian reserve. Materials and methods. The study included 147 infertile women with stages III–IV ovarian endometriotic cysts (OEC). Group I consisted of 49 patients who underwent surgery followed by the expectation of spontaneous pregnancy and IVF. Group II comprised 98 patients with personalized management, including preoperative hormonal therapy with GnRH agonist or dienogest, surgery for OEC followed by the expectation of spontaneous pregnancy against the background of cyclic dydrogesterone administration (IIA subgroup, n=40) or complex treatment with dienogest, NSAID, cavity magneto-therapy, central color rhythm therapy, electrical nerve stimulation and expectation of spontaneous pregnancy and IVF (IIB subgroup, n=43). Patients in the IIC subgroup with a critical ovarian reserve (n=15) underwent IVF with oocyte donation. Results. Perioperative hormonal treatment was statistically significantly associated with the preservation and quality of the ovarian reserve, OEC size reduction by 42.5%, and endometrial receptivity and cytokine status normalization. A differentiated approach to the management of infertile patients with severe forms of OEC was associated with a 2-fold increase in the pregnancy rate (ABI 20.2% [95% CI 2.7–34.5], RR 2.04 [95% CI 1.04–4.01], p <0.001) and a 2.9-fold increase in live birth rate (ABI 23.9% [95% CI 7.6–36.7], RR 2.96 [95% CI 1.24–7.08], p <0.001). Conclusion. The study findings objectified the rationality of personalized management of patients with severe OEC compared with patients without an individual approach to infertility treatment.Tezikov Yu.V., Strizhakov A.N., Lipatov I.S., Kalinkina O.B., Aravina O.R., Amosov M.S.
Keywords
Ovarian endometriotic cysts (OEC) are among the most common benign tumors in reproductive age women [1]. Incidence of infertility in patients with OEC is high and, according to research evidence, women with this pathology are 24–45% less likely to have a live birth in assisted reproductive technologies (ART) than women with infertility of unknown origin (OR 0.76 (95% CI 0.59–0.98), p=0.035) [2, 3]. OEC has always presented a problem in choosing strategies for infertility management.
Today, there are several views on the management of infertility and OEC. The difficulty in choosing fertility treatment for women with OEC is related to several aspects. The most important among them is the safety and quality of the ovarian reserve and the state of the endometrium, its readiness for the complete formation of the early placenta, and the maternal-placental-fetal unit [1, 3]. The presence of pelvic pain and very large endometriomas may require surgery followed by assisted reproductive technologies (ART) if pregnancy does not occur after 9–15 months [4]. However, surgery for such endometriotic lesions can alter ovarian reserve and the response to ovarian stimulation in ART. Several studies have shown that OECs do not damage the ovarian reserve and rarely impair the quality of oocytes [5, 6]. Other studies reported that the presence of OEC significantly affects iron metabolism and the activity of follicular fluid enzymes, increases oxygen free radicals concentration, and leads to a deterioration in the quality of eggs [7]. Some studies [6, 8] suggest a link between the size of endometriomas and the state of the ovarian reserve, which is usually not considered when preparing for ovarian surgery.
At the same time, careful removal of the OEC can increase the effectiveness of ART [4, 5]. However, contrary findings were noted by other studies suggesting that cystectomy for OEC can impair ovarian response to controlled ovarian hyperstimulation in ART cycles and significantly reduce ovarian reserve [9]. In stage III–IV OEC, surgical treatment is a necessary step to restore the organ anatomy. In case of the persistence of infertility, it is recommended to decide on the use of ART [8]. Some research papers report convincing data on the positive effect on the ovarian reserve and endometrial receptivity of adjuvant hormonal therapy after surgery, aimed at inhibiting the progression of the endometriotic process and preventing relapse [10, 11]. In the available literature, there is no unified algorithm for managing patients with severe OEC in terms of the recommended expectation of spontaneous pregnancy and the timing of starting ART. There is also no evidence base on preserving the ovarian reserve and receptor sensitivity of the eutopic endometrium at the stages of the desired pregnancy preparation.
To objectify the effect of surgical treatment of OEC on the ovarian reserve, improve ovarian protection and endometrium preparation for pregnancy, and develop a program for managing infertile patients with severe ovarian endometriosis, we conducted a prospective study.
The study aimed to develop a personalized approach to managing infertile patients with stage III–IV ovarian endometriosis based on the clinical picture, the state of the endometrium, and ovarian reserve.
Materials and methods
The prospective study included 147 infertile women with stages III–IV ovarian endometriotic cysts. Group I included 49 patients who underwent surgery for stage III–IV ovarian endometriosis. Surgical treatment consisted of laparoscopic exfoliation of ovarian cysts with concomitant cauterization of visible endometriotic foci and adhesiolysis. If spontaneous pregnancy does not occur after six months of expectant management, the patients were referred for infertility treatment using ART according to the current clinical protocol [1]. Group II comprised 98 infertile patients with stages III–IV OEC. All patients received a gonadotropin-releasing hormone agonist (GnRH agonist) (3.75 mg intramuscular Buserelin, once every four weeks, starting in the first five days of the menstrual cycle) for three months or synthetic progestogens (oral daily dienogest 2 mg) at least 4–5 months. Before surgery, 43.9% (43/98) of patients with severe pain received GnRH agonist, 56.1% (55/98) with moderate and mild pain received dienogest. After surgery, the patients of group II were divided into three subgroups categorized by ovarian reserve and eutopic endometrium receptor activity, which determined the personalized management strategies.
Subgroup IIA consisted of 40 patients with reduced ovarian reserve (AMH not less than 0.5 and FSH not more than 10 IU/ml) and preserved eutopic endometrium receptor activity, in whom expectant strategy for spontaneous pregnancy was chosen. These patients received dydrogesterone in a cyclic regimen in the postoperative period to support the II phase of the menstrual-ovarian cycle (10 mg t.d.s. from the 11th to the 25th day of the cycle). At the onset of pregnancy, the drug administration was prolonged to reduce the risk of spontaneous miscarriage. In the absence of pregnancy for six months, ART was used to treat infertility. Group IIB included 43 patients also with moderately reduced ovarian reserve but impaired endometrial receptivity. After surgical treatment, patients in this group received complex therapy, including dienogest 2 mg daily for 3–4 months to increase the endometrial receptor sensitivity and prevent the progression of endometriosis, non-steroidal anti-inflammatory drugs (NSAID) rectal diclofenac 200 mg daily up to one month to correct the pro-inflammatory status. To improve lymph- and microcirculation, nerve conduction, relieve pain, reduce stress and the severity of psychosomatic reactions from day five after the operation, patients received physiotherapeutic treatment including ten procedures using the Androgin apparatus (FSR certificate No. 2008/02913 dated 05.06 .2008, RF), cavity magneto-therapy, central color rhythm therapy, short-pulse electroanalgesia (percutaneous electroneurostimulation) [12]. If spontaneous pregnancy does not occur after six months of expectant management, the patients were referred for infertility treatment using ART. Group IIC consisted of 15 patients with critically reduced ovarian reserve after surgical treatment (AMH below 0.5 ng/ml) and normal/reduced eutopic endometrium receptor function, referred for the treatment of infertility by ART with oocyte donation. The control group III consisted of 35 healthy women with intact ovaries and male factor infertility, treated with ART. Stimulation of ovulation in IVF cycles was carried out according to the standard method using the long protocol of GnRH agonist.
Criteria for inclusion in groups I and II were: histologically confirmed diagnosis of OEC, reproductive age up to 35 years, first surgical treatment of ovarian endometriosis, consent to participate in the study, and data processing. Exclusion criteria from the study were obesity and concomitant severe somatic comorbidities, autoimmune diseases, sexually transmitted infections (STIs), chronic endometritis, severe male factor infertility (azoospermia requiring testicular puncture), as well as non-compliance with administered therapy revealed during the survey of patients.
Patients were examined at the stage of diagnosis and clarification of the cause of infertility before surgery (group I) or preoperative hormonal therapy (group II), on day three after surgery, and before ART. Anamnesis was collected from all women with an assessment of complaints and menstrual function. Pain was evaluated on a visual analogue scale (VAS, where 0 = no pain, 1–2 = mild pain, 3–5 = moderate pain, 6 or more = severe pain [1]). Based on surgery outcomes, the fertility index was calculated in the study groups [4]. During follow-up, all patients underwent pelvis ultrasound examination with Doppler (Philips Affinity 70, USA) and measuring antral follicle count (AFC), based on the recommendations of S.G. Khachkuzov [13]. Enzyme-linked immunosorbent assay, chemiluminescence immunoassay on Architect equipment (Abbot, England) was used on up to day 7 of the menstrual cycle to determine tumor markers – CA-125, HE-4 with the calculation of the risk index for ovarian malignancy (ROMA index) [1]; blood hormones - LH, FSH, TSH, prolactin, AMG, estradiol; in the second phase of the cycle (19-23 days): venous blood progesterone, cytokines interleukin (IL)-1β, IL-6, alpha-2-microglobulin fertility (glycodelin) in menstrual blood, pipelle endometrial biopsy was performed for histological and immunohistochemical analyses to rule out chronic endometritis. Expression of estrogen receptors (ER) and progesterone (RP) in endometrial samples (Biocare test system, Germany) was determined by monoclonal antibodies against estrogens (clone SP1, IgG isotype) and progesterone (clone PGR 16, IgG1 isotype). The counting of cells expressing ER and RP was carried out according to G.A. Frank et al. [14]. Analysis of the vaginal and cervical canal discharge for STIs and microflora was carried out by the real-time polymerase chain reaction.
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics 25 PS software, license No. 5725-A54. The distribution of continuous variables was tested for normality using the Shapiro-Wilk and the Kolmogorov-Smirnov tests. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD), and the statistical significance of the differences was determined by one-way analysis of variance (ANOVA). Tukey's test was used for post-hoc comparisons for significant differences. The equality of variance was assessed by Levene's test. Non-normally distributed data were expressed as medians and IQR [25 quartiles (Q1) –75 quartile (Q3)]. Kruskal–Wallis test was used for comparing numerical data between groups, followed by pairwise comparison using the Mann–Whitney U-test with Bonferroni correction for multiple comparisons. The critical level of significance when testing statistical hypotheses were considered at p<0.05. When using the Bonferroni correction for pairwise comparison of 4 groups, the number of comparison pairs is six and the new critical level corresponded to p<0.008; for pairwise comparison of 3 groups, the number of comparison pairs is three, and the new critical level corresponded to p<0.017. The Wilcoxon T-test for paired samples was used to compare paired groups (before and after treatment). The Pearson χ² test with the Yates correction was used to compare categorical variables. Correlation analysis was conducted by calculating Spearman's rank correlation coefficients. The sample size was estimated according to Altman's nomogram, taking into account the preliminary study data. With the given parameters to ensure statistical power of 80%, considering an increase in live birth rate by 30% to be clinically significant, a sufficient number of groups will be from 34 observations. The effectiveness of personalized treatment was assessed effect size indicators: EER – experimental event rate, taking into account the onset of pregnancy and live birth; CER – control event rate in group I; RR – relative risk; RBI – relative benefit increase; ABI - absolute benefit increase; NNT is the number needed to treat to prevent an adverse outcome in one patient. A 95% confidence interval (95% CI) was calculated [15, 16].
Results and discussion
The medical and social characteristics and intraoperative data of the study participants are presented in Table 1. At the stage of inclusion in the prospective study, which, taking into account the duration of pregnancy, lasted up to 22 months, the patients of the comparison groups did not differ in age, duration of infertility and parity, social status, body mass index (BMI), thyroid function, and prolactin levels. Patients in groups I and II did not differ in terms of clinical data, the severity of pain, the initial size of OEC, endometrial thickness, the concentration of glycodelin, interleukins, characteristics of ovarian reserve (AFC, FSH, AMH, estradiol), levels of tumor markers and ROMA index. These characteristics had significant differences in comparison with group III (control group) (p<0.001).
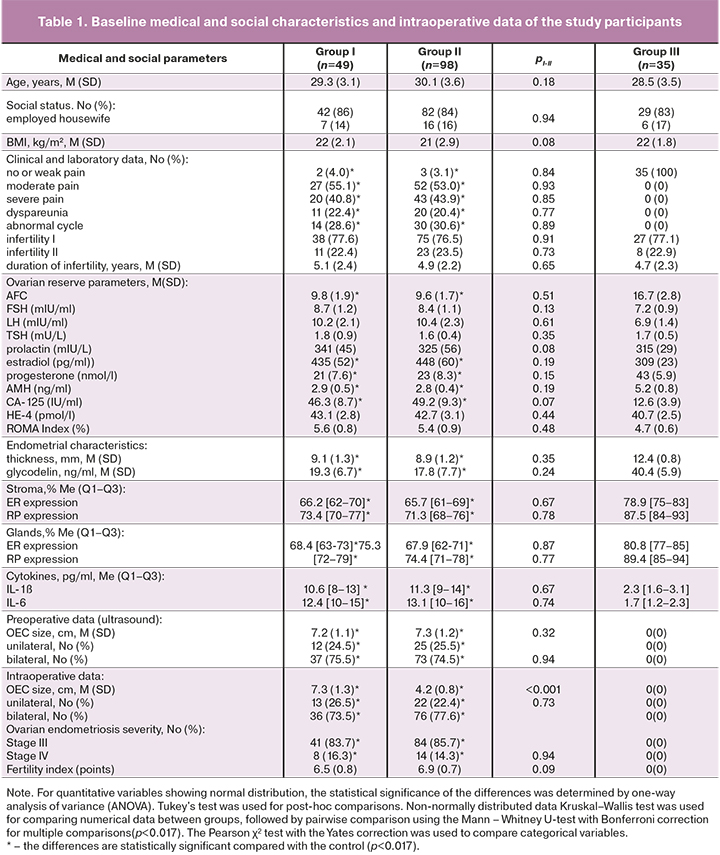
After a pipelle biopsy of the endometrium, no statistically significant differences were found in the levels of expression of RP and RE by the eutopic endometrium of glands and stroma in patients with OEC (pI-II>0.05). Patients in the control group had a statistically significant higher expression of ER and RP in the stroma and the glands compared to that in patients with severe OEC and infertility (p<0.001) (Table 1).
Analysis of intraoperative findings showed a decrease in the mean OEC size in group II compared with the baseline ultrasound data before the start of hormonal treatment (from 7.3 (1.2) cm to 4.2 (0.8) cm). This change was statistically significantly different from intraoperative OEC sizes in group I without preoperative hormonal treatment [4.2 (0.8) cm versus 7.3 (1.3) cm, p<0.001)]. The severity of endometriosis, uni- or bilateral location of OEC did not differ (Table 1), as well as in the fertility index taking into account intraoperative and anamnestic data did not show statistically significant differences in groups I and II [scores 6.5 (0, 8) vs. 6.9 (0.7) (p=0.09)]. The fertility index values indicate the possibility of spontaneous pregnancy in women of the comparison groups with a probability of 40% [1, 4].
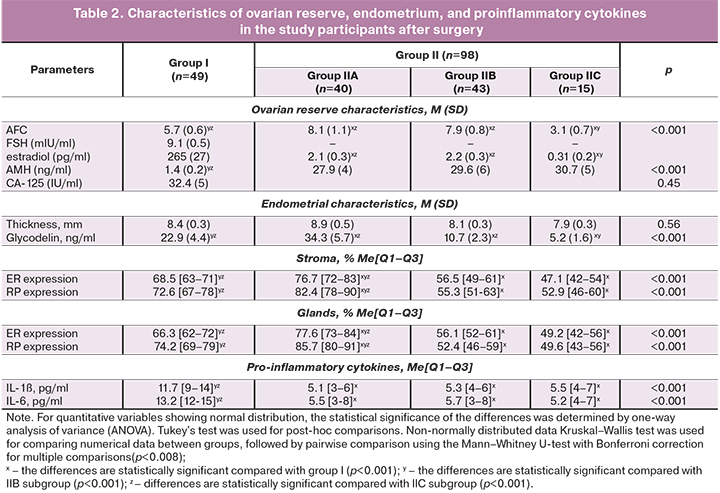
Findings of patients in group I and IIA, IIB, IIC subgroups of group II after surgery are presented in Table 2.
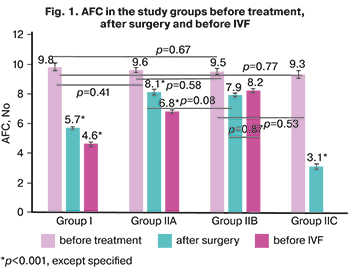 In group I, there was a statistically significant increase in the level of FSH and a decrease in estradiol concentration after surgery compared with the baseline parameters (p=0.04 and p=0.03, respectively), which is due to the aggressive effect of surgical intervention on the ovaries on the ovarian reserve (decrease AFC from 9.8 (1.9) to 5.7 (0.6), p<0.001, and AMH level from 2.9 (0.5) ng/ml to 1.4 (0.2) ng/ml, p<0.001). Intra-group dynamics and inter-group comparisons of the AFC are presented in Figure 1.
In group I, there was a statistically significant increase in the level of FSH and a decrease in estradiol concentration after surgery compared with the baseline parameters (p=0.04 and p=0.03, respectively), which is due to the aggressive effect of surgical intervention on the ovaries on the ovarian reserve (decrease AFC from 9.8 (1.9) to 5.7 (0.6), p<0.001, and AMH level from 2.9 (0.5) ng/ml to 1.4 (0.2) ng/ml, p<0.001). Intra-group dynamics and inter-group comparisons of the AFC are presented in Figure 1.
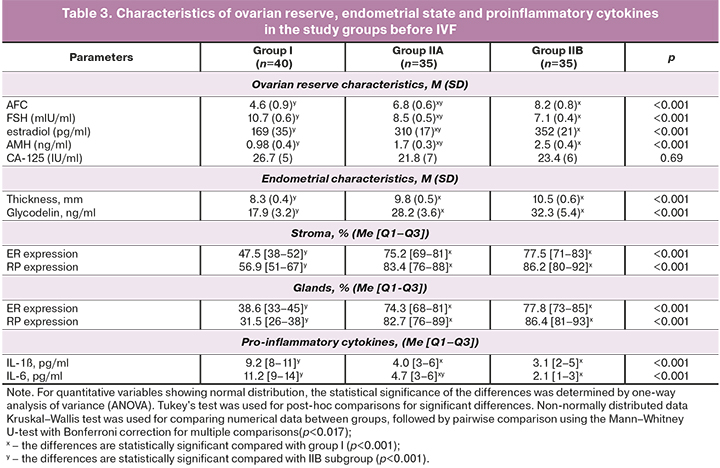
The findings about the adverse impact of surgical treatment of stage III–IV OEC on the ovarian reserve served as the basis for a personalized approach to the management of patients in group II in terms of achieving the desired pregnancy. Patients in subgroup IIC with a significantly reduced ovarian reserve were immediately referred for ART with oocyte donation. In group II, 15 out of 98 (15.3%) of patients with critical ovarian reserve required ART with oocyte donation, despite the preoperative hormonal therapy. This frequency of urgent ART use in group II was comparable to group I, where oocyte donation was performed in 8 out of 49 patients (16.3%), (pI-IIC=0.79). Patients in IIB subgroup (43/98 – 43.9%) with moderately reduced ovarian reserve and reduced endometrial receptivity (for ER stroma and glands pIIB-III<0.001; for RP stroma and glands pIIB-III<0.001; for endometrial thickness pIIB-III=0.01) received complex postoperative treatment with subsequent spontaneous pregnancy, in the absence of which infertility was treated with IVF. Patients in IIА subgroup (40/98 – 40.8%) also with moderately reduced ovarian reserve, but with normal receptivity of the endometrium (for ER stroma pIIА-III=0.86, glands pIIА-III=0.92; for RP stroma pIIА-III=0.79, glands pIIA-III=0.84) were sent to achieve spontaneous pregnancy; if it did not occur they were sent for IVF. The characteristics of patients of group I and subgroups of group II before IVF are presented in Table 3.
Comparing the findings on ovarian reserve in severe OEC with the results of studies by other authors [16], it becomes evident that the question of adequate ovarian protection is relevant and timely. Several studies have convincingly shown that infertility in patients with OEC can be caused by altered folliculogenesis, decreased preovulatory steroidogenesis of granulosa cells, and activation of the immune response against follicles [17]. Consequently, maintaining the maximum possible ovarian reserve during infertility treatment is the key to achieving pregnancy.
It is well known that sex steroid genes are highly expressed in the eutopic endometrium of women with endometriosis. Increased secretion of progesterone after ovulation is required to downregulate genes associated with DNA replication, a mechanism by which endometrial proliferation is rapidly stopped in the early secretory phase [18]. Insufficient endometrial secretion and the inability to suppress cell cycle genes are likely key factors in implantation failure, increasingly recognized in endometriosis-associated infertility [17]. The putative characteristics of the implantation window include upregulation of progesterone target genes that facilitate embryo attachment and regulate the local immune response. At the same time, it is widely known that glycodelin, an immunomodulator regulated by progesterone, decreases during the implantation window in patients with OEC [8]. Abnormal secretory transformation of endometrial cells in OEC is additionally confirmed by the data obtained during the study on the level of fertility alpha-2-microglobulin (p<0.001), a decrease in the expression of ER and RP, both in the stromal and glandular components of the eutopic endometrium in patients with severe forms of OEC, compared with the control group (p<0.001).
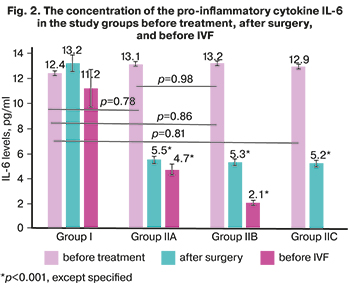 The exact mechanism of progesterone resistance in endometriosis remains unclear. However, it has been proven that a decrease in progesterone levels leads to increased local pro-inflammatory cytokines, chemokines, which interfere with biological pregnancy, activate tissue destruction and menstruation. The anti-inflammatory properties of progesterone in uterine cells have been well studied [11, 17]. Undoubtedly, the cytokines themselves also play an essential role in the formation of progesterone resistance. Tumor necrosis factor (TNFα), IL-6, IL-1ß directly reduce the levels of both RP isoforms, possibly through epigenetic modifications [18]. The findings on cytokine levels in the dynamics of prospective observation of patients of group I and subgroups of group II with severe OEC are presented in Tables 1–3 and Figure 2.
The exact mechanism of progesterone resistance in endometriosis remains unclear. However, it has been proven that a decrease in progesterone levels leads to increased local pro-inflammatory cytokines, chemokines, which interfere with biological pregnancy, activate tissue destruction and menstruation. The anti-inflammatory properties of progesterone in uterine cells have been well studied [11, 17]. Undoubtedly, the cytokines themselves also play an essential role in the formation of progesterone resistance. Tumor necrosis factor (TNFα), IL-6, IL-1ß directly reduce the levels of both RP isoforms, possibly through epigenetic modifications [18]. The findings on cytokine levels in the dynamics of prospective observation of patients of group I and subgroups of group II with severe OEC are presented in Tables 1–3 and Figure 2.
Correlation analysis between indicators of ovarian reserve (AFC, AMG, FSH), endometrial receptivity (thickness, ER, RP, glycodelin), and the concentration of IL-1β, IL-6 showed a strong negative correlation in group I (r from 0.84 to 0, 9, at p<0.001) and a moderate positive correlation in IIA and IIB subgroups (r from 0.72 to 0.79, at p<0.001), which confirms a close immune and hormonal interactions in the regulation of the reproductive system [ 19, 20].
The female reproductive organs are innervated by sympathetic, parasympathetic, and sensory afferent nerves. Their distribution and immunoreactivity to various peptides indicate a role in hemodynamics and smooth muscle contraction. In the uterus of patients with severe OEC and severe chronic pelvic pain, there is an increased number of nerve fibers and perivascular proliferation of nerve fibers [10]. Besides, small nerve fibers were found in the functional layer of the eutopic endometrium in patients with endometriosis but not in controls [8]. Clinical symptoms of endometriosis range from severe dysmenorrhea, dyspareunia, dysuria, to severe chronic pelvic pain. Obviously, the disease seriously affects patients’ quality of life, social life, sexuality, and psychological well-being. This served as a pathogenetic rationale for applying a personalized approach to the management of infertile patients with stage III–IV ovarian endometriosis.
Analysis of the parameters affecting infertility treatment outcomes in the study groups revealed the following patterns. At each stage of the examination, patients in group I had statistically significant differences in the levels of AFC and AMH, which confirms the alteration of the ovarian reserve due to surgical trauma. At the same time, in IIA and IIB subgroups, we did not find significant differences in the level of AFC and AMH after surgery, compared with baseline [for IIA subgroup – 8.1 (1.3) versus 9.6 (1.7), p=0.08, and 2.1 (0.3) ng/ml versus 2.8 (0.4) ng/ml, p=0.13; for IIB – 7.9 (0.8) versus 9.6 (1.7), p=0.06, and 2.2 (0.3) ng/ml versus 2.8 (0.4) ng/ml, p=0.11, respectively], which is explained by the ovario-protective effect of preoperative hormonal therapy with GnRH agonist or dienogest. Besides, the AFC level in subgroup IIB, in contrast to subgroup IIA, as a result of complex treatment before IVF was stable in comparison with postoperative data [8.2 (0.8) and 7.9 (0.8), p=0, 87], and the AMH level is even higher [2.5 (0.4) ng/ml and 2.2 (0.3) ng/ml, p=0.06], which is undoubtedly associated with quantitative and qualitative preservation of the ovarian reserve, anti-inflammatory effect, increased sensitivity to progesterone, blocking the progression of endometriosis, improvement of lymph- and microcirculation, nerve conduction, antinociceptive, anti-stress mechanisms of complex treatment with the use of non-steroidal anti-inflammatory drugs, central dienogest, hip magneto-therapy, color stimulation therapy.
There were significant differences in endometrial thickness both in intergroup comparisons and within the studied groups. The endometrial thickness after surgery did not differ in subgroup IIB and IIC (p=0.06). It was statistically lower than that in subgroup IIA (p=0.01), which is confirmed by effective preoperative hormonal correction of endometrial receptor expression in this subgroup. At the same time, the endometrial thickness increased statistically significantly in IIA and IIB subgroups before IVF (p=0.01 and p<0.001 – respectively), which is due to the support of dydrogesterone of the second phase of the menstrual-ovarian cycle in women in IIA subgroup and the effect of dienogest on the endometrium in women IIB subgroups.
Against the background of staged personalized treatment (preoperative hormonal therapy, postoperative hormonal treatment in subgroup IIA, and complex treatment in subgroup IIB), the level of proinflammatory cytokines significantly decreased in group II. It remained low before IVF (Fig. 1).
Protocol parameters and IVF outcomes in the groups are presented in Table 4.
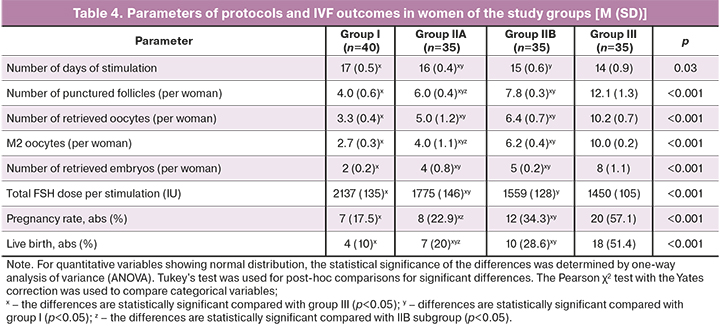
The spontaneous pregnancy rate in women of group I within six months after surgery was only 2% (1/49), which can be explained by the effect of surgical trauma on the ovarian reserve and low receptivity of the endometrium. According to the results of the postoperative assessment of the ovarian reserve, 8 (8/49 – 16.3%) patients underwent ART with oocyte donation with a favorable outcome (live birth). Of 40 women who entered the IVF program, pregnancy occurred in 7 (17.5%) patients, of whom three (3/7 – 42.9%) had a spontaneous miscarriage, in four (4/40 – 10%), the pregnancy ended with the birth of a live child. In group I, the pregnancy rate was 32.7% (16/49), except for IVF using oocyte donation – 19.5% (8/41). Live births occurred in 26.5% (13/49), without IVF with oocyte donation – in 12.5% (5/41) women.
In subgroup IIA (n=40), after preoperative hormonal therapy, surgical treatment of OEC, against the background of cyclic use of dydrogesterone for six months, spontaneous pregnancy occurred in 12.5% (5/40) of women. Thirty-five women entered the IVF program, of whom pregnancy occurred in 8 (22.9%), of whom miscarriage – in 1 (1/8 – 12.5%) patient, live birth – in 7 (7/35 – 20%) of patients. In total, pregnancies in subgroup IIA occurred in 32.5% (13/40) of women; live births occurred in 30% (12/40) of patients.
In subgroup IIB (n=43), after preoperative hormonal treatment, surgery, and complex therapy, spontaneous pregnancy within 6–8 months occurred in 18.6% (8/43) of women. Thirty-five women entered the ART program. Pregnancy occurred in 12 (12/35 – 34.3%) patients, of whom miscarriage occurred in 2 (2/12 – 16.7%), and live births in 10 (10/35 – 28.6%). In total, pregnancies in subgroup IIB occurred in 46.5% (20/43) of women; live births took place in 41.9% (18/43) of patients.
In the IIC subgroup, all 15 patients with critical ovarian reserve underwent IVF with oocyte donation with favorable perinatal outcomes.
In total, in group II, the pregnancy rate was 48.9% (48/98), except for IVF with the use of oocyte donation – 39.8% (33/83), live births occurred in 45.9% (45/98), without IVF with the use of oocyte donation – in 36.1% (30/83) women.
In the control group, including women receiving ART for male infertility factor the pregnancy rate was 57.1% (20/35) and 51.4% (18/35) live birth.
Therefore, the effectiveness of infertility treatment in patients with severe forms of OEC in group II with personalized management was higher than in group I of patients without an individualized management strategy. Thus, the pregnancy rate in group II was 1.5 times higher (and doubled after excluding women with oocyte donation). The live birth rate was1.7 times higher (and 2.9 times higher after excluding women with oocyte donation).
To calculate the effect of therapeutic intervention, infertility treatment outcomes were taken into account in patients in groups II and I except for women immediately referred to IVF with oocyte donation due to the critical level of ovarian reserve. The number of patients with confirmed pregnancy was 33 and 8. Pregnancy did not occur in 50 and 33. Live birth occurred in 30 and 5, live birth was absent in 53 and 36, the total number of patients in the groups was 83 and 41, respectively.
Key treatment effect size characteristics in group II with a differentiated approach compared with group I without a personalized approach are presented in Table 5.
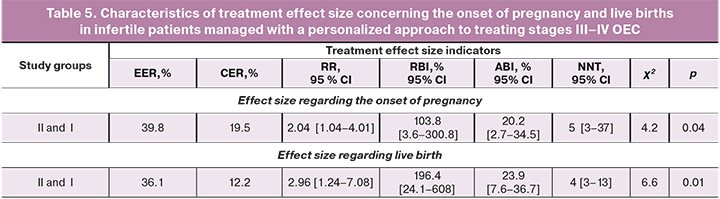
The NNT indicates that a differentiated approach to managing patients with severe forms of OEC, compared with the absence of a personalized approach, resulted in the onset of pregnancy and live birth in every 5 and 4 women, respectively. The RR indicating the ratio of pregnancy rates in each group shows that with a personalized approach, pregnancies and live births occur 2.04 and 2.96 times more often than in the absence of differentiated management of patients with severe OEC stages. The absolute benefit increase indicates a 20.2% and 23.9% increase in pregnancy rate and live birth by 20.2% and 23.9% in group II. Therefore, the chance of giving birth to a living child in patients of group II is higher than in group I. Based on the obtained treatment effect size indicators, we can state a significant effect of personalized therapeutic measures in group II.
OEC, and especially stage III–IV ovarian endometriosis, is a complex therapeutic problem regarding the progression of the disease, anti-relapse treatment, and overcoming infertility [4, 6, 8, 10, 11]. Different approaches to managing infertility in OEC reported in meta-analyzes and individual studies are objective, since this group of patients is heterogeneous. At the same time, the effectiveness of overcoming infertility depends on several individual factors including age, clinical course of the disease, the size of the OEC, uni- or bilateral ovarian lesions, quantitative and qualitative characteristics of the ovarian reserve, the state of endometrial receptivity, the presence of comorbidities, a history of surgical interventions on the ovary, the recurrence rate, etc. [1, 3]. Currently, there are various directions in the treatment of infertility in ovarian endometriosis. They include surgical or hormonal methods followed by the expectation of spontaneous pregnancy, a surgical method with adjuvant hormonal treatment or hormonal cycle support and subsequent expectation of spontaneous pregnancy, initial / urgent ART, individual selection of various IVF protocols, the use of ovulation induction protocols with embryo cryopreservation, and other options for ART. The lack of a specific algorithm for managing patients with unfulfilled reproductive potential and severe OEC is associated with individual risk factors and the optimal use of surgical and reproductive approaches.
Conclusion
The proposed differentiated approach to managing patients with stage III–IV OEC suggests the personification of treatment strategy depending on the clinical course of the disease, ovarian reserve, and the state of the endometrium.
The results of a prospective study comparing the effectiveness of infertility treatment in women with stages III–IV OEC by various approaches showed that differentiated, personalized management according to the developed algorithm resulted in a double pregnancy rate and a 2.9-fold higher live birth rate compared with patients without an individual approach to managing infertility. The study findings objectify the rationality of personalized management of infertile patients with severe OEC and optimize the possibility of fulfilling reproductive potential in this patient category.
References
- Адамян Л.В., ред. Эндометриоз: Диагностика, лечение и реабилитация. Клинические рекомендации по ведению больных. М.; 2016. 66с. [Adamyan, L.V., ed. Endometriosis: Diagnostics, treatment and rehabilitation, clinical recommendations about maintaining patients. Moscow; 2016. 66 p. (in Russian)].
- Hong S.B., Lee N.R., Kim S.K., Kim H., Jee B.C., Suh C.S. et al. In vitro fertilization outcomes in women with surgery induced diminished ovarian reserve after endometrioma operation: Comparison with diminished ovarian reserve without ovarian surgery. Obstet. Gynecol. Sci. 2017; 60(1): 63-8. https://dx.doi.org/10.5468/ogs.2017.60.1.63.
- Чернуха Г.Е., Марченко Л.А., Гусев Д.В. Поиск оптимальных решений и пересмотр тактики ведения пациенток с эндометриозом. Акушерство и гинекология. 2020; 8: 12-20. [Chernykha G.E., Marchenko L.A., Gusev D.V. Searching for optimal decisioms and revising management tactics for patients with endometriosis. Obstetrics and Gynecology. 2020; 8: 12-20. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.8.12-20.
- Andrew S., Cook G., Adamson D. The role of the endometriosis fertility index (EFI) and endometriosis scoring systems in predicting infertility outcomes. Curr. Obstet. Gynecol. Rep. 2013; 2: 186-94. https://dx.doi.org/10.1007/s13669-013-0051-x.
- De Ziegler D., Pirtea P., Carbonnel M., Poulain M., Cicinelli E., Bulletti C. et al. Assisted reproduction in endometriosis. Best Pract. Res. Clin. Endocrinol. Metab. 2019; 33(1): 47-59. https://dx.doi.org/10.1016/j.beem.2018.10.001.
- Tanbo T., Fedorcsak P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 2017; 96(6): 659-67. https://dx.doi.org/10.1111/aogs.13082.
- Coccia M.E., Rizzello F., Capezzuoli T., Evangelisti P., Cozzi C., Petraglia F. Bilateral endometrioma excision: surgery-related damage to ovarian reserve. Reprod. Sci. 2019; 26(4): 543-50. https://dx.doi.org/10.1177/1933719118777640.
- Safdarian L., Ghalandarpoor Attar S.N., Aleyasin A., Aghahosseini M., Sarfjoo F.S., Hosseinimousa S. Investigation of anti-mullerian hormone (AMH) level and ovarian response in infertile women with endometriosis in IVF cycles. Int. J. Reprod. Biomed. 2018; 16(11): 719-22.
- Khan K.N., Fujishita A., Kitajima M., Hiraki K., Nakashima M., Masuzaki H. Occult microscopic endometriosis: undetectable by laparoscopy in normal peritoneum. Hum. Reprod. 2014; 29(3): 462-72. https://dx.doi.org/10.1093/humrep/det438.
- Murji A., Biberoğlu K., Leng J., Mueller M.D., Römer T., Vignali M. et al. Use of dienogest in endometriosis: a narrative literature review and expert commentary. Curr. Med. Res. Opin. 2020; 36(5): 895-907. https://dx.doi.org/10.1080/03007995.2020.1744120.
- Nguyen T.T., Hachisuga T., Urabe R., Ueda T., Kurita T., Kagami S. Immunohistochemical analysis of the effect of dienogest on ovarian endometriotic cysts. J UOEH. 2016; 38(4): 271-8. https://dx.doi.org/10.7888/juoeh.38.271.
- Арсланян К.Н., Маланова Т.Б., Стругацкий В.М. Физиотерапия в практике акушера-гинеколога: клинические аспекты и рецептура. М.: МЕДпресс-информ; 2013. 248с. [Arslanyan K.N., Malanova T.B., Strugazkiy V.M. Physiotherapy in the practice of an obstetrician-gynecologist: clinical aspects and formulation. Moscow: MEDpress-inform; 2013. 248 p. (in Russian)].
- Хачкузов С.Г. УЗИ в гинекологии. Симптоматика. Диагностические трудности. Руководство для врачей. CПб.: ЭЛБИ-СПб; 2016. 672c. [Khachkuzov S.G. Ultrasoundingynecology. Symptomatology. Diagnostic difficulties. Manual for doctors. Saint-Petersburg: ELBI-SPb; 2016. 672p. (in Russian)].
- Kumar G.L., Rudbeck L., ред. Иммуногистохимические методы. Руководство. Пер. с англ. Франк Г.А., Мальков П.Г., ред. М.; 2011. 223с. [Kumar G.L., Rudbeck L., eds. Immunohistochemical staining methods. Education guide. 5th ed. Carpenteria, CA: Dako North America; 2009.]
- Котельников Г.П., Шпигель А.С. Доказательная медицина. Научно обоснованная медицинская практика: монография. 2-е изд. М.: ГЭОТАР-Медиа; 2012. 239с. [Kotelnikov G.P., Shpigel A.S. Evidence-based medicine. Evidence-based medical practice: monograph. 2nd ed. Moscow: GEOTAR Media; 2012. 239 p. (in Russian)].
- Липатов И.С., Тезиков Ю.В., Санталова Г.В., Овчинникова М.А. Профилактика рецидивов герпетической инфекции у беременных и внутриутробного инфицирования плода вирусом простого герпеса. Российский вестник акушера-гинеколога. 2014; 14(4): 63-8. [Lipatov I.S. Tezikov Yu.V. Santalova G.V. Ovchinnikova M.A. Prevention of recurrent herpes infection in pregnant women and intrauterine infection of the fetus with the herpes simplex virus. Russian Bulletin of the obstetrician-gynecologist. 2014; 14(4): 63-8. (in Russian)].
- Пшеничнюк Е.Ю., Асатурова А.В., Адамян Л.В., Зайцев Н.В. Иммуногистохимические предикторы рецидивирования эндометриоидных кист яичников после лапароскопического оперативного лечения. Архив патологии. 2018; 80(4): 14-20. [Pshenichnyuk E.Yu., Asaturova A.V., Adamyan L.V., Zaitsev N.V. Immunohistochemical predictors of recurrence of endometrioid ovarian cysts after laparoscopic surgical treatment. Pathology Archive. 2018; 80 (4): 14-20. (in Russian)].
- Tamura H., Yoshida H., Kikuchi H., Josaki M., Mihara Y., Shirafuta Y. et al. The clinical outcome of dienogest treatment followed by in vitro fertilization and embryo transfer in infertile women with endometriosis. J. Ovarian Res. 2019; 12(1): 123. https://dx.doi.org/10.1186/s13048-019-0597-y.
- Máté G., Bernstein L.R., Török A.L. Endometriosis is a cause of infertility. Does reactive oxygen damage to gametes and embryos play a key role in the pathogenesis of infertility caused by endometriosis? Front. Endocrinol. (Lausanne). 2018; 9: 725. https://dx.doi.org/10.3389/fendo.2018.00725.
- Zhou W.J., Yang H.L., Shao J., Mei J., Chang K.K., Zhu R. et al. Anti-inflammatory cytokines in endometriosis. Cell. Mol. Life Sci. 2019; 76(11): 2111-32. https://dx.doi.org/10.1007/s00018-019-03056-x.
Received 15.12.2020
Accepted 25.12.2020
About the Authors
Yurii V. Tezikov, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology No 1, Samara State Medical University, Ministry of Health of Russia.E-mail: yra.75@inbox.ru. ORCID: 0000-0002-8946-501X. 89 Chapaevskaya str., Samara, 443099, Russia.
Alexander N. Strizhakov, Academician of the RAS, Merited Doctor of the RF, Head of the Department of Obstetrics, Gynecology and Perinatology, I.M. Sechenov
First MSMU, Ministry of Health of Russia (Sechenov University). 8-2 Trubetskaya str., Moscow, 119991, Russia.
Igor S. Lipatov, Dr. Med. Sci., Professor at the Department of Obstetrics and Gynecology No 1, Samara State Medical University, Ministry of Health of Russia.
E-mail: i.lipatoff2012@yandex.ru. ORCID: 0000-0001-7277-7431. 89 Chapaevskaya str., Samara, 443099, Russia.
Olga B. Kalinkina, Dr. Med. Sci., Associate Professor, Professor at the Department of Obstetrics and Gynecology No 1, Samara State Medical University,
Ministry of Health of Russia. Е-mail: maiorof@mail.ru. ORCID: 0000-0002-1828-3008. 89 Chapaevskaya str., Samara, 443099, Russia.
Oxana R. Aravina, Teaching Assistant at the Department of Obstetrics and Gynecology No 1, Samara State Medical University, Ministry of Health of Russia.
E-mail: dr.aravina@gmail.com. ORCID: 0000-0002-2107-1508. 89 Chapaevskaya str., Samara, 443099, Russia.
Mikhail S. Amosov, Teaching Assistant at the Department of Obstetrics and Gynecology No 1, Samara State Medical University, Ministry of Health of Russia.
E-mail: og1samsmu @mail.ru. ORCID: 0000-0002-2107-1508. 89 Chapaevskaya str., Samara, 443099, Russia.
For citation: Tezikov Yu.V., Strizhakov A.N., Lipatov I.S., Kalinkina O.B., Aravina O.R., Amosov M.S. Optimizing management strategy in infertile patients with ovarian endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 7: 122-132 (in Russian)
https://dx.doi.org/10.18565/aig.2021.7.122-132



