Analysis of heparan sulfate proteoglucans content in eutopic and heterotopic endometrium in ovarian endometriosis
Aim. To analyze the content of heparan sulfate proteoglycans (HSPGs) in eutopic and heterotopic endometrium in women with ovarian endometriosis (OE). Materials and methods. Surgical samples of endometrioid ovarian cysts and endometrial biopsy specimens obtained from 11 women of reproductive-age in the proliferative phase of the ovarian cycle with stage III OE (according to the American Fertility Society classification) were analyzed using real-time semi-quantitative PCR and immunohistochemistry (IHC). The expression patterns of core proteins of three HSPGs and the enzyme heparanase (HPSE) were assessed. Nonparametric statistical methods were used for data processing. Results. Real-time PCR analysis of the relative expression of core proteins of HSPGs in endometrial heterotopias revealed a 2-fold increase in total transcriptional activity of the gene encoding perlecan (HSPG2) and HPSE compared to eutopic endometrium (p<0,05). IHC analysis of these molecules in surgical samples confirmed these data. The relative expression of HPSE in heterotopias positively correlated with concentration of the tumor marker CA125 in blood serum. Conclusion. HSPG2 and HPSE participate in the pathomorphogenesis of OE, leading to extracellular matrix modification and impairment of intercellular communications. They can be used as markers of stage III OE.Timofeeva Yu.S., Sokolоv D.К., Grigorieva E.V., Volchek А.V., Kazanskaya G.M., Sukhovskikh A.V., Tsidulko A.Yu., Evseeva Ya.M., Кuleshov V.M., Marinkin I.О., Aidagulovа S.V.
Keywords
Endometriosis is a complex syndrome caused by estrogen-dependent chronic progradient inflammatory process that mainly affects the ovaries, as well as other pelvic organs and tissues [1]. Endometriosis is of great social importance, since it occurs in 6-10% of women, including those under 20 years old [2]. At the same time, the endometriosis tend to gradually increase with a prevalence of almost 15% among women worldwide. [3]. Clinical manifestations of pathology include chronic pelvic pain, dysmenorrhea and infertility, the latter is registered in 30–50% of cases; and a long-term asymptomatic course of endometriosis is in 20–25% of patients [4].
One of the theories of the pathogenesis of endometriosis is associated with the migration of endometrial tissue fragments outside the uterine mucosa, mainly to the lower part of abdominal cavity due to repeated ovulatory menstrual episodes [2]. Moreover, recurrence of endometriosis on the postoperative scar may be due to retrograde menstruation [5]. However, about 90% of women, who also have retrograde menstruation, do not suffer from endometriosis [6].
According to the published data [7], development of endometriosis may be associated with preservation of ectopic "primitive" endometrial cells from the period of organogenesis; during pubertal development these cells differentiate into estrogen-dependent, functionally active endometrial implants, or heterotopias. There is an opinion [8], that the main source of lesions of extragenital endometriosis are stem cells originated from bone marrow, which can migrate in the systemic circulation and cause endometriosis of various extrapelvic organs.
Nevertheless, for the persistence of endometrioid heterotopias, regardless of the source of their origin, not only a certain level of estrogen is required, but also specific microenvironmental conditions, which largely depend on large molecules of the extracellular matrix – proteoglycans. [9].
Heparan sulfate proteoglycans (HSPGs) are protein-carbohydrate molecules, each of them consisting of a core protein, to which unbranched carbohydrate chains of negatively charged sulfated glycosaminoglycans are covalently attached [9]. Syndecan-1 (SDC1) is one of the family members of HSPGs known as CD138 – a marker used to identify plasma cells, as well as chronic endometritis [10].
The attention to the role of the extracellular matrix and proteoglycans in human reproductive function is steadily increasing [11, 12]. The studies using mice with a deficiency or knockout of the main proteins of proteoglycans or enzymes responsible for the biosynthesis revealed the role of these molecules in the reproductive disorders, and this can be extrapolated to human pathology [10].
HSPGs mediate signaling processes that regulate cell proliferation, differentiation, migration, as well as tumor transformation [13]. Transmembrane and pericellular HSPGs, in particular, SDC1, glypican-1/GPC1 or the perl gene play an important role in embryonic morphogenesis, physiological and pathological processes due to their ability to bind and modify the activity of numerous growth factors and cytokines involved in intercellular and cell-matrix interactions [14,15].
Heparanase (HPSE) is the enzyme of biodegradation of HSPGs – the only human endoglycosidase that shortens the extracellular carbohydrate chains of HSPGs. This leads to the release of growth factors, including angiogenic ones. Then matrix metalloproteinase-9 "cuts" core proteins SDC1 from the cell surface [16].
Aim of the study: to analyze the content of HSPGs и HPSE in eutopic and heterotopic endometrium in women with stage Ш ovarian endometriosis.
Materials and methods
The surgical samples of 3 family members of HSPGs и HPSE from 11 patients aged 30,7±6,3 years with stage III endometriotic cysts (according to criteria of L.V. Adamyan et al. [17] were analyzed. The women underwent surgery in the period from 2016 to 2017 in the Department of Gynecology of Novosibisk Regional Clinical Hospital, which is the clinical base of the Department of Obstetrics and Gynecology of Novosibirsk State Medical University of the Ministry of Health of Russia. Surgical treatment included removal of endometriotic cysts, excision of lesions of endometriosis in the pelvis and separation of adhesions.
Inclusion criteria were indications for elective surgery in accordance with clinical recommendations [18], including the presence of ovarian endometriotic cysts >3 cm in diameter, proliferative phase of menstrual cycle, histologically verified OE and the patient’s informed consent. Exclusion criteria were pregnancy, intake of hormonal drugs for a minimum of 3 months before surgery, oncopathology, immunodeficiency disorders, decompensated extragenital pathology.
Serum levels of СА125 marker were analyzed using ARCHITECT CA-125 II Reagent Kit (“Abbott”, USA). The fragments of approximately 0.3 cm3 were immediately cut off from each sample of resected endometriotic cysts and endometrial biopsy specimens, and placed in RNALater solution (ThermoFisher Scientific), then were cooled to +4°C and stored at -20°C for subsequent study using semi-quantitative real-time polymerase chain reaction (RT-PCR). The remaining fragments of native tissue were immediately fixed in buffered formalin and embedded into paraffin medium Histomix for histological and immunohistochemical (IHC) analysis.
RNA extraction from the samples was performed using QIAzol Lysis Reagent (Qiagen, USA) with further purification using on RNeasy Plus Mini Columns (Qiagen, USA) in accordance with the manufacturer's instruction. The quality of the obtained RNA was assessed using agarose gel electrophoresis. Thereafter, 0.2 μg of RNA and RevertAid First Strand cDNA Synthesis kit (ThermoFisher Scientific, USA) were used to perform cDNA synthesis. Gene expression of SDC1, GPC1, HSPG2, and HPSE by RT-PCR in triplicate reactions was analyzed using BioMaster HS-qPCR SYBR Blue (Biolabmix, Russia) and CFX-96 Touch amplifier (Bio-Rad, USA). PCR was performed under the following conditions: initial heating at 95°C for 3 min, then 40 cycles at 95°C for 10 sec, at 59°C for 20 sec and at 72°C for 30 sec. The total volume for PCR was 25 μl. 2ΔCt method was used to quantify gene expression. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was a reference gene. Working stock of primers is shown in the Table.
For IHC analysis, 4–5 μm-thick paraffin sections were deparrafined in changes of xylene and ethanol. The antigens were unmasked in a citrate buffer solution (pH 6.0; 10 mM sodium citrate, 0.05% Tween-20) by heating to 95–98°C for 20 min in the microwave oven.
To visualize the total HSPGs, mouse monoclonal antibodies to the carbohydrate chains of mammalian heparan sulfate (HS) (Millipore, IgG1, clone T320.11, cat. N MAB2040, dilution 1: 100) were used as primary antibodies. IHC staining was carried out using mouse primary antibodies to SDC1 core protein (“Thermo Scientific”, IgG1/κ, clone Ml15; ready to use reagents cat. N MS-1793-RQ); mouse monoclonal antibodies to human HSPG2/perlecan (“Abcam”, IgG1, clone А74, cat. N ab23418, 1:100); rabbit polyclonal antibodies to human GPC1 antibody (“Abcam”, IgG, cat. N ab226855, 1:100) and rabbit polyclonal antibodies to HPSE (“Abcam”, IgG, cat. N ab85543, 1:100). IHC staining specificity was confirmed using positive controls specified in the instructions for antibody specificity. The absence of nonspecific antibody binding was verified using negative controls under experimental conditions.
IHC reaction products for SDC1 were visualized using UltraVision Quanto Detection System HRP kit (ThermoScientific), for other HSPGs and HPSE «Mouse and Rabbit Specific HRP/DAB (ABC) Detection IHC kit (“Abcam”, cat. N ab64264)» with diaminobenzidine (DAB) was used; the nuclei were stained using hematoxylin. IHC reaction products were studied using Axio Scope.A1 microscope with digital camera AxioCam MRc5 (С.Zeiss) at 400x magnification.
Statistical analysis
The statistical significance of differences between the groups was assessed using the Mann–Whitney test (at critical level of significance p<0.05) and OriginPro 8.5 software. Spearman's correlation coefficient was used to assess the correlation between HPSE expression in heterotopias and tumor marker CA-125 in blood serum.
Results and Discussion
Real-time PCR analysis showed that in patients with stage III OE in proliferative phase of the cycle, relative expression of core proteins of three key members of HSPGs – transmembrane syndecan SDC1 and glypican GPC1 and HSPG2/perlecan localized in the epithelial basement membranes was statistically significantly higher only for HSPG2/perlecan (p=0.047) in surgical specimens of heterotopias compared to eutopic endometrium. No significant changes were found for the other two members of the HSPGs family (Fig.1). Moreover, the presence of mRNA of these members of the HSPGs family in the eutopic endometrium in women of reproductive age showed no endometrial pathology [10].
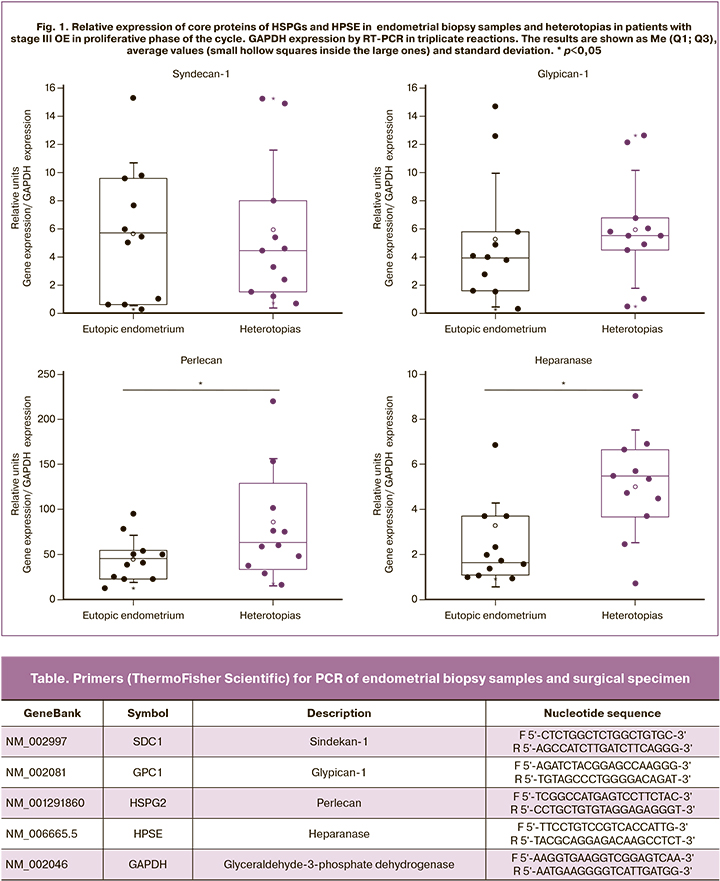
Along with HSPG2/perlecan, the expression of HPSE – the enzyme of biodegradation of carbohydrate chains of all HSPGs significantly increased (by 2 times, p=0.001) in samples of heteroropias compared to eutopic endometrium. It was previously shown, that the expression of HPSE both at mRNA and protein levels significantly increased in tumors with aggressive course and in tissues with active proliferation [9]. Other enzymes of biodegradation of the extracellular matrix, for example, metalloproteinases, were considered to be an important and promising component of the pathogenesis of endometriosis in non-invasive diagnostics [19]. The authors of this study conclude that further search for panels of biomarkers for endometriosis is relevant, since proteomics or genomics failed to meet the expectations, and isolated biomolecules or a group of biomarkers with sufficient specificity and sensitivity have not yet been identified.
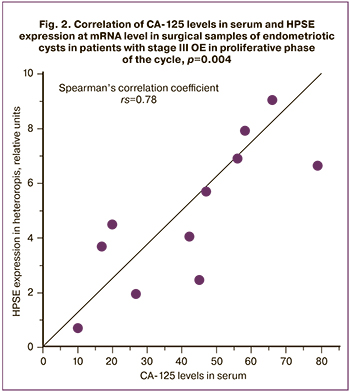 A possible relationship between expression of core proteins of HSPGs and CA125 levels has not been previously studied. We have analyzed PCR results of endometroid heterotopias M (SD)=5.56 (2.59) (0.70– 9.04) and the levels of tumor screening marker CA125 in blood serum in patients with stage III OE (41.64 (23.05), which varied from 10 to 79 U/mL. A positive correlation was established between the relative expression of HPSE in pathological foci and the content of the specified screening marker (Spearman's correlation coefficient rs=0.78, CI 95%: 0.168–1, p=0.004) (Fig. 2). This requires further study.
A possible relationship between expression of core proteins of HSPGs and CA125 levels has not been previously studied. We have analyzed PCR results of endometroid heterotopias M (SD)=5.56 (2.59) (0.70– 9.04) and the levels of tumor screening marker CA125 in blood serum in patients with stage III OE (41.64 (23.05), which varied from 10 to 79 U/mL. A positive correlation was established between the relative expression of HPSE in pathological foci and the content of the specified screening marker (Spearman's correlation coefficient rs=0.78, CI 95%: 0.168–1, p=0.004) (Fig. 2). This requires further study.
The remaining fragments of endometrial biopsy specimens from the patients were studied using IHC. In the proliferative phase of the cycle, a heterogeneous expression (from pronounced to insignificant) of the total HSPGs in gland epithelial cells was detected (Fig. 3, a). Along with this, the endometrial stroma contained a large amount of IHC reaction products. Analysis of core proteins expression of three family members of HSPGs showed only the traces of SDC1 in endometrial extracellular matrix (Fig. 3, b) and overt plasma cell response; uneven epithelial localization of HSPG2/perlecan (Fig 3, c); as well as moderate expression of GPC1 in the endometrial epithelial and stromal cells (Fig. 3, d). Despite the fact that the total HSPGs in the proliferative phase of the cycle in patients with stage III OE were visualized using antibodies to carbohydrate chains, they also contained core proteins GPC1 and HSPG2/Perl as part of a complex molecule. These data are generally consistent with PCR results of the same clinical samples (Fig.1).
HPSE is the enzyme of biodegradation of carbohydrate chains HS in eutopic endometrium that was detected in single superficial epithelial cells and small foci of the periglandular stroma (Fig.3, e). IHC analysis confirmed [20] that in patients with edometriosis, HPSE expression was at minimal level in eutopic endometrium in the first phase of the cycle and increased at the end of secretory phase of the cycle promoting desquamation.
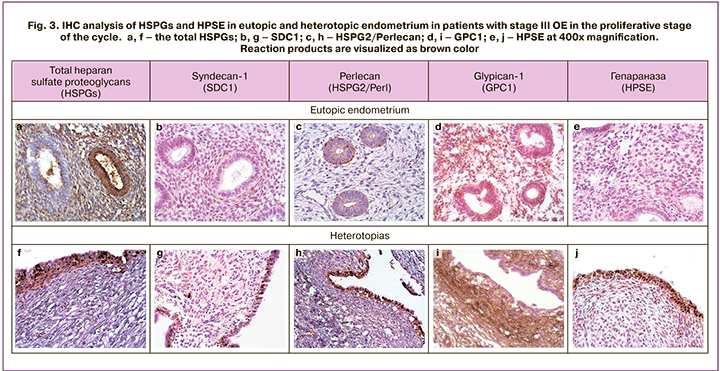
IHC analysis of resected endometriotic ovarian cysts focused on the epithelium and cytogenic stroma, where DAB-positive total HSPGs were found. (Fig. рис. 3, f). SDC1 expression was insignificant, and mainly was detected in basal and basolateral membranes of epithelial cells (Fig. 3, g). High HSPG2/perl expression was in heterotopic epithelium (Fig. 3, h) and low expression was in the stroma and fibrous capsules. On the contrary, GPC1 expression was pronounced in these areas in the absence of response in the epithelium (Fig. 3, i).
It should be noted, that HSPG2/Perl can act as a potent pro-angiogenic factor, either directly representing vascular endothelial growth factor A (VEGF-A) and its VEGFR-2 receptors, or indirectly – after partial cleavage of carbohydrate chains of HSPGs by HPSE (similarly, it refers to other growth factors associated with HSPGs). Both mechanisms can initiate VEGFR-2 signaling to stimulate proliferation, migration and enhancement of vascular permeability [21].
At the same time expression of HPSE in the epithelial component of heterotopias was high (Fig.3, j). According to some authors [20], HPSE is high-expressed in heterotopias regardless of the phase of the menstrual cycle. Previously we have shown [22] that pronounced expression of HPSE in the epithelium of endometriotic ovarian cysts in patients of early reproductive age with stage III OE was associated with inflammatory cell infiltration and the presence of pain syndrome. However, SDC1 expression in the epithelium was low, although statistically significant patterns were not identified. According to IHC results [23], in eutopic endometrium, the level of SDC1 expression in the epithelium was higher in the secretory phase of the cycle than in the proliferative phase, while stromal fibroblasts did not differ between the phases of menstrual cycle.
Conclusion
Thus, in stage III OE, the relative expression of core proteins of HSPG2/pelican and HPSE in endometrial heterotopias in the proliferative phase of cycle significantly exceeds their relative expression in endometrial biopsy samples from the same patients. A positive correlation was established between the relative expression of HPSE at mRNA level in the endometroid heterotopias and tumor marker CA-125 in blood serum.
The results of the study indicate the involvement of extracellular glycosylated molecules of the HSPGs family in the pathomorphogenesis of external genital endometriosis. Endoglycosidase HPSE modifies the extracellular matrix of endometrial tissue by cleaving the carbohydrate chains of HSPGs. This may contribute to the spread of endometrioid heterotopias.
References
- Bulun S.E., Yilmaz B.D., Sison C., Miyazaki K., Bernardi L., Liu S. et al. Endometriosis. Endocr. Rev. 2019; 40(4): 1048-79. https://dx.doi.org/10.1210/er.2018-00242.
- Zondervan K.T., Becker C.M., Koga K., Missmer S.A., Taylor R.N., Viganò P. Endometriosis. Nat. Rev. Dis. Primers. 2018; 4(1): 9. https://dx.doi.org/10.1038/s41572-018-0008-5.
- Moga M.A., Balan A., Dimienescu O.G., Burtea V., Dragomir R.M., Anastasiu V.C. Circulating miRNAs as biomarkers for endometriosis and endometriosis-related ovarian cancer – An overview. J. Clin. Med. 2019; 8(5): 735. https://dx.doi.org/10.3390/jcm8050735.
- Bulletti C., Coccia M.E., Battistoni S., Borini A. Endometriosis and infertility. J.Assist. Reprod. Genet. 2010; 27(8): 441-7. https://dx.doi.org/10.1007/s10815-010-9436-1.
- Canis M., Bourdel N., Chauvet P., Gremeau A.S., Botchorishvili R. Recurrences of endometriosis after surgery may be the consequence of retrograde menstruation on the postoperative scar. Hum. Reprod. 2020; 35(5): 1246-7. https://dx.doi.org/10.1093/humrep/deaa053.
- Halme J., Hammond M.G., Hulka J.F., Raj S.G., Talbert L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 1984; 64: 151-4.
- Laganà A.S., Garzon S., Götte M., Viganò P., Franchi M., Ghezzi F., Martin D.C. The pathogenesis of endometriosis: Molecular and cell biology insights. Int. J. Mol. Sci. 2019; 20(22): 5615. https://dx.doi.org/10.3390/ijms20225615.
- Pluchino N., Taylor H.S. Endometriosis and stem cell trafficking. Reprod. Sci. 2016; 23(12): 1616-9. https://dx.doi.org/10.1177/1933719116671219.
- Karamanos N.K., Theocharis A.D., Neill T., Iozzo R.V. Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019; 75-76: 1-11. https://dx.doi.org/10.1016/j.matbio.2018.08.007.
- Kitaya K., Tada Y., Hayashi T., Taguchi S., Funabiki M., Nakamura Y., Yasuo T. Diverse functions of uterine proteoglycans in human reproduction (review). Mol. Med. Rep. 2012; 5(6): 1375-81. https://dx.doi.org/10.3892/mmr.2012.826.
- Зиганшина М.М., Абдурахманова Н.Ф., Павлович С.В., Гвоздева А.Д., Бовин Н.В., Сухих Г.Т. Гликом эндометрия в менструальном цикле и рецептивность эндометрия. Акушерство и гинекология. 2017; 12: 17-24. [Ziganshina M.M., Abdurakhmanova N.F., Pavlovich S.V., Gvozdeva A.D., Bovin N.V. Sukhikh G.T. Endometrial glycome in the menstrual cycle and endometrial receptivity. Obstetrics and Gynecology. 2017; 12: 17-24. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.12.17-24.
- Chelariu-Raicu A., Wilke C., Brand M., Starzinski-Powitz A., Kiesel L., Schüring A.N., Götte M. Syndecan-4 expression is upregulated in endometriosis and contributes to an invasive phenotype. Fertil. Steril. 2016; 106(2): 378-85. https://dx.doi.org/10.1016/j.fertnstert.2016.03.032.
- Theocharis A.D., Manou D., Karamanos N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019; 286(15): 2830-69. https://dx.doi.org/10.1111/febs.14818.
- Suhovskih A.V., Tsidulko A.Y., Kutsenko O.S., Kovner A.V., Aidagulova S.V., Ernberg I., Grigorieva E.V. Transcriptional activity of heparin sulfate biosynthetic machinery is specifically impared in benign prostate hyperplasia and prostate cancer. Front. Oncol. 2014; 4: 79. https://dx.doi.org/10.3389/fonc.2014.00079.
- Kazanskaya G.M., Tsidulko A.Y., Volkov A.M., Kiselev R.S., Suhovskih A.V., Kobozev V.V., Gaytan A.S., Aidagulova S.V., Krivoshapkin A.L., Grigorieva E.V. Heparan sulfate accumulation and perlecan/HSPG2 up-regulation in tumour tissue predict low relapse-free survival for patients with glioblastoma. Histochem. Cell. Biol. 2018; 149(3): 235-44. https://dx.doi.org/10.1007/s00418-018-1631-7
- Manon-Jensen T., Itoh Y., Couchman J.R. Proteoglycans in health and disease: The multiple roles of syndecan shedding. FEBS J. 2010; 277(19): 3876-89. https://dx.doi.org/10.1111/j.1742-4658.2010.07798.x.
- Адамян Л.В., Куликов В.И., Андреева Е.Н. Эндометриозы. Руководство для врачей. М.: Медицина; 2006. 416с. [Adamyan L.V., Kulikov V.I., Andreeva E.N. Endometriosis: A guide for doctors. M.: Medicine, 2006. 416 p. (in Russian)].
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Эндометриоз. 2016. ID: КР259. [Ministry of Health of the Russian Federation. Clinical recommendations. The endometriosis. 2016. ID: КР259. (in Russian)].
- Anastasiu C.V., Moga M.A., Neculau A.E., Bălan A., Scârneciu I., Dragomir R.M. et al. Biomarkers for the noninvasive diagnosis of endometriosis: state of the art and future perspectives. Int. J. Mol. Sci. 2020; 21(5): 1750. https://dx.doi.org/10.3390/ijms21051750.
- Xu X., Ding J., Ding H., Shen J., Gattuso P., Prinz R.A. et al. Immunohistochemical detection of heparanase-1 expression in eutopic and ectopic endometrium from women with endometriosis. Fertil. Steril. 2007; 88(5):1304-10. https://dx.doi.org/10.1016/j.fertnstert.2006.12.081.
- Iozzo R.V., Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 2015; 42: 11-55. https://dx.doi.org/10.1016/j.matbio.2015.02.003.
- Маринкин И.О., Тимофеева Ю.С., Кулешов В.М., Волчек А.В., Макаров К.Ю., Омигов В.В., Айдагулова С.В. Особенности экспрессии синдекана-1 и гепараназы в эпителии эндометриоидных кист яичников третьей стадии. Акушерство и гинекология. 2018; 11: 86-91. [Marinkin I.O., Timofeeva Yu.S., Kuleshov V.M., Volchek A.V., Makarov K.Yu., Omigov V.V., Aidagulova S.V. Specific features of the expression of syndecan-1 and heparanase in the epithelium of Stage III ovarian endometrioid cysts. Obstetrics and Gynecology. 2018; 11: 86-91. (in Russian)]. https://dx.doi.org/10.18565/aig.2018.11.86-91
- Lai T.H., King J.A., Shih I.M., Vlahos N.F., Zhao Y. Immunological localization of syndecan-1 in human endometrium throughout the menstrual cycle. Fertil. Steril. 2007; 87(1): 121-6. https://dx.doi.org/10.1016/j.fertnstert.2006.06.042.
Received 02.07.2020
Accepted 24.11.2020
About the Authors
Yulia S. Timofeeva, assistent of the Obstetrics and Gynecology Department, NSMU. Tel.: +7(906)194-77-55. E-mail: dr.yustimofeeva@gmail.com.630091, Russia, Novosibirsk, Krashy Ave., 52.
Dmitriy K. Sokolov, postgraduate student, Laboratory of Glycobiology, Institute of Molecular Biology and Biophysics FRC FTM. Tel.: +7(913)716-93-31.
E-mail: dmit_s95@mail.ru.630117, Russia, Novosibirsk, Timakova str., 2/12.
Elvira V.Grigorieva, PhD, Head of Laboratory of Glycobiology, Institute of Molecular Biology and Biophysics FRC FTM. Tel.: +7(383)333-40-25.
E-mail: elv_grig@mail.ru.630117, Russia, Novosibirsk, Timakova str., 2/12.
Alexander V. Volchek, junior researcher, Laboratory of Cellular Biology and Fundamental Basis of Reproduction of NSMU Central scientific laboratory.
Tel.: +7(913)003-17-22. E-mail: alexander@volcheck.ru.630091, Russia, Novosibirsk, Krashy Ave., 52.
Galina M. Kazanskaya, PhD, researcher of Laboratory of Glycobiology, Institute of Molecular Biology and Biophysics FRC FTM. Tel.: +7(962)837-59-61.
E-mail: g_kazanskaya@meshalkin.ru.630117, Russia, Novosibirsk, Timakova str., 2/12.
Anastasiya V. Suhovskih, PhD, researcher of Laboratory of Glycobiology, Institute of Molecular Biology and Biophysics FRC FTM. Tel.: +7(923)177-70-63.
E-mail: anastasia-suhovskih@mail.ru.630117, Russia, Novosibirsk, Timakova str., 2/12.
Alexandra Yu.Tsidulko, researcher of Laboratory of Glycobiology, Institute of Molecular Biology and Biophysics FRC FTM. Tel.: +7(913)016-86-36.
E-mail: alexandra.tsidulko@gmail.com.630117, Russia, Novosibirsk, Timakova str., 2/12.
Yanina M. Evseeva, student, NSMU. Tel.: +7(913)772-01-28. E-mail: janinaevseeva@yahoo.com.630091, Russia, Novosibirsk, Krashy Ave., 52.
Vitaliy M. Kuleshov, MD, professor, professor of the Obstetrics and Gynecology Department, NSMU. Tel.: +7(383)341-04-36. E-mail: kuleshov_vm@mail.ru.
630091, Russia, Novosibirsk, Krashy Ave., 52.
Igor O. Marinkin, MD, professor, Head of the Obstetrics and Gynecology Department of NSMU, Rector of NSMU. Tel.: +7(383)222-32-04. E-mail: rector@ngmu.ru.
630091, Russia, Novosibirsk, Krashy Ave., 52.
Svetlana V. Aidagulova, PhD, professor, Head of Laboratory of Cellular Biology and Fundamental Basis of Reproduction of NSMU Central scientific laboratory.
Tel.: +7(913)909-22-51. E-mail: s.aydagulova@gmail.com. ORCID: 0000-0001-7124-1969.630091, Russia, Novosibirsk, Krashy Ave., 52.
For citation: Timofeeva Yu.S., Sokolоv D.К., Grigorieva E.V., Volchek А.V., Kazanskaya G.M., Sukhovskikh A.V., Tsidulko A.Yu., Evseeva Ya.M., Кuleshov V.M., Marinkin I.О., Aidagulovа S.V. Analysis of heparan sulfate proteoglucans content in eutopic and heterotopic endometrium in ovarian endometriosis.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 3: 110-116 (in Russian)
https://dx.doi.org/10.18565/aig.2021.3.110-116



