Еndometrial microbiota of women with chronic endometritis and idiopathic infertility
Objective. To assess the composition of the microorganisms of the uterine cavity of women with chronic endometritis (CE) and idiopathic infertility.Tapilskaya N.I., Budilovskaya O.V, Krysanova A.A., Tolibova G.KH., Kopylova A.A., Tsypurdeeva N.D., Gzgzyan A.M., Savicheva A.M., Kogan I.YU.
Materials and methods. The study included 145 women of reproductive age with idiopathic infertility. All the patients underwent hysteroscopic examination followed by biopsy histopathological evaluation for immunohistochemical (IHC) and microbiological («Femoflor 16» and «Femoflor Screen» test, DNA-technology, Moscow) studies.
Results. According to the results of the IHC study, the CE was detected in 113 (77.9%) patients. The most common microorganisms identified in the uterine cavity were Lactobacilli (66.21%), as well as Staphylococcus (44.83%), Enterobacteriaceae family (22.1%), Ureaplasma spp. (19.3%), Streptococcus spp. (15.2%), Gardnerella vaginalis (11.03%), Atopobium vaginae (10.34%). The presence of Ureaplasma spp. in the uterine cavity significantly increasing the risk of developing CE by 4,5 times (RR=4.483; 95% CI 1.003–20.029). Presence of Atopobium vaginae and Staphylococcus spp. increases the risk of developing pronounced CE by 7 times (RR=6.959; 95% CI 0.856–56.602) and 2.5 times (OS=2.5; 95% CI 1.034–6.043), respectively.
Conclusion. The risk of developing CE increases with the persistence of certain microorganisms in the uterine cavity.
Keywords
Recent advances in microbiology have challenged the concept that the upper reproductive tract is usually a sterile site [1]. Today, the international scientific community has to decide which microorganisms found in the endometrium may be considered as infectious agents causing subclinical inflammation, which is classified in the ICD-10 as a chronic inflammatory disease of the uterus [2, 3].
For practicing obstetrician-gynecologists, it is not always possible to identify a definite cause for infertility. As a result, these patients are diagnosed with idiopathic infertility and undergo assisted reproductive technologies (ART). However, undiagnosed chronic endometritis (CE) can be the cause of recurrent implantation failure and spontaneous miscarriages, even despite the euploid embryo transfer into the endometrial cavity [4, 5].
The study aimed to investigate uterine microbial composition in women with chronic endometritis and idiopathic infertility and develop treatment regimens.
Materials and methods
The study comprised 145 reproductive-age women who were referred for consultation with a reproductologist at the D.O. Ott Research Institute for Obstetrics and Gynecology from April 2015 to January 2019 and underwent a hysteroscopy followed by an investigation of uterine microbial composition. All patients underwent a hysteroscopy with endometrial biopsy. Biopsy specimens were evaluated by immunohistochemical (IHC) testing for CE markers and pathomorphological analysis. All patients signed informed consent to participate in the study.
Inclusion criteria for study patients were age from 25 to 40 at the time of enrollment, diagnosis of idiopathic primary infertility before admission, the presence of a permanent sexual partner aged ≤ 50, a full clinical examination of an infertile couple under the order No. 107n of the Ministry of Healthcare of Russia dated August 30, 2012, “On the Procedure for the Use of Assisted Reproductive Technologies, Contraindications, and Restrictions to their Application.” (as amended on June 11, 2015, and on February 1, 2018 .), and no infertility or impaired fertility of any other genesis.
Criteria for exclusion from the study were as follows: patient age under 25 and over 40, spouse age over 50, 2 or more regular sexual partners, infertility or impaired fertility of any other genesis, including tubal peritoneal infertility, anovulatory infertility, male infertility, including with subfertile semen analysis results, any non-inflammatory gynecological diseases requiring surgical treatment and/or prescription of drug therapy, diabetes mellitus and other disorders of carbohydrate metabolism, uncompensated thyroid gland disorders and other endocrine glands (pituitary, adrenal glands, parathyroid glands, ovaries) disorders, including those requiring hormone replacement therapy, psychiatric diseases, alcoholism, drug addiction, malignant neoplasms, including intraepithelial dysplasia of any location, and/or previous or identified during the study lymphoproliferative diseases, body mass index (BMI) over 29.9 or under 18.5 kg/m2, HIV-positive patients or patients at high risk of infection who receive prophylactic antiretroviral therapy, patients with viral hepatitis B, C, including those infected in the past, any other diseases that, according to the researchers, affect or are able to influence the study results, and patient reluctance to participate in the study and/or low compliance.
In total, the study comprised 145 women who were eligible to participate in the study.
As shown in the table 1, most of the study participants were aged 35 [31; 38], had higher education, and BMI in the range of 25–29.9 kg/m2. There were similar proportions of women who smoke and do not smoke.
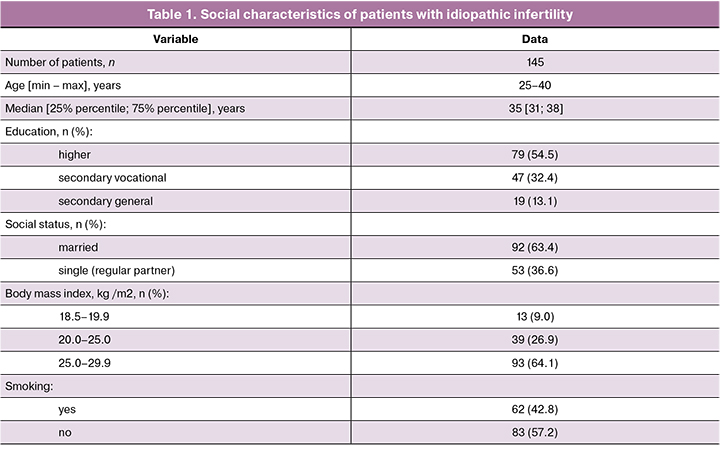
Before hysteroscopy, blood samples were drawn from the antecubital vein to test for antibodies to HIV-1, 2, markers of viral hepatitis B and C, and syphilis. Laboratory testing also included blood count, blood biochemistry and coagulation, and urinalysis.
All patients underwent hysteroscopy with endometrial biopsy followed by histological and IHC examination. In 38 women, hysteroscopy was performed on days 7-9 and in 107 on days 19–22 of the menstrual cycle [6]. Histological and IHC studies were performed according to standard methods. The diagnosis and CE severity were assessed using a combination of antibody CD8 + [clone CD8/144B], CD20 + [clone L26], CD4 + [clone 4B12], CD138 + [clone M115], in a standard dilution of 1:50; CD34 + [clone QBEnd-10] in a standard 1:25 dilution from Dako Cytomation (Denmark), because the number of pro-inflammatory cells does not depend on the phase of the menstrual cycle. The severity of CE was classified by Tolibova G.Kh. et al. [7].
All patients underwent hysteroscopy with endometrial biopsy followed by a microbiological examination of biopsy specimens for the presence of opportunistic and pathogenic microorganisms (chlamydia, gonococci, Mycoplasma genitalium) and viruses (herpes simplex virus type 1 and 2, cytomegalovirus) using a real-time polymerase chain reaction (DNA-Technology Femoflor 16 and Femoflor Screen PCR Detection Kits, DNA technology, Moscow).
The patients received systemic antibiotic therapy according to the composition of microorganisms and the presence of chronic endometritis.
After antimicrobial therapy, the patients were followed up by telephone questionnaires; by July 2019, the median follow-up was 18.6 (from 8 to 40.4) months.
This study was descriptive, not comparative (except for an intragroup analysis); therefore, no statistical hypotheses were previously determined. Statistical analysis was performed using Microsoft Office Excel and the PAST3 software. The normality of the distribution was tested by the Shapiro-Wilk test. Quantitative variables not showing normal distribution were expressed as the median (Me) and interquartile range [25–75 quartiles]. Qualitative variables were summarized as counts and percentages. Statistical analysis of qualitative variables (the frequency of detection of a microbial agent in each subgroup) was performed using the Chi-square test (χ2) for contingency tables or Fisher’s exact when the expected cell count was less than 5. The relationship between the persistence of the microorganism and the presence or absence of the disease (CE, severe CE) was determined by calculating the odds ratio (OR) with a 95% confidence interval (95% CI). The critical level of significance when testing statistical hypothesis was considered at p < 0.05.
The study was carried out under the principles of the Helsinki Declaration of the World Association “Ethical principles for medical research involving human subjects”, the current procedures and standards for the provision of medical care, and other applicable regulatory requirements for conducting clinical trials and observational studies in the Russian Federation. The patient monitoring protocol and examination program were approved by the local ethics committee.
Results
Diagnostic hysteroscopy revealed endometrial polyps and uterine synechiae in 18 (12.4%) and 1 (0.7%) patients, respectively.
According to pathomorphological findings, simple endometrial hyperplasia without atypia and endometrial hyperplasia with endometrial polyps were detected in 18 (12.4%) and 3 (2.1%) patients, respectively. Adenomyosis was diagnosed in 2 (1.4%) cases. IHC analysis detected CE was in 113 (77.9%) patients. Of them, mild, moderate, and severe CE was found in 11 (9.7%), 42 (37.2%), and 60 (53.1%) patients, respectively.
Molecular biological testing of endometrial biopsy samples by real-time PCR using the Femoflor-16 assay showed that the most common microorganisms detected in the uterine cavity were lactobacilli (66.21%), staphylococci (44.83%), microorganisms of the Enterobacteriaceae family ( 22.1%), and Streptococcus spp. (15.2%) (Table 2). Detection rates of microorganisms associated with bacterial vaginosis were as follows: Ureaplasma sp. (19.3%), Mycoplasma hominis (9.65%), Gardnerella vaginalis (11.03%), Atopobium vaginae (10.34%). Yeast-like fungi of the genus Candida were found in 6 (4.14%) patients. The remaining microorganisms were detected much less frequently. All biopsy specimens were tested for herpes simplex and cytomegaly viruses. These viruses were detected only in 2 (0.7%) women. Chlamydia, gonococcus, Mycoplasma genitalium were not found in any patient.
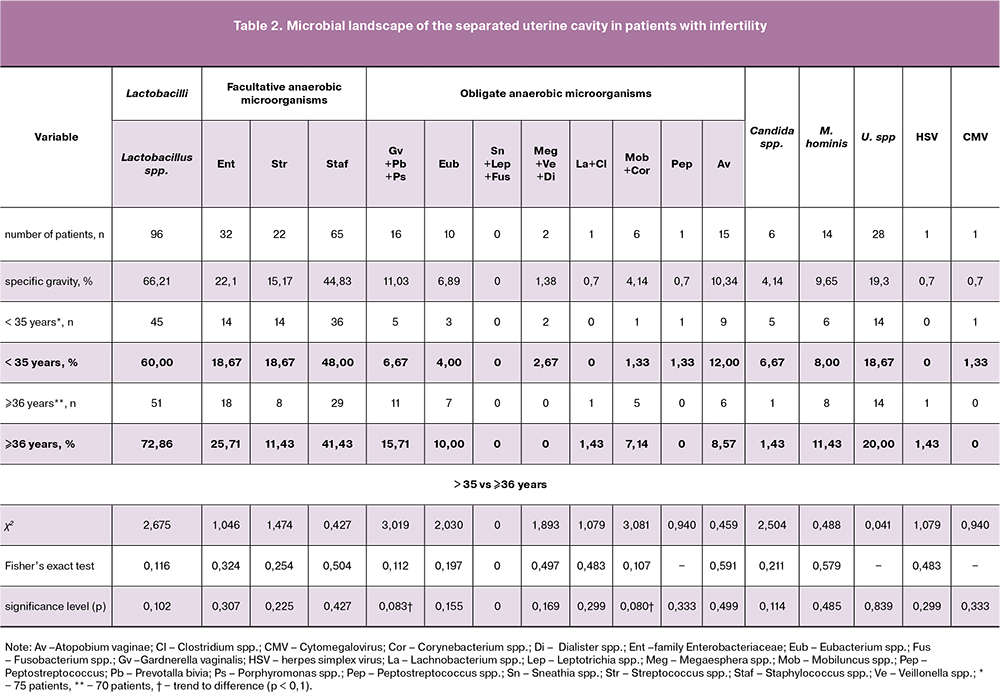
Only in 11 (7.6%) endometrial biopsy specimens no microorganisms were detected. In 21 samples, lactobacilli were the only isolated microorganisms. In the remaining cases, specimens contained from 1 (24.1%) to 9 (1.4%) microbial agents. Most often (31.1%), biopsy specimens contained two genera of microorganisms; less frequently, there were 3 (14.5%), 4 (12.4%), 5 (4.1%), and 6 (2.8%) of them (Table 3). Staphylococci that were most often detected in the endometrium co-existed with bacteria of the Enterobacteriaceae family and with streptococci. Ureaplasma spr. most commonly co-existed with Staphylococcus spp. (53.6%), Mycoplasma hominis (35.7%), and Gardnerella vaginalis (25.0%). Mycoplasma hominis was also most often combined with staphylococci (78.6%). Gardnerella vaginalis most often co-existed with Staphylococcus spp. (62.5%) and Eubacterium spp. (56.3%).
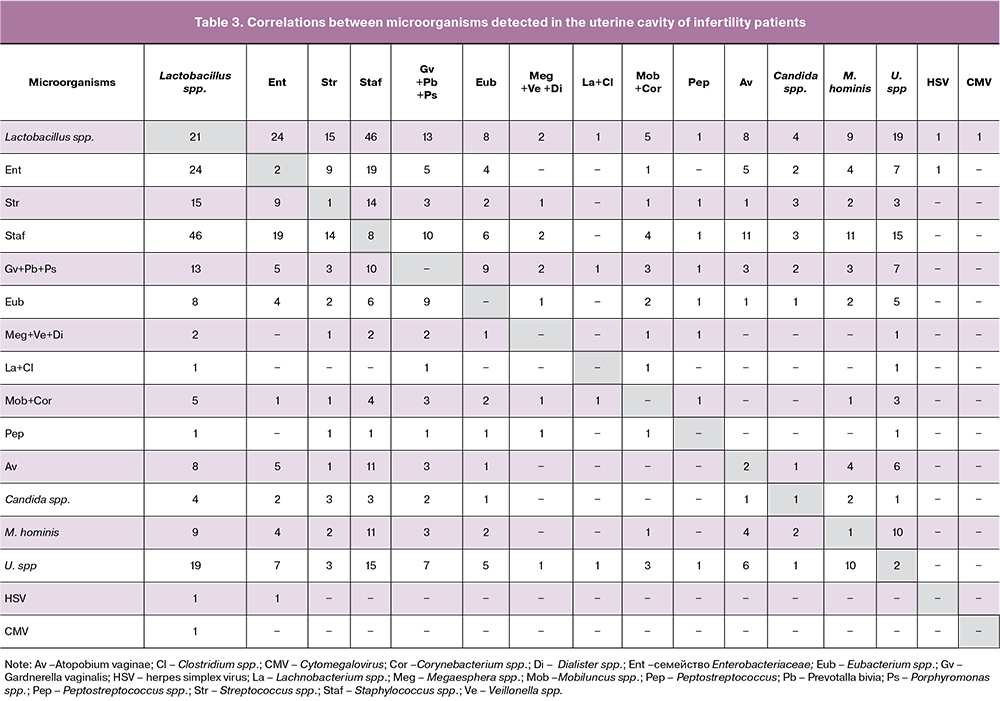
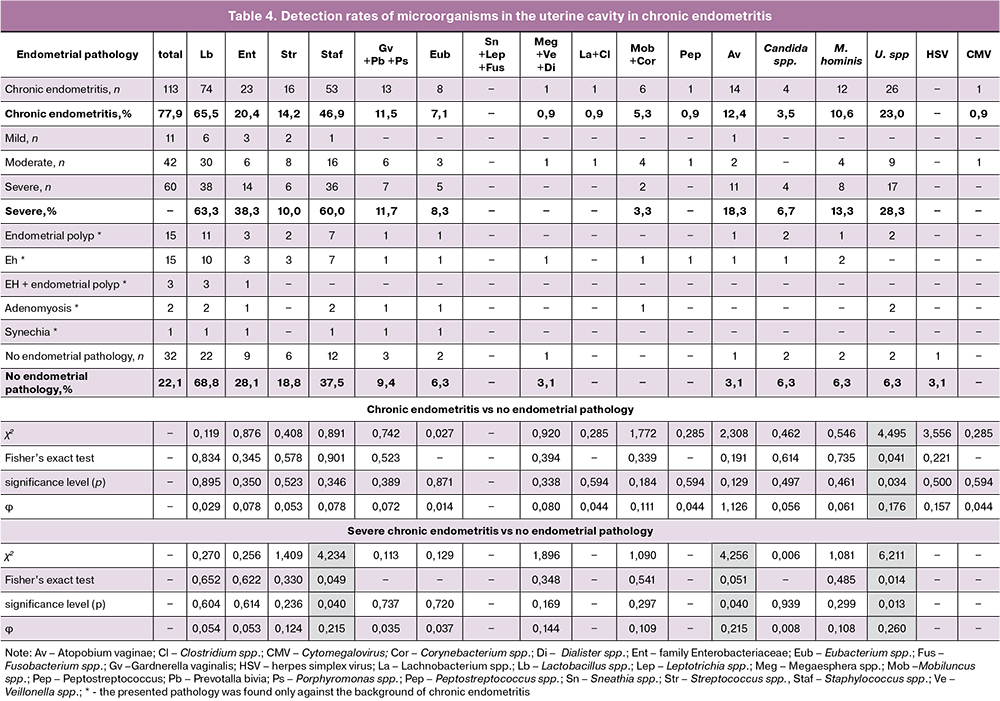
Table 4 summarizes detection rates of microorganisms in the uterine cavity of patients with chronic endometritis. The detection rate of Ureaplasma spr. by IHC analysis was statistically significantly higher (92.9%; χ2 = 4.495, p = 0.034) in patients with CE (26 positive out of 113 specimens) than in patients without CE (2/32 positive). In the presence of severe CE, ureaplasma was detected in 17 (60.7%) cases; χ2 = 4.495, p = 0.034. Patients with severe CE had significantly higher detection rates of Atopobium vaginae (93.3%) and Staphylococcus spp (81.5%), p = 0.04. The presence of ureaplasmas in the uterine cavity significantly increases the risk of developing CE (OR = 4.483; 95% CI 1.003‒20.029), while the risk of developing severe CE increases almost 6 times (OR = 5.93; 95% CI 1.274‒27.595). The presence of Atopobium vaginae and Staphylococcus spp. was also associated with a 7-fold (OR = 6.959; 95% CI 0.856– 56.602) and a 2.5-fold (OR = 2.5; 95% CI 1.034–6.043) higher risk of developing severe CE, respectively.
All patients with chronic endometritis were treated. If opportunistic pathogens such as staphylococci, gardnerella, Atopobium vaginae were detected in the uterine cavity and a diagnosis of CE was made, the patients were administered amoxicillin with clavulanic acid (Flemoclav Solutab); patients with genital mycoplasmas and/or ureaplasma received josamycine (Wilprafen) because these microorganisms were associated with a manifold (2.5–7 times) higher risk of developing chronic endometritis. Treatment with these drugs showed clinical effectiveness: 11% of women had a spontaneous pregnancy and 28.3% after ART procedures.
During the follow-up, 16 (11.0%) spontaneous pregnancies were registered within 4–10 months after antibiotic therapy. Another 29 pregnancies were diagnosed after ART among 41 (28.3%) participants who entered the protocol. No microbiological testing was performed after antimicrobial therapy because it was not included by the study protocol given its invasiveness.
The ESHRE 2019 Annual Meeting in Vienna addressed the causes and treatment strategies for idiopathic infertility as essential and yet poorly studied problems. However, the role of the infectious factor was discussed as one of the most likely causes of unexplained infertility [8].
Bouet P. et al. [9] reported that 14–27% of patients with recurrent implantation failure had immunohistochemically confirmed CE associated with the persistence of bacterial agents and, as a rule, without clinical manifestation of infection. Kushnir V.A. et al. [10] found that the prevalence of CE among infertile patients with recurrent implantation failure was 45% [10]. Cicinelli E. et al. [11] conducted a retrospective study among women under 40 who had a history of three or more spontaneous miscarriages of unknown etiology. In this cohort, 57.8% of patients had hysteroscopic signs of CE, of which in 91.3% of cases, the diagnosis was confirmed histologically. In our study, the prevalence of CE was 77.9%, which is a result of meeting the study eligibility criteria. In 68% of patients with CE, opportunistic pathogens were isolated from the uterine cavity, such as Streptococcus agalactiae (serological group B), E. coli, Enterococcus faecalis, and/or mycoplasmas [11].
According to other studies, CE is most often associated with Enterococcus faecalis, Enterobacteriaceae, Streptococcus spp., Staphylococcus spp., Gardnerella vaginalis, and Mycoplasma spp. as well as Ureaplasma urealyticum, Chlamydia trachomatis, and Neisseria gonorrhoeae [12, 13].
In our study, no microorganisms or only lactobacilli were detected in 22.1% of cases; in the remaining cases, opportunistic pathogens were identified, of which the most common were the Enterobacteriaceae family (31.7%), Staphylococcus spp. (16.6%) and Ureaplasma sp. (13.1%). It is noteworthy that in our study, a monoculture of lactobacilli was detected in 21 (14.5%) samples. There is currently no complete understanding of whether the presence of Lactobacillus spp. in the uterine cavity is a “physiological phenomenon,” or a high prevalence of these microorganisms in patients with CE is caused by the disruption of protective physiological barriers and/ or a functional deficiency of cellular immunity components [5].
Investigation of reproductive tract microbiota composition using NGS technology showed that in patients with infertility, the microbiome of the endometrium was distinctly different from vaginal microbiota. In other words, this is not about the colonization of the uterine cavity with vaginal bacteria. Therefore, the study of the uterine cavity microflora is of great importance, especially in infertile patients, since this medium provides for embryo implantation in the uterine cavity [14]. According to Moreno I. et al. [15], there was a high degree of concordance (76.92%) between nucleic acid amplification test and histological and culture examinations in the identification of the uterine cavity microbial composition. The sensitivity and the specificity of this test was 75% and 100%, respectively.
Many authors postulate that the histological diagnosis is not specific, extremely subjective, time- and resourceconsuming and, most importantly, does not predict reproductive outcomes [16, 17]. Given these limitations, the examination of the endometrial microbiome is an attractive diagnostic option, which determines the further therapeutic strategy and the choice of appropriate antimicrobial therapy [11, 12, 18]. This allows the detection of cultivable and uncultivable bacteria that colonize the endometrium, even without histological signs of infection. The molecular test shows promise as a faster and more reliable tool for simplified diagnosis of chronic endometritis [11].
Conclusion
The prevalence of CE in patients with idiopathic infertility was 77.9%. Detection of opportunistic pathogens such as staphylococci, gardnerella, Atopobium vaginae, genital mycoplasmas, and ureaplasmas in endometrial biopsy specimens warrants administration of antimicrobial agents because these microorganisms were associated with a manifold (2.5–7 times) higher risk of developing CE. Antibacterial therapy showed high clinical effectiveness: 11% of women had a spontaneous pregnancy and 28.3% after ART procedures.
References
- Тапильская Н.И., Карпеев С.А., Кузнецова И.В. Хронический эндометрит – субклиническое воспалительное заболевание органов малого таза. Гинекология. 2014; 16(1): 104-9. [Tapilskaya N.I., Karpeev S.A., Kuznetsova I.V.Subclinical inflammatory diseases of the pelvic organs: chronic endometritis. Gynecology/Ginekologiya. 2014; 16(1): 104-9. (in Russian).]
- Савичева А.М., Тапильская Н.И., Шипицына Е.В., Воробьева Н.Е. Бактериальный вагиноз и аэробный вагинит как основные нарушения баланса вагинальной микрофлоры. особенности диагностики и терапии. Акушерство и гинекология. 2017; 5: 24-31. [Savicheva A.M., Tapilskaya N.I., Shipitsyna E.V., Vorobyeva N.E. Bacterial vaginosis and aerobic vaginitis as major vaginal microflora balance disorders: diagnostic and therapeutic characteristics. Obstetrics and Gynecology/Akusherstvo ginekologiya. 2017; (5): 24-31. (in Russian).] https://dx.doi.org/10.18565/aig.2017.5.24-31.
- Kitaya K., Takeuchi T., Mizuta S., Matsubayashi H., Ishikawa T. Endometritis: new time, new concepts. Fertil. Steril. 2018; 110(3): 344-50. https://dx.doi.org/10.1016/j.fertnstert.2018.04.012.
- Puente E., Alonso L., Laganà A.S., Ghezzi F., Casarin J. Carugno J. Chronic endometritis: old problem, novel insights and future challenges. Int. J. Fertil. Steril. 2020; 13(4): 250-6. https://dx.doi.org/10.22074/ijfs.2020.5779.
- Kimura F., Takebayashi A., Ishida M., Nakamura A., Kitazawa J., Morimune A. et al. Review: Chronic endometritis and its effect on reproduction. J. Obstet. Gynaecol. Res. 2019; 45(5): 951-60. https://dx.doi.org/10.1111/jog.13937.
- Цыпурдеева Н.Д., Шипицына Е.В., Савичева А.М., Гзгзян А.М., Коган И.Ю. Состав микробиоты эндометрия и степень выраженности хронического эндометрита у пациенток с неэффективными протоколами экстракорпорального оплодотворения. есть ли связь? Журнал акушерства и женских болезней. 2018; 67(2): 5-15. [Tsypurdeeva N.D., Shipitsyna E.V., Savicheva A.M., Gzgzyan A.M., Kogan I.Yu. Composition of endometrial microbiota and chronic endometritis severity in patients with in vitro fertilization failures. Is there any connection? Journal of Obstetrics Women’s Diseases/Zhurnal akusherstva i zhenskikh boleznej. 2018; 67(2): 5-15. (in Russian).]
- Bouet P.E., El Hachem H., Monceau E., Gariépy G., Kadoch I.J., Sylvestre C. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil. Steril. 2016; 105(1): 106-10. https://dx.doi.org/10.1016/j.fertnstert.2015.09.025.
- Европейское общество по вопросам репродукции человека и эмбриологии (ESHRE). Идиопатическое бесплодие. Доступно по: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Guidelines-in-development/Unexplained-infertility [European Society of Human Reproduction and Embryology (ESHRE). Unexplained-infertility. (in Russian).] Available at: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Guidelines-in-development/Unexplained-infertility
- Kushnir V.A., Solouki S., Sarig-Meth T., Vega M.G., Albertini D.F. , Darmon S.K. et al. Systemic inflammation and autoimmunity in women with chronic endometritis. Am. J. Reprod. Immunol. 2016; 75(6): 672-7. https://dx.doi.org/10.1111/aji.12508.
- Cicinelli E., Matteo M., Tinelli R., Lepera A., Alfonso R., Indraccolo U. et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum. Reprod. 2015; 30(2): 323-30. https://dx.doi.org/10.1093/humrep/deu292.
- Cicinelli E., De Ziegler D., Nicoletti R., Colafiglio G., Saliani N., Resta L. et al.Chronic endometritis: correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil. Steril. 2008; 89(3): 677-84. https://dx.doi.org/10.1016/j.fertnstert.2007.03.074.
- Cicinelli E., De Ziegler D., Nicoletti R., Tinelli R., Saliani N., Resta L. et al. Poor reliability of vaginal and endocervical cultures for evaluating microbiology of endometrial cavity in women with chronic endometritis. Gynecol. Obstet. Invest. 2009; 68(2): 108-15. https://dx.doi.org/10.1159/000223819.
- Pinto V., Matteo M., Tinelli R., Mitola P.C., De Ziegler D., Cicinelli E. Altered uterine contractility in women with chronic endometritis. Fertil. Steril. 2015; 103(4): 1049-52. https://dx.doi.org/10.1016/j.fertnstert.2015.01.007.
- Moreno I., Cicinelli E., Garcia-Grau I., Gonzalez-Monfort M., Bau D., Vilella F. et al. The diagnosis of chronic endometritis in infertile asymptomatic women: a comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am. J. Obstet. Gynecol. 2018; 218(6): 602. e1-602. e16. https://dx.doi.org/10.1016/j.ajog.2018.02.012.
- Park H.J., Kim Y.S., Yoon T.K., Lee W.S. Chronic endometritis and infertility. Clin. Exp. Reprod. Med. 2016; 43(4): 185-92. https://dx.doi.org/10.5653/cerm.2016.43.4.185.
- Haggerty C.L., Ness R.B., Amortegui A., Hendrix S.L., Hillier S.L., Holley R.L. et al. Endometritis does not predict reproductive morbidity after pelvic inflammatory disease. Am. J. Obstet. Gynecol. 2003; 188(1): 141-8. https://dx.doi.org/10.1067/mob.2003.87.
- Cicinelli E., Resta L., Nicoletti R., Tartagni M., Marinaccio M., Bulletti C, Colafiglio G. Detection of chronic endometritis at fluid hysteroscopy. J. Minim. Invasive Gynecol. 2005; 12(6): 514-8. https://dx.doi.org/10.1016/j.jmig.2005.07.394.
Received 31.01.2020
Accepted 07.02.2020
About the Authors
Natalya I. Tapilskaya, MD, PhD, DSci (Medicine), Professor, Leading research scientist. The Department of Reproduction of the Research Institute of Obstetrics, Gynecology and Reproductology named after D.O. Ott, Professor of the Department of Obstetrics and Gynecology of Saint Petersburg State Pediatric Medical University Ministryof Healthcare of the Russian Federation. Tel.: +7 (812) 328-98-22. E-mail: tapnatalia@yandex.ru. ORCID 0000-0001-5309-0087.
3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation; 2 Litovskaya str., Saint Petersburg, 194100, Russian Federation.
Olga V. Budilovskaya, Researcher of the department of the medical microbiology of the Research Institute of Obstetrics, Gynecology and Reproductology named
after D.O. Ott, Assistant of the Department of Clinical Laboratory Diagnostics of Saint Petersburg State Pediatric Medical University Ministry of Healthcare
of the Russian Federation. Tel.: +7 (812) 328-98-22. E-mail: o.budilovskaya@gmail.com. ORCID 0000-0001-7673-6274.
3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation; 2 Litovskaya str., Saint Petersburg, 194100, Russian Federation.
Anna A. Krysanova, Researcher of the Department of Medical microbiology of the Research Institute of Obstetrics, Gynecology and Reproductology named after D.O. Ott, Assistant of the Department of Clinical Laboratory Diagnostics of Saint Petersburg State Pediatric Medical University Ministry of Healthcare of the Russian Federation.
Tel.: +7 (812) 328-98-22. E-mail: krusanova.anna@mail.ru.
3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation; 2 Litovskaya str., Saint Petersburg, 194100, Russian Federation.
Gulrukhsor Kh. Tolibova, MD, PhD, Head of Immunohistochemistry Laboratory, Research Institute of Obstetrics, Gynecology and Reproductology named after D.O. Ott.
Tel.: +7 (812) 328-98-22, E-mail: gulyatolibova@yandex.ru. ORCID 0000-0002-6216-6220.
3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation.
Anastasiya A. Kopylova, Postgraduate student of the Department of reproduction of the Research Institute of Obstetrics, Gynecology and Reproductology named
after D.O. Ott. Tel.: +7 (812) 328-98-22, E-mail: iagmail@ott.ru.
3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation.
Natalia D. Tsypurdeeva, PhD, Researcher of the Department of reproduction of the Research Institute of Obstetrics, Gynecology and Reproductology named after D.O. Ott. Tel.: +7 (812) 328-98-22. E-mail: tsypurdeevan@mail.ru.
3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation.
Alexander M. Gzgzyan, MD, PhD, DSci (Medicine), Head of the Department of reproduction of the Research Institute of Obstetrics, Gynecology and Reproductology named after D.O. Ott, Professor of the Department of Obstetrics, Gynecology and Reproduction, Medical Faculty, St. Petersburg State University.
Tel.: +7 (812) 328-98-22. E-mail: iagmail@ott.ru. ORCID 0000-0003-3917-9493.
3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation; 7-9 Universitetskaya nab., Saint Petersburg, 199034, Russian Federation.
Alevtina M. Savicheva, MD, PhD, Professor, Head of the Department of Medical microbiology of the Research Institute of Obstetrics, Gynecology and Reproductology named after D.O. Ott, Head of the Department of Clinical Laboratory Diagnostics of Saint Petersburg State Pediatric Medical University Ministry of Healthcare of the Russian Federation. Tel.: +7 (812) 328-98-22. E-mail: savitcheva@mail.ru. ORCID 0000-0003-3870-5930.
3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation; 2 Litovskaya str., Saint Petersburg, 194100, Russian Federation.
Igor Yu. Kogan, MD, PhD, DSci (Medicine), Professor, Corresponding Member of RAS, Director of the Research Institute of Obstetrics, Gynecology and Reproductology named after D.O. Ott, Professor of the Department of Obstetrics, Gynecology and Reproduction, Medical Faculty, St. Petersburg State University. E-mail: ikogan@mail.ru. ORCID ID 0000-0002-7351-6900. 3 Mendeleyevskaya Line, Saint Petersburg, 199034, Russian Federation; 7-9 Universitetskaya nab., Saint Petersburg,
199034, Russian Federation.
For citation: Tapilskaya N.I., Budilovskaya O.V., Krysanova A.A., Tolibova G.Kh., Kopylova A.A., Tsypurdeeva N.D., Gzgzyan A.M., Savicheva A.M., Kogan I.Yu. Еndometrial microbiota of women with chronic endometritis and idiopathic infertility.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 4: 72-81. (In Russian).
https://dx.doi.org/10.18565/aig.2020.4.72-81



