New markers for early prediction of preeclampsia
Relevance: Preeclampsia is a dangerous condition that can be life threatening for the mother and baby. Globally, it is diagnosed in 10 million women annually, representing 3 to 8% of all pregnancies. Currently, there is no confirmed effective treatment for preeclampsia. In this regard, the possibility of predicting this complication is relevant. Objective: To investigate the prognostic value of matrix metalloproteases-2 and metalloproteases-9 as early markers of preeclampsia. Materials and methods: In this prospective study, 72 patients were tested for matrix metalloproteinase-2 and matrix metalloproteinase-9. Thirty-four of them (study group) subsequently developed preeclampsia during pregnancy (20 and 14 patients had moderate and severe preeclampsia, respectively); 38 patients with uncomplicated pregnancy constituted the control group. Results: In pregnant women who developed preeclampsia, matrix metalloproteinase-2 levels at 11-13 weeks' gestation were 155±73.4 ng/mL and were statistically significantly higher than in pregnant women without hypertensive disorders (75.0±32.8 ng/mL). Concentration of matrix metalloproteinase-9 in pregnant women with preeclampsia was significantly lower than in controls [749±296 ng/mL and 1667±552 ng/mL (P<0.001)]. In the first trimester, the matrix metalloproteinase-2 cut-off value to predict the development of preeclampsia was ≥102 ng/mL (sensitivity, 88.24%; specificity, 82.76%). For matrix metalloproteinase-9, concentration of ≤980 ng/mL in the first trimester predicts the development of preeclampsia with a sensitivity of 85.29% and specificity of 84.48%. Conclusion: The study identified cut-off values for matrix metalloproteases-2 and metalloproteases-9 to predict the development of preeclampsia in the first trimester.Timokhina E.V., Strizhakov A.N., Belotserkovtseva L.D., Fedyunina I.A., Zinin V.N., Pesegova S.V.
Keywords
Preeclampsia is a common and life-threatening complication of pregnancy with grave consequences for both the mother and offspring. Each year, 10 million women worldwide develop pre-eclampsia, accounting for 3 to 8% of all pregnancies. Approximately 76,000 pregnant women and 500,000 fetuses and newborns die from preeclampsia each year worldwide [1–3].
In developing nations, preeclampsia is one of the leading causes of maternal mortality, accounting for up to 20% of all maternal deaths [4]. Severe preeclampsia complicates 1.4% of all pregnancies worldwide. This condition usually occurs long before the full term and therefore requires early delivery. This leads to the birth of a preterm newborn, often with growth restriction and all the consequences of prematurity [1–4].
The financial impact of preeclampsia cannot be overlooked. For example, in the United States, the estimated cost of pre-eclampsia including the care of the mother and children for the first 12 months after delivery was US $2.18 billion, which is one third of all postnatal care costs [5].
The next aspect of the impact of preeclampsia on population health is the long-term maternal and child risks. Preeclampsia in pregnancy significantly increases the risk of distant cardiovascular disease (myocardial infarction, stroke), metabolic disorders (type I and type II diabetes mellitus), and sudden death later in life [2, 6]. Evidence has emerged that hypertensive disorders in pregnancy are associated with a higher risk of serious mental disorders in offspring in adulthood [7].
Currently, there is no confirmed effective treatment for preeclampsia except to deliver the baby, which means preterm birth and all the problems of a preterm newborn, especially with extremely low birth weight. In this regard, the issue of predicting preeclampsia is relevant.
The main pathological processes leading to the development of preeclampsia occur as early as the first trimester. This is a sequential impairment of syncytiotrophoblast invasion, defective remodeling of spiral arteries with subsequent placental oxidative stress, and imbalance of pro- and anti-angiogenic factors [1, 3, 8]. Extracellular proteolytic enzyme – matrix metalloproteinases (MMPs) of types 2, 3, 7, 9, 13 play a vital role in these stages of pregnancy development [9, 10]. MMPs are a family of enzymes that degrade extracellular matrix molecules [10, 11]. MMPs produced by trophoblast cells play a major role for successful implantation: MMP-2, -3, -7, -9, -13 [5, 6]; MMP-2 and MMP-9 are the key effectors of this process [9-11]. MMP-2, MMP-9, MMP-3, and MMP-13 take part in the process of spiral arteries remodeling. They ensure the degradation of endothelin and adrenomedullin, leading to the vasodilation of spiral arteries and the formation of adequate placental blood flow later on.
Several studies indicate the involvement of MMP in the key processes of the first trimester of pregnancy responsible for the subsequent development of preeclampsia [9-12]. At the same time, to date, there are no studies on the practical application of MMP to predict pre-eclampsia. There are few studies [13–15] that confirm a direct correlation of MMP-2 and an inverse correlation of MMP-9 with the development of preeclampsia, but they offer no real guidelines for use in clinical practice.
Therefore, we conducted a study to investigate the prognostic role of MMP-2 and MMP-9 in the development of preeclampsia and establish their cut-off concentrations to predict preeclampsia in clinical practice.
Materials and methods
Study design
To determine the prognostic value of MMP-2 and MMP-9 as early markers of preeclampsia, we conducted a prospective study of 310 patients aged 18 to 45 years with singleton pregnancies. The patients were included in the study at 10–11 weeks of gestation.
The inclusion criteria were informed consent of the patient to participate in the study, age over 18 years and singleton pregnancy. The non-inclusion criteria were pregnancy after assisted reproductive technology, multiple pregnancies, connective tissue dysplasia, type I and II diabetes, cancer, autoimmune diseases, hereditary thrombophilia, infectious diseases (HIV infection, hepatitis), taking medications, psychostimulant drugs, smoking, and drug addiction.
Peripheral venous blood samples were collected from the study subjects to determine the concentration of MMP-2 and MMP-9 types at 11-13 weeks of gestation. The sampling was performed simultaneously with the patients' first screening examination. The patients were under our observation throughout pregnancy until delivery. Blood samples were taken after the end of the study patients' pregnancy.
Informed consent was obtained from each woman to participate in the study and collect venous blood samples. The study was approved by the Research Ethics Committee of the I.M. Sechenov First Moscow State Medical University (Sechenov University), the study protocol No10-17 dated 16.11.2017. During the study, 18 patients (5.8%) were excluded. Of these, six were found to be at high risk of fetal chromosomal abnormalities by biochemical screening and non-invasive prenatal tests. A patient was found to have a fetal congenital defect. For this reason, these patients underwent termination of pregnancy for medical indications. Spontaneous miscarriage occurred in 11 patients before 22 weeks of pregnancy. Preterm births unrelated to preeclampsia and/or placental insufficiency occurred in 29 patients (8.7%).
Preeclampsia was diagnosed in 34 patients (10.9%). Of these, 20 pregnant women (58.8%) were classified as having moderate and 14 (41.2%) as having severe preeclampsia. To make the diagnosis of preeclampsia, we used the criteria of the Clinical Guidelines of the Russian Ministry of Health "Hypertensive Complications of Pregnancy, Childbirth, and the Postpartum Period. Preeclampsia. And Eclampsia."
Patients with the following criteria were classified as moderate preeclampsia: arterial hypertension – systolic BP 140–159 mm Hg or diastolic BP 90–109 mm Hg that occurred at >20 weeks in a woman with a normal history of BP and proteinuria greater than 0.3g/L in a 24-hour urine collection.
Preeclampsia was considered severe if one and/or more of the criteria for severe preeclampsia were present: severe arterial hypertension with diastolic BP ≥110 mmHg, systolic BP ≥160 mmHg, proteinuria greater than 0.3 g/L in a 24-hour urine collection, and symptoms of central nervous system disorders (visual impairment, headache); renal dysfunction (oliguria <500 ml/day, increased creatinine level); pulmonary edema; sudden appearance of edema of face, arms, legs; edema of the optic disc; impaired liver function; epigastrium/upper right quadrant pain; severe thrombocytopenia (<100×106/L); HELLP syndrome; fetal distress (fetal growth restriction, small fetus, negative non-stress test).
Pregnant women with preeclampsia constituted the study group (n=34). The remaining 229 patients had a pregnancy without hypertensive disorders that ended in full-term delivery. Of these, 38 patients were randomly selected by blind selection and tested for blood concentrations of MMP-2 and MMP-9. These women formed a control group (n=38). Therefore, altogether, we tested levels of MMP-2 and MMP-9 in 72 pregnant women.
Laboratory studies
The blood samples were placed in sterile tubes at 4∘ C and centrifuged for 15 min at 1500 ×g. Plasma samples were then stored at -80°C until analysis. MMP-2 and MMP-9 levels were assayed after delivery, when patients were assigned to groups based on whether they had preeclampsia or an uncomplicated pregnancy.
MMP-2 and MMP-9 levels were determined using an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol (Cloud-Clone Corp. Houston, USA). Kits designed for quantification of MMP-2 (SEA100Hu, minimum detectable concentration of 0.27 ng/mL) and MMP-9 (SEA553Hu, minimum detectable concentration of 0.055 ng/mL) by enzyme-linked immunosorbent assay (ELISA) were used. The samples were collected, stored, and analyzed according to the manufacturer's instructions (Cloud-Clone Corp. Houston, USA).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 26.0 software. The normality of the distribution was tested by the Shapiro–Wilk test. Quantitative variables that showed normal distribution were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise, the medians (Me) with the interquartile range (Q1; Q3) were reported. Normally distributed continuous variables were compared between two groups with a Student’s t test. Differences were considered statistically significant at p<0.05. The MMP concentrations did not meet the normality assumptions and were compared with a nonparametric Mann–Whitney U test. Differences were considered statistically significant at p<0.05. Categorical data were described as counts and percentages and compared by Fisher's exact test or Pearson's χ2 test. To assess the diagnostic accuracy of MMR concentration in the first trimester in predicting the development of preeclampsia, ROC-curve analysis was used. It was used to determine the optimal cut-off value of a quantitative variables, which allows to classify patients according to the risk of outcome and has the best combination of sensitivity and specificity. The quality of the prognostic model obtained by this method was assessed based on the area under the ROC curve with standard error and 95% confidence interval (CI) and the level of statistical significance. An adjusted Wald method was used to calculate the 95% CI of proportion.
Results
The levels of MMP-2 and MMP-9 were measured in 72 patients. Thirty-four of them developed preeclampsia later in pregnancy (20 and 14 patients had moderate and severe preeclampsia, respectively); they constituted the study group. Thirty-eight patients whose pregnancies ended at full term without hypertensive disorders and placental insufficiency/fetal growth restriction constituted the control group.
Clinical characteristics
The clinical characteristics of the study participants are shown in Table 1. The mean age of the women in the study group was 30.5 (6.31) years and was statistically significantly higher than that of the control group patients [25.2 (5.12)] years. Body mass index in the study group was significantly higher [28.1 (2.73) kg/m2] than in the control group [22.9 (2.41) kg/m2] (p≤0.001). The frequency of previous chronic arterial hypertension in the study group was 2.9%.
There were 23/34(67.6%) and 29/38(77.4%) primiparas in the study and control groups, respectively. Multiparas constituted 1/34(32.4%) and 12/38(22.6%), respectively. The differences were statistically insignificant (p<0.05).
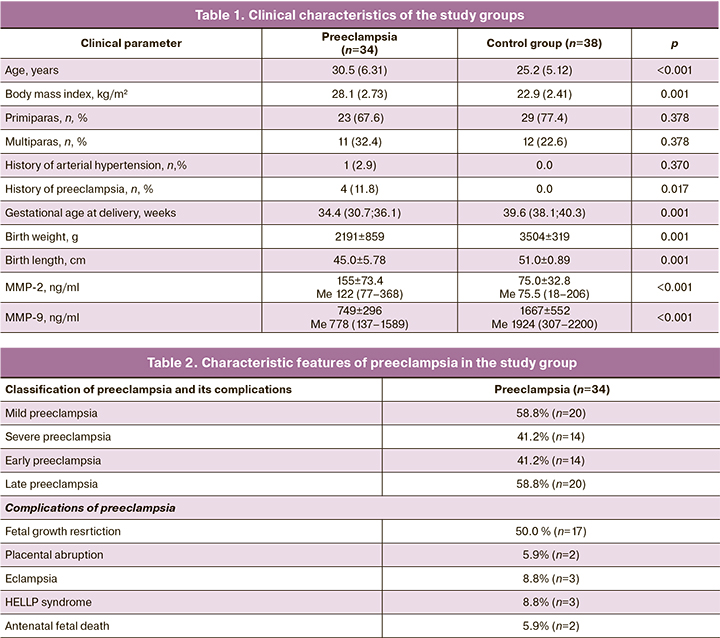
It should be noted that 4/34 (11.8%) of multigravida mothers in the study group also had complicated preeclampsia.
The gestational age at delivery was 34.4 (30.7; 36.1) weeks in the study group and 39.6 (38.1; 40.3) weeks in the control group (p<0.001).
Characteristic features of preeclampsia in the patients of the study group are presented in Table 2.
In 14/34 (41.2%) patients, preeclampsia was classified as early, that is, it developed and required delivery before 34 weeks of pregnancy (28.1; 33.8 weeks). Late preeclampsia [>34 (35.8; 39.5) weeks] was observed in 20/34 (58.8%) patients. Fourteen (41.2%) patients had severe preeclampsia.
Complications of preeclampsia observed in our study included [2/34 (5.9%)], eclampsia [3/34 (8.8%)], HELLP syndrome [3/34 (8.8%)], and antenatal fetal death [2/34 (5.9%)]. Half of the patients with preeclampsia had concomitant placental insufficiency and fetal growth restriction.
Findings of MMR plasma level
The MMR-2 and MMR-9 concentrations at 11–13 weeks' gestation are presented in Table 1.
In pregnant women with subsequent preeclampsia, MMP-2 levels at 11–13 weeks' gestation were 155±73.4 ng/mL and were statistically significantly higher than in pregnant women with no subsequent hypertensive disorders, 75.0±32.8 ng/mL (p<0.001) (Fig. 1).
In the study group, MMP-9 concentration were a statistically significantly lower compared to controls [749±296 ng/ml and 1667±552 ng/ml (p<0.001)] (Fig. 2).
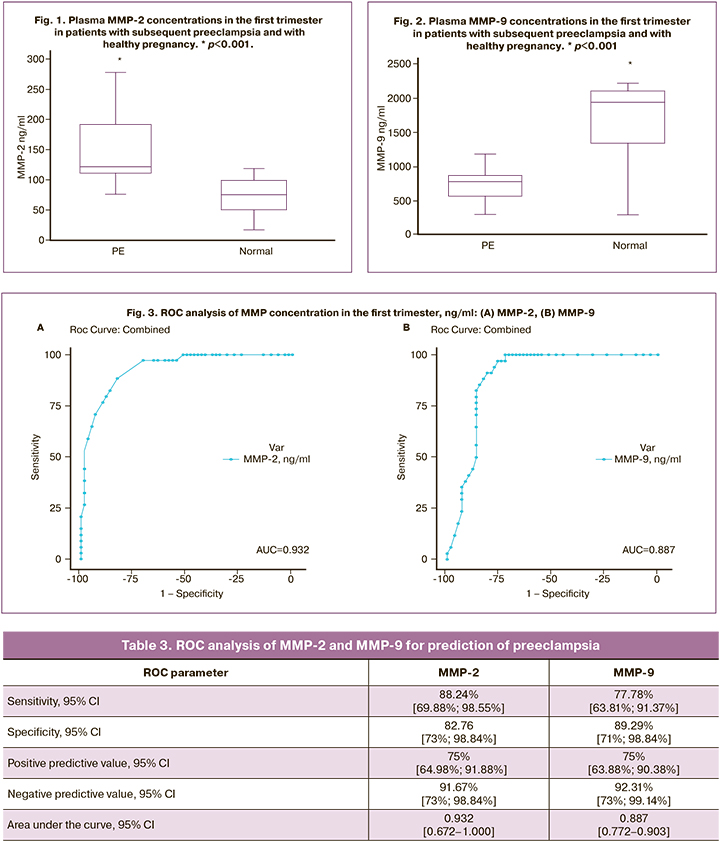
Determination of MMP thresholds for predicting preeclampsia - ROC analysis
To establish the prognostic value of MMP-2 and MMP-9 for preeclampsia, we performed an ROC analysis. The results are presented in Table 3.
Considering blood concentrations of MMP-2 and MMP-9 at 11–13 weeks of gestation as a prognostic point, the ROC-analysis established the threshold values (Table 3, Fig. 3).
The ROC analysis performed for MMP-2 yielded a threshold value of ≥102 ng/mL, which predicts the development of preeclampsia with a sensitivity of 88.24% and specificity of 82.76% (Table 3). Our ROC analysis allowed us to define a MMP-9 concentration ≤980 ng/mL as the cut-off for predicting preeclampsia with a sensitivity of 77.78% and specificity of 89.29% (Table 3).
Discussion
Currently, preeclampsia remains a complex and unresolved problem.
First, there is no downward trend in the incidence of this complication in both developed and developing countries globally. It remains a major cause of maternal mortality and presents a substantial challenge even in countries with advanced health care systems [2, 4]. Secondly, the pathophysiological mechanisms underlying preeclampsia begin to be developed in the first trimester, many weeks before the clinical manifestation of this complication. Deviations from the normal course of the processes of implantation, trophoblast invasion, gestational transformation of the spiral arteries necessary for the adequate growth and development of the fetus and the adaptation of the maternal body to pregnancy – all this further leads to preeclampsia. In the first trimester, these processes do not have any clinical manifestations that make clinicians alert. However, the pregnant woman is already in the risk zone, which is realized in the second half of pregnancy.
Thirdly, when the diagnosis of preeclampsia is made, there are no effective methods for its treatment. The only approach is delivery, because only the removal of all the elements of the fetus from the mother's body will stop the cascade of multiple mechanisms running, damaging blood vessels and target organs.
Considering the above issues, it is relevant to search for predictors of preeclampsia with high specificity for its prediction, already from the first weeks of pregnancy. The prediction of preeclampsia is based on the identification of risk factors, assessment of ultrasound parameters, and biochemical markers [1–3, 8].
Current biochemical markers (placental growth factor, endothelin, plasma protein-13) are characterized by insufficient sensitivity and specificity [1, 3, 8].
Therefore, the search for early markers of preeclampsia with high sensitivity and specificity, working already from the first trimester, is relevant.
Since MMPs are involved in the key processes of the first trimester of pregnancy that ensure its normal development later on (implantation, trophoblast invasion, gestational transformation of the spiral arteries), an increase or decrease in MMP levels will indicate an impaired course of these processes. Therefore, in order to predict preeclampsia, changes in concentration and metalloprotease activity should be studied.
In our study, we found that at 11–13 weeks' gestation, MMR-2 concentrations were statistically significantly higher in the group of pregnant women who subsequently develop preeclampsia than in healthy pregnancies (155±73.4 ng/mL and 75.0±32.8 ng/mL (p<0.001).
In contrast, among pregnant women with subsequent preeclampsia the MMP-9 concentration was significantly lower than that in the control group [749±296 ng/mL and 1667±552 ng/mL (p<0.001), respectively].
MMP-2 and MMP-9 are known to be involved in the processes of blastocyst implantation. They are responsible for collagen matrix breakdown and successful invasion of extravillous cytotrophoblasts into the endometrium. Their deficiency can disrupt this key process of pregnancy and further lead to disruption of chorion and placenta formation [9, 11].
This is also confirmed by the results of our work.
MMP-9 is involved in the remodeling of spiral arteries at early pregnancy stages. Our findings on the reduced level of MMP-9 in pregnant women with subsequent development of preeclampsia confirm that a decrease in this enzyme disrupts the physiological transformation of the spiral arteries, which leads to further development of preeclampsia. Therefore, a decrease in the level of MMP-9 at 11–13 weeks gestation can be considered as a marker for predicting preeclampsia.
MMP-2 plays the leading role in implantation. Chen at al. investigated the role of MMP-2 and MMP-9 in the rat placenta during the first days of gestation. They found a correlation between decreased MMP-2 and MMP-9 and trophoblast invasion defect, insufficient rearrangement of spiral arteries, impaired placental angiogenesis and type IV collagen accumulation, which subsequently leads to placental ischemia and preeclampsia [15].
Our study found elevated blood levels of MMP-2 in pregnant women who subsequently developed preeclampsia at 11–13 weeks. The differences in the data can be explained by the fact that we assessed MMP-2 levels at 11–13 weeks of gestation, and, possibly, at this gestational age, the malformed placenta already triggers endothelial dysfunction, and MMP-2 is its early and sensitive marker.
Palei A.C. et al. data are consistent with our results and indicate that MMP-2 and MMP-9 levels are abnormally elevated already from the first trimester in pregnant women with subsequent development of preeclampsia [16].
Martinez-Fierro M.L. et al. conducted a study of MMP-2 in urine. They showed that its level increases as early as the 12th–16th week in patients with the further development of preeclampsia. An increase in MMP-2 concentration at 12 weeks predicted the development of preeclampsia with a sensitivity of 100% and specificity of 62.5% and after week 16 with a sensitivity of 87.5% and specificity of 74.1% [17].
A number of studies have investigated the practical application of MMP to predict preeclampsia in the second and third trimesters of pregnancy. For example, Feng H. et al. [14] analyzed not only MMP-2 and MMP-9 levels, but also their ratios in pregnant women in the second trimester from week 20 as potential biomarkers of preeclampsia. The authors showed that not only individual MMP-2 and MMP-9 levels but also MMP-2/MMP-9 ratios were significantly higher in pregnant women who developed preeclampsia later.
The results of our study are in agreement with those of these authors. We also found an increase in MMP-2 levels and a decrease in MMP-9 levels, which will certainly lead to an increase in their ratio. However, a significant advantage of our work is the evaluation of these biomarkers already in the first trimester at 11–13 weeks, simultaneously with the prenatal screening.
It should be noted that studies on the role of MMR in the first trimester of pregnancy are lacking. Many of them have been conducted in animal models, so further studies are needed to clarify the role of MMP-2 and MMP-9, as potential markers of preeclampsia.
Conclusion
In summary, in the present clinical study we tried to establish the value of MMP-2 and MMP-9 as biomarkers for early prediction of preeclampsia starting from the first trimester of pregnancy.
Key processes that ensure proper placental formation and successful pregnancy, such as implantation, trophoblast invasion, and subsequent gestational transformation of the spiral arteries, begin from 5–6 weeks of gestation and continue until the final placental formation by 18 weeks of pregnancy.
In this work, we sampled blood from patients at 11–13 weeks' gestation and determined the plasma concentration of MMP. This timing of inclusion in the study is due to the fact that at this gestational age all pregnant women undergo prenatal screening, which includes mandatory blood sampling.
Consequently, we can identify a limitation of our study – the time of biomaterial sampling. MMP-2 and MMP-9 concentrations may vary from week 5 to week 11 of pregnancy in parallel with the stage of placental formation.
Therefore, we are currently conducting a study with MMP analysis at 5–6, 11–13, and 18–19 weeks' gestation to establish the role of each MMP in implantation, invasion, and gestational transformation of the spiral arteries.
A second limitation of our study is that we established the importance of MMPs as biomarkers of preeclampsia as a whole, without dividing it into early and late stages. In clinical practice, there are two different variants of the course of preeclampsia. Early preeclampsia is characterized by a higher incidence of life-threatening complications with grave consequences for both the mothers and the child and is also more resource-intensive. Therefore, differential prediction of early and late preeclampsia is relevant. Further study of the practical application of MMP in this matter is planned. Specific prediction of both early and late preeclampsia is promising, including other MMPs as well as their inhibitors in the research spectrum.
References
- Poon L.C., Shennan A., Hyett J.A., Kapur A., Hadar E., Divakar H. et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019; 145(Suppl. 1): 1-33. https://dx.doi.org/10.1002/ijgo.12802.
- NICE. Hypertension in pregnancy: diagnosis and management NICE guideline Published: 25 June 2019. Available at: www.nice.org.uk/guidance/ng133
- Тимохина Е.В., Стрижаков А.Н., Зафириди Н.В., Губанова Е.С. Инновационный подход к прогнозированию и терапии преэклампсии – мировой опыт. Акушерство и гинекология. 2019; 5: 5-10. [Timokhina E.V., Strizhakov A.N., Zafiridi N.V., Gubanova E.S. An innovative approach to the prediction and treatment of preeclampsia – world experience. Obstetrics and Gynecology. 2019; 5: 5-10. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.5.5-10.
- Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO Available at: https://www.who.int/reproductivehealth/publications/maternal-mortality-2000-2017/en/#
- Li R., Tsigas E.Z., Callaghan W.M. Health and economic burden of preeclampsia: no time for complacency. Am. J. Obstet. Gynecol. 2017; 217(3): 235-6. https://dx.doi.org/10.1016/j.ajog.2017.06.011.
- Theilen L.H., Meeks H., Fraser A., Esplin M.S., Smith K.R., Varner M.W. Long-term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am. J. Obstet. Gynecol. 2018; 219(1): 107.e1-107.e6. https://dx.doi.org/10.1016/j.ajog.2018.04.002.
- Lahti-Pulkkinen M., Girchenko P., Tuovinen S., Sammallahti S., Reynolds R.M., Lahti J. et al. Maternal hypertensive pregnancy disorders and mental disorders in Children. Hypertension. 2020; 75(6): 1429-38. https://dx.doi.org/10.1161/HYPERTENSIONAHA.119.14140.
- Bartsch E., Medcalf K.E., Park A.L., Ray J.G.; High Risk of Pre-eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016; 353: i1753. https://dx.doi.org/10.1136/bmj.i1753.
- Laskowska M. Altered maternal serum matrix metalloproteinases MMP-2, MMP-3, MMP-9, and MMP-13 in severe early- and late-onset preeclampsia. Biomed. Res. Int. 2017; 2017: 6432426. https://dx.doi.org/10.1155/2017/6432426.
- Su M.T., Tsai P.Y., Tsai H.L., Chen Y.C., Kuo P.L. miR-346 and miR-582-3p-regulated EG-VEGF expression and trophoblast invasion via matrix metalloproteinases 2 and 9. Biofactors. 2017; 43(2): 210-9. https://dx.doi.org/10.1002/biof.1325.
- Espino Y., Sosa S., Flores-Pliego A., Espejel-Nuñez A., Medina-Bastidas D, Vadillo-Ortega F. et al. New insights into the role of matrix metalloproteinases in preeclampsia. Int. J. Mol. Sci. 2017; 18(7): 1448. https://dx.doi.org/10.3390/ijms18071448.
- Erez O., Romero R., Maymon E., Chaemsaithong P., Done B., Pacora P. et al. The prediction of late-onset preeclampsia: Results from a longitudinal proteomics study. PLoS One. 2017; 12(7): e0181468. https://dx.doi.org/10.1371/journal.pone.0181468.
- Plaks V., Rinkenberger J., Dai J., Flannery M., Sund M., Kanasaki K. et al. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc. Natl. Acad. Sci. USA. 2013; 110(27): 11109-14. https://dx.doi.org/10.1073/pnas.1309561110.
- Feng H., Wang L., Zhang M., Zhang Z., Guo W., Wang X. Ratio of matrix metalloproteinase-2 to -9 is a more accurate predictive biomarker in women with suspected pre-eclampsia. Biosci. Rep. 2017; 37(2): BSR20160508. https://dx.doi.org/10.1042/BSR20160508.
- Lin C., He H., Cui N., Ren Z., Zhu M., Khalil R.A. Decreased uterine vascularization and uterine arterial expansive remodeling with reduced matrix metalloproteinase-2 and -9 in hypertensive pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2020; 318(1): H165-80. https://dx.doi.org/10.1152/ajpheart.00602.2019.
- Palei A.C., Sandrim V.C., Duarte G., Cavalli R.C., Gerlach R.F., Tanus-Santos J.E. Matrix metalloproteinase (MMP)-9 genotypes and haplotypes in preeclampsia and gestational hypertension. Clin. Chim. Acta. 2010; 411(11-12): 874-7. https://dx.doi.org/10.1016/j.cca.2010.03.002.
- Martinez-Fierro M.L., Perez-Favila A., Garza-Veloz I., Espinoza-Juarez M.A., Avila-Carrasco L., Delgado-Enciso I. et al. Matrix metalloproteinase multiplex screening identifies increased MMP-2 urine concentrations in women predicted to develop preeclampsia. Biomarkers. 2018; 23(1): 18-24. https://dx.doi.org/10.1080/1354750X.2017.1279214.
Received 14.03.2022
Accepted 27.05.2022
About the Authors
Elena V. Timokhina, Dr. Med. Sci., Professor, Department of Obstetrics, Gynecology and Perinatology, I.M. Sechenov First Moscow State Medical University,Ministry of Health of Russia (Sechenov University), +7(499)782-30-45, timokhina_e_v@staff.sechenov.ru, 119991, Russia, Moscow, B. Pirogovskaya str., 2-4.
Alexander N. Strizhakov, Academician of RAS, Head of Department of Obstetrics, Gynecology and Perinatology, I.M. Sechenov First Moscow State Medical University,
Ministry of Health of Russia (Sechenov University), +7(499)782-30-45, strizhakov_a_n@staff.sechenov.ru, 119991, Russia, Moscow, B. Pirogovskaya str., 2-4.
Larisa D. Belotserkovtseva, Dr. Med. Sci., Professor, Merited Doctor of the Russian Federation, Head of Obstetrics, Gynecology and Perinatology Department,
Surgut State University; Chief Physician, Surgut Clinical Perinatal Center, +7(950)538-08-48, info@surgut-kpc.ru,
628415, Russia, Khanty-Mansiysk Autonomous Okrug – Ugra, Surgut, Gubkin str., 1/2.
Irina A. Fedyunina, PhD, Associate Professor at the Department of Obstetrics, Gynecology and Perinatology, I.M. Sechenov First Moscow State Medical University,
Ministry of Health of Russia (Sechenov University), +7(499)782-30-45, fedyunina_i_a@staff.sechenov.ru, 119991, Russia, Moscow, B. Pirogovskaya str., 2-4.
Vadim N. Zinin, PhD, Head of Gravitational Blood Surgery Department, Surgut Clinical Perinatal Center,
628415, Russia, Khanty-Mansiysk Autonomous Okrug – Ugra, Surgut, Gubkin str., 1/2.
Svetlana V. Pesegova, Post-Graduate Student, Department of Obstetrics, Gynecology and Perinatology, I.M. Sechenov First Moscow State Medical University,
Ministry of Health of Russia (Sechenov University), +7(499)782-30-45, pesegova_s_v@staff.sechenov.ru, 119991, Russia, Moscow, B. Pirogovskaya str., 2-4.
Corresponding author: Elena V. Timokhina, timokhina_e_v@staff.sechenov.ru
Authors' contributions: Timokhina E.V., Strizhakov A.N., Belotserkovtseva L.D. – conception and design of the study; Pesegova S.V., Fedyunina I.A. – data collection, manuscript drafting; Timokhina E.V., Zinin V.N. – manuscript reviewing and editing; Timokhina E.V. – statistical analysis and visualization.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was supported by the Russian Foundation for Basic Research, grant no. 19-315-90088/19.
Acknowledgement: The authors express their gratitude to the S.S. Yudin State Clinical Hospital of the Moscow City Health Department for assistance and support in the study and to the staff of the Central Laboratory and Diagnostic Service
of the I.M. Sechenov First MSMU for performing the laboratory tests.
Ethical Approval: The study was approved by the Research Ethics Committee of the I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Timokhina E.V., Strizhakov A.N., Belotserkovtseva L.D., Fedyunina I.A., Zinin V.N., Pesegova S.V. New markers for early prediction of preeclampsia.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 6: 50-58 (in Russian)
https://dx.doi.org/10.18565/aig.2022.6.50-58



