Potential of first trimester plasma lipidome in high-risk pregnancy groups
Minaeva E.A., Starodubtseva N.L., Shmakov R.G., Chagovets V.V., Tokareva A.O., Novoselova A.V., Kukaev E.N., Frankevich V.E.
Objective: To evaluate the relationship between plasma lipids, changes in their concentration during early pregnancy, and the risk of preeclampsia (PE) in pregnant women at a high risk for placenta-mediated complications. Materials and methods: This prospective case-control study included 66 pregnant women, including a group at high risk for placenta-associated complications based on medical history and first trimester screening (n=38) and a control group (n=28). Lipid extracts of blood plasma from the first trimester of pregnancy were analyzed using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Lipids were identified using Lipid Match R-script by accurate mass using the Lipid Maps database and characteristic tandem mass spectra (MS/MS). Based on lipids identified as having a statistically significant correlation with clinical data (Spearman's criterion), a model of projections to latent structures was constructed using two predictive axes. From lipids with a variable projection value greater than 1 in the model, those with which the model based on logistic regression had the lowest Akaike information criterion and coefficients that were significantly different from zero were selected. Results: Correlation analysis according to high-risk groups identified 48 lipids, for which the level of correlation with at least one clinical parameter was average or above average (R>0.6), belonging to the classes of phosphatidylcholines, phosphatidylinositols, sphingomyelins, and triglycerides. Two logistic regression models were constructed to identify patients at high risk for PE by plasma lipid levels in the first trimester of pregnancy with optimal sensitivity and specificity of 0.91 and 0.91 for the positive ion regimen (phosphatidylethanolamine PE 16:0_22:6 and phosphatidylcholine PC 18:0_18:1) and optimal sensitivity and specificity of 0.82 and 0.96 for the negative ion mode (sphingomyelins SM d24:0/18:1 and SM d22:1/20:4). Survival function analysis yielded a relative risk for the group defined as high-risk using the model based on lipid profile in the positive ion mode of 33.5 with CI 4.7–241, and a relative risk for the PE screening outcome of 2.67 with CI 0.74–9.64. Conclusion: Changes in the first-trimester plasma lipid spectrum, mainly phosphatidylcholines, lysophosphatidylcholines, phosphatidylethanolamine, triglycerides, and sphingomyelins, are associated with the risk of PE in the high-risk group. This allows us to propose logistic regression models based on first-trimester plasma marker lipid levels as a refinement after the first-trimester screening. In the future, the data obtained may contribute to the improvement of preventive measures for placenta-associated disorders and the timely monitoring of their development.
Authors' contributions: Chagovets V.V., Minaeva E.A., Shmakov R.G., Starodubtseva N.L., Frankevich V.E. – conception and design of the study; Tokareva A.O., Chagovets V.V., Minaeva E.A., Kukaev E.N., Starodubtseva N.L. – collection and processing of the material; Tokareva A.O., Chagovets V.V., Starodubtseva N.L., Frankevich V.E. – statistical analysis; Tokareva A.O., Chagovets V.V., Minaeva E.A., Starodubtseva N.L., Kukaev E.N. – drafting of the manuscript; Shmakov R.G., Frankevich V.E., Starodubtseva N.L. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was performed within the framework of the experimental scientific research "Improving the management and timing of delivery of pregnant women with fetal growth retardation based on the study of molecular-genetic and metabolomic factors with the subsequent introduction of modern methods of diagnostics of the severity of the course of this gestational complication" 121040600408-4.
Acknowledgments: The authors would like to thank the Laboratory of Biological Material Collection and Storage (Biobank) for providing the samples.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Minaeva E.A., Starodubtseva N.L., Shmakov R.G., Chagovets V.V., Tokareva A.O., Novoselova A.V., Kukaev E.N., Frankevich V.E. Potential of first trimester plasma lipidome in high-risk pregnancy groups. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (10): 108-118 (in Russian) https://dx.doi.org/10.18565/aig.2023.229
Keywords
Severe preeclampsia (PE) is associated with significant perinatal and neonatal complications. The underlying vascular disturbances, oxidative stress, and endothelial damage can contribute to fetal growth restriction (FGR). Intrauterine fetal death is a life-threatening complication that requires careful attention and efficient prognosis and prevention of PE. Interestingly, in low- and middle-income countries, the incidence of antenatal fetal death in women with PE is three times higher than in high-income countries [1].
Pregnant women diagnosed with early severe PE are at a significantly higher risk of preterm labor since delivery is currently the only effective treatment for managing these complications. Approximately 25% of cases of PE require delivery before 37 weeks of gestation [2, 3]. Prematurely born infants face elevated risks of neonatal mortality and morbidity such as the development of necrotizing enterocolitis, retinopathy of prematurity, bronchopulmonary dysplasia, intraventricular hemorrhage, and neurodevelopmental disorders. The adverse outcomes experienced by neonates can be attributed to prematurity or can arise directly as a consequence of PE. Both these factors are often involved in severe early onset PE cases. In a publication by Osmond C. and Barker D.J. in 2000, they hypothesized that fetal environment has an impact on adult health. Their hypothesis suggests that inadequate intrauterine nutrient intake due to placental insufficiency may increase the risk of insulin resistance, diabetes mellitus, coronary heart disease, and hypertension in adulthood [4].
A growing body of new evidence points to an increased risk of developing long-term adverse health outcomes in women with PE [5]. The American Heart Association notes an increased risk of cardiovascular disease in mothers with PE and recommends careful collection of obstetric history to assess cardiovascular risk [6, 7]. A 2007 meta-analysis that included nearly 200,000 pregnant women with PE showed a relative risk of 3.7, 2.16, and 1.81 for developing hypertension, coronary heart disease, and stroke, 10–15 years after a given pregnancy. A subsequent 2016 meta-analysis showed a threefold increased risk of chronic hypertension and a two-fold increased risk of cardiovascular disease and stroke in mothers with PE compared to women with uncomplicated pregnancies [8]. Al-Nasiry S. et al. in their study showed that women with PE are 2 times more likely to develop future metabolic syndrome [9].
Inhibin-A is a glycoprotein hormone produced by placental trophoblasts [10, 11]. It is used for the combined screening of chromosomal abnormalities [12]. Several studies have shown that women who develop PE have higher inhibin A levels in the first trimester. However, its sensitivity was too low to be used as the sole marker for predicting PE [13].
Pregnancy-associated plasma protein A (PARP-A) is mainly produced by placental syncytiotrophoblasts [14]. Its decrease is associated with pregnancy complications, such as PE, fetal growth restriction, preterm labor, and spontaneous miscarriage [15, 16]. PAPP-A2 is a homolog of PAPP-A with 46% amino acid similarity. PAPP-A2 is also expressed by syncytiotrophoblasts and specifically cleaves insulin-like growth factor-binding protein 5 (IGFBP-5), whereas PAPP-A cleaves IGFBP-4. Unlike PAPP-A, cleavage of IGFBP-5 by PAPP-A2 does not require IGF, which is required for normal placentation. Maternal serum PAPP-A2 concentrations have been shown to be significantly higher in advanced PE than in uncomplicated pregnancies. PAPP-A2 plays an important role in normal placentation, and changes in its concentration may be associated with placenta-associated pregnancy complications [17].
There are data on PE prediction using maternal plasma proteins in domestic and international studies [18, 19]. Tarca A.L. et al. in their 2019 study found that multiprotein models obtained from the 16th to 22nd week of gestation can predict early PE with a sensitivity of 71% with a false positive rate of 10% [20]. In a 2017 article. Sergeyeva V.A. et al. also found 12 peptides that can unambiguously distinguish healthy patients from the group of PE or PE on the background of chronic arterial hypertension [21]. Starodubtseva N. et al. found SERPINA1 peptide in urine, which has a similar diagnostic pattern to previously known PE markers such as sFlt-1/PLGF. Assessment of SERPINA1 peptide in urine can be used as a diagnostic test for PE severity to determine further pregnancy management strategies or the need for urgent surgical delivery [22].
One of the topical issues in modern obstetrics is the prediction of placenta-associated complications. Two groups of prediction models, biochemical and ultrasonic, have been proposed at different times. Biochemical markers are predominantly represented by peptides secreted by the placenta. These biomarkers include pro- and angiogenic factors, including placental growth factor (PlGF), soluble fms-like tyrosine kinase 1 (sFlt-1), RARP-A, RARP-A2, and N-terminal pro-B-type natriuretic peptide (NT-proBNP), which are sensitive markers of heart failure and late-onset PE [23–25].
To date, determination of the plasma lipid profile of pregnant women for the prediction of major obstetric syndromes is a promising area. Studies on the plasma lipid profile of women in the first trimester of pregnancy have demonstrated changes in the levels of some classes of plasma lipids, especially triglycerides, in women who developed PE. In turn, induced hyperlipidemia causes endothelial maternal vascular dysfunction. Women with a history of placenta-associated complications have significant differences in lipid parameters and increased susceptibility to lipoprotein oxidation compared with women whose previous pregnancy was uneventful. Thus, the measurement of serum lipid profiles may have good prognostic value [26, 27]. Hypertriglyceridemia results from the physiological increase in estrogen levels during pregnancy. Estrogens induce the hepatic biosynthesis of endogenous triglycerides. Hypertriglyceridemia may also occur during the pathogenesis of hypertensive disorders during pregnancy [28]. Yu L. et al. report that women with elevated triglyceride levels have a 4-fold higher risk of developing PE than women with normal laboratory values [29]. Gofman J.W. et al. in their 2011 study suggested that changes in lipid levels between 28th and 32nd week of pregnancy can help predict the development of PE. Serum triglyceride levels were higher in women with severe PE (210.57±14.09 mg/dL) than in women who developed moderate PE (195.33±14.38 mg/dL) and in the control group (152.30±9.22 mg/dL). Elevated serum triglyceride levels were significantly higher (p<0.01) in patients who developed PE [30]. In a 2018 study, Ahmed et al. observed that PE was associated with hypertriglyceridemia, increased levels of low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) cholesterol, and decreased levels of high-density lipoprotein (HDL). The greater the severity of PE, the higher the serum triglyceride, cholesterol, LDL, and VLDL levels, and the lower the HDL levels. The authors noted that it is critical that the serum lipid profile be assessed throughout pregnancy as it may aid in the early detection of obstetric complications [31].
It can be assumed that a detailed study of the lipid profile of pregnant women will identify markers for early prediction of placenta-associated diseases for closer observation by an obstetrician-gynecologist, monitoring of clinical, laboratory, and functional examination methods, and timely administration of treatment.
This study aimed to evaluate the relationship between plasma lipids, changes in their concentration during early pregnancy, and the risk of preeclampsia in pregnant women at high risk for placenta-mediated complications.
Materials and methods
This prospective case-control study included 66 pregnant women, including a group at high risk for placenta-associated complications (group 1, n=38) based on medical history (n=12) and first trimester screening (n=34) and a control group (group 2, n=28). Patients were selected from women who provided blood plasma samples at the time of referral for the first screening of pregnancy to the outpatient department of Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of the Russian Federation. Inclusion criteria were age of the patients 18–45 years; singleton pregnancy; gestational age at randomization 7–13 weeks; complicated obstetric history (development of PE, FGR, and HELLP-syndrome in the previous pregnancy); and risk of PE and FGR identified by the first prenatal screening. The inclusion criteria were multiple pregnancies, transplanted organs, autoimmune diseases, and cancer. Exclusion criteria were fetal chromosomal abnormalities, fetal congenital malformations, and refusal to continue participation in the study. The diagnoses of PE and FGR were established based on criteria regulated by the national clinical guidelines [32].
All patients signed an informed consent form to participate in this study. Observations were carried out in accordance with the order of the Ministry of Health of the Russian Federation No. 1130n dated 20.10.2020 "On Approval of the Procedure for the Provision of Medical Care in the Profile of Obstetrics and Gynecology" and was carried out from the moment of the first treatment until delivery.
Lipid extraction from blood plasma samples was performed using a modified Folch method:480 μl of CHCl3/CH3OH (1/1) was added to 40 μl of plasma and incubated in an ultrasonic bath for 10 min. The mixture was stirred for 10 s and centrifuged for 5 minutes at 15000 G. The chloroform-methanol layer containing the lipids was withdrawn into a separate vial. To the aqueous layer, 250 μl CHCl3/ CH3OH (1/1) was added and centrifuged for 5 min at 15000 G. The lower chloroform-methanol layer was re-sampled and combined with the previously sampled layer. The lipid solution was dried in a stream of nitrogen and re-dissolved in 200 μL of isopropanol/acetonitrile (1/1) for further liquid chromatography-mass spectrometric analysis.
Lipid extracts were analyzed by high-performance liquid chromatography-mass spectrometry using a Dionex UltiMate 3000 liquid chromatograph (Thermo Scientific, Germany) coupled to a Maxis Impact qTOF mass analyzer with an ERI ion source (Bruker Daltonics, Germany). The samples were separated by reversed-phase chromatography on a Zorbax C18 column (150×2.1 mm, 5 μm, Agilent, USA) with a linear gradient of 30 to 90% eluent B (acetonitrile/isopropanol/water solution, 90/8/2 o/o/o, supplemented with 0.1% formic acid and 10 mmol/L ammonium formate) in 20 min. An acetonitrile/water solution (60/40, o/o) supplemented with 0.1% formic acid and 10 mM ammonium formate was used as eluent A. The elution flow rate was 40 μL/min and the injection volume was 3 μL. Mass spectra were obtained in positive ion mode in the range of m/z 100–1700 and negative ion mode in the range of m/z 100-1000 with the following settings: capillary voltage 4.1 kV for positive ion mode and 3.0 kV for negative ion mode, atomizing gas pressure 0.7 bar, drying gas flow rate 6 L/min, and drying gas temperature 200°C.
Lipids were identified using Lipid Match R-script by accurate mass using Lipid Maps database and by characteristic tandem mass spectra (MS/MS).
Statistical analysis
Plasma lipid levels were tested for statistically significant correlations with clinical characteristics, screening results, medical history, numerical screening value, body mass index, pregnancy number, duration of last pregnancy, and biochemical parameters in the third trimester, including blood levels, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), proteinuria level, platelet count, sFlt-1/PLGF ratio, and Spearman's test. Based on the lipids that were identified as having a statistically significant correlation, a latent structure projection model was constructed using two predictive axes. A discriminant model was constructed using orthogonal projections onto the latent structures "group in which PE did not occur"/"group in which PE occurred.” From lipids with a projection value of the variable greater than 1 in the model, the lipids with which the model based on logistic regression had the lowest Akaike information criterion and coefficients that were statistically significantly different from zero were selected. The sensitivity and specificity of the model were assessed using cross-validation for a single subject with the selection of the cutoff threshold based on the maximum sum of the sensitivity and specificity values.
For the results obtained using the lipid profile-based model during cross-validation and on the basis of screening, survival functions were constructed based on Kaplan–Meier estimation, and logarithmic rank significance criteria were calculated.
Statistical analysis was performed using R 4.1.3 language tools in the Rstudio 1.1.463 environment with connected packages ropls, survival, and survminer. ggplot2, pROC, corrplot.
Results and discussion
The blood plasma samples used in this study were obtained from 66 women during the first trimester screening at 7–13 weeks of gestation, 38 of whom were at high risk for placenta-associated disorders (group 1), and 28 of whom were controls (group 2). Of the 38 high-risk patients, 11 developed PE at 22–37 weeks of gestation. All patients in the high-risk group for placenta-mediated disorders received acetylsalicylic acid at a daily dose of 150 mg for prophylaxis from 12–16 to 36 weeks of gestation, according to clinical guidelines [32].
Twelve of the 38 pregnant women (31.5%) in group 1 had a history of PE and/or GDM. These patients were subdivided into subgroups based on the development of early severe PE (esPE) – 5/38 (13.2%) and late severe PE (lsPE) – 4/38 (10.5%). FGR was observed in 3/38 (7.9%) women.
Seven of the 38 patients in group 1 (18.4%) had chronic arterial hypertension in the medically corrected stage (Table 1). 11/38 (28.9%) pregnant women developed PE and FGR. These patients were categorized into subgroups according to the development of esPE: 6/38 (15.7%), lsPE; 1/38 (2.6%), late moderate PE (lmPE); and 4/38 (10.5%). FGR was observed in 5/38 (13.1%) patients who developed PE and in 1/38 (2.6%) patients without PE (Table 1). It should be noted that in 7/38 (18.4%) multiparous women, this pregnancy was again complicated by placenta-mediated diseases. Gestational arterial hypertension developed in 2/38 (5.3%) patients in the first group.
Early delivery was performed because of the increasing severity of PE and/or worsening of maternal and fetal conditions. It was performed in of 7/38 (18.4%) patients in the first group. Preterm labor occurred in 7/38 (18.4%) pregnant women in this group: early preterm labor in 3/38 (7.9%) and late preterm labor in the remaining 4/38 (10.5%) women.
The second group comprised 28 healthy pregnant women. No placenta-associated diseases or preterm deliveries were observed.
In blood plasma samples from the first trimester of pregnancy, 149 lipids were identified in the positive ion mode and 160 lipids were identified in the negative ion mode. Of these, 60 lipids in the positive ion mode belonged predominantly to the classes of phosphatidylcholines and lysophosphatidylcholines, including those with simple ester bonds, phosphatidylethanolamines, triglycerides, and sphingomyelins, and 59 lipids in the negative ion mode, belonging to the classes of phosphatidylcholines, including those with simple ether linkages, lysophosphatidylcholines, phosphatidylethanolamines, phosphatidylethanolamines, lysophosphatidylethanolamines, oxidized lipids, phosphatidylinositols, and sphingomyelins, had a statistically significant correlation with clinical group membership.
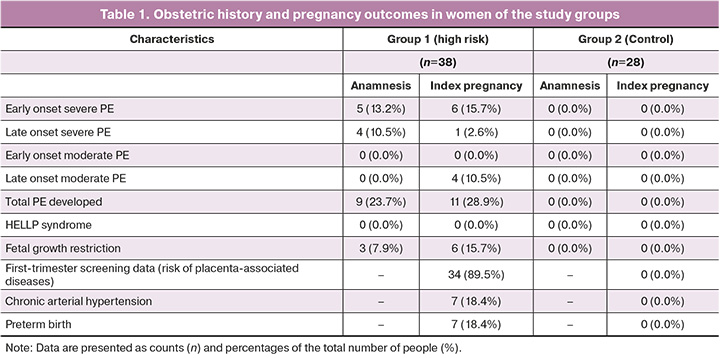
Correlation analysis according to high-risk groups identified 48 lipids, for which the level of correlation with at least one clinical parameter was average or above average (R>0.6), belonging to the classes of phosphatidylcholines, phosphatidylinositols, sphingomyelins, and triglycerides (Fig. 1).
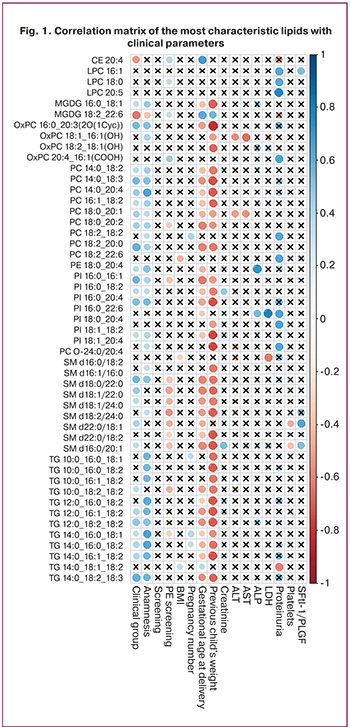
Based on the lipids that were significantly correlated with the clinical group, PLS models were built, with a score plot demonstrating a better overlap of points from the risk group for PE, in which PE did not occur during pregnancy, with the control group than in the risk group, in which PE occurred (Fig. 2).
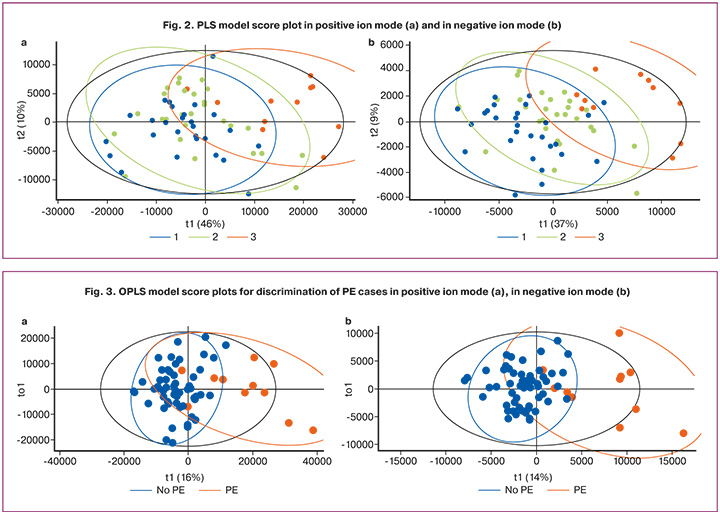
Given the close proximity of the clusters of the control group and the high-risk PE group, for which PE did not occur, OPLS models were constructed to discriminate PE cases from the rest, with the proportion of described response variables R2Y 0.55 and 0.59, and the proportion of predicted response variables Q2Y 0.32 and 0.46, positive ion mode, and negative ion mode, respectively (Fig. 3).
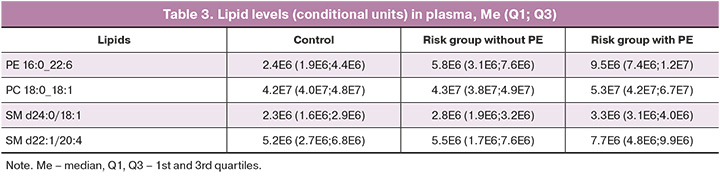
The final model (Table 2) included the lipids in Table 3, which allowed the model to be constructed based on logistic regression with optimal sensitivity and specificity of 0.91 and 0.91, respectively, for the positive ion mode and optimal sensitivity and specificity of 0.82 and 0.96, respectively, for the negative ion mode (Fig. 4).
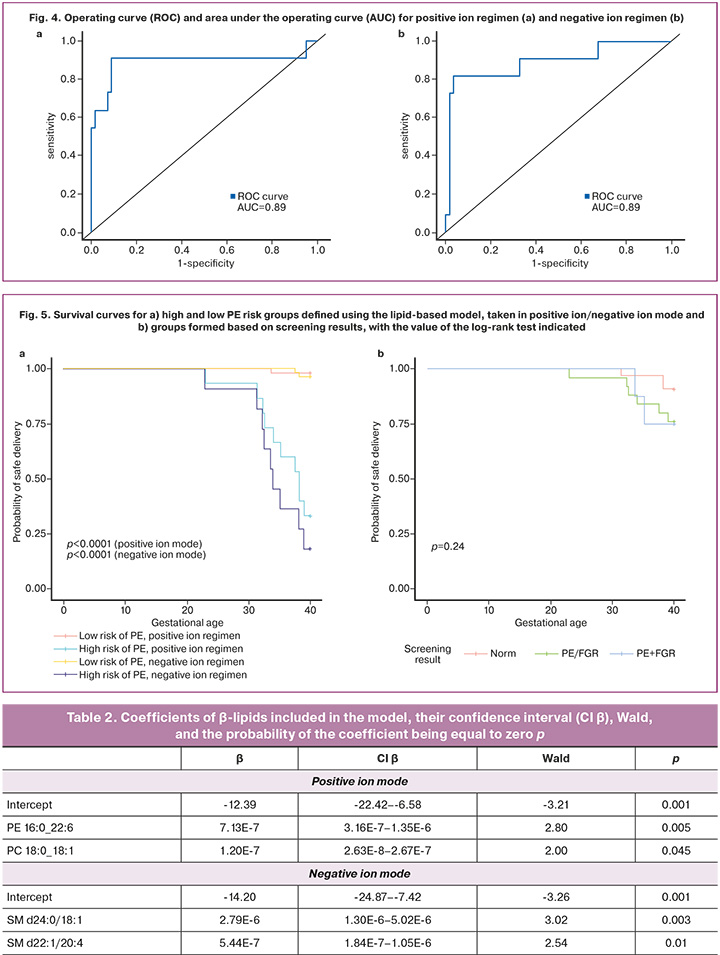
Survival function analysis yielded a relative risk for the group defined as high-risk using the lipid profile-based model in the positive ion regimen of 33.5 with CI 4.7–241, and a relative risk for the group defined as high-risk using the lipid profile-based model in the negative ion regimen of 20.5 with CI 5.1–82.2 (Fig. 5a). Survival function analysis constructed from the screening outcome information showed a relative risk for the PE/FGR screening outcome of 2.67 with CI 0.74–9.64 and for the PE+FGR screening outcome of 2.78 with CI 0.53–14.12 (Fig. 5b).
Early diagnosis and prediction at the molecular level of such life-threatening complications for both the mother and fetus are subjects of active interaction between clinicians and researchers. To date, the main problem in modern obstetrics is the lack of reliable screening markers to identify pregnant women at a high risk of PE, which makes it impossible to initiate preventive therapy before the manifestation of symptoms. Over the past few decades, many attempts have been made to identify the mechanisms underlying PE development. Serum markers, specific proteins, peptides, long non-coding RNAs, and microRNAs have been shown to contribute to disease development. Nevertheless, all discoveries remain theoretical, which explains the substantial gap between laboratory findings and clinical applications. The course and outcomes of both the present and previous pregnancies were thoroughly analyzed, and a panel of lipids capable of predicting the development of PE in the current pregnancy was sought.
A statistically significant correlation was observed between dyslipidemia in pregnant women (especially phosphatidylcholines, lysophosphatidylcholines, phosphatidylethanolamines, triglycerides, sphingomyelins, oxidized lipids, and phosphatidylinositols) and clinical and laboratory parameters of PE development. In particular, more than 80% of blood plasma lipids from the first trimester of pregnancy identified as markers showed a positive correlation (rs>0,7) with the severity of PE and the presence of obstetric complications in the history, and a high level of negative correlation with the weight of the baby in the previous pregnancy (for repeat mothers) and the term of delivery. Attention is drawn to a group of sphingomyelins, the levels of which in the blood are significantly negatively correlated with the results of biochemical screening in the first trimester of pregnancy. For a number of marker lipids (phosphatidylcholines, lysophosphatidylcholines, phosphatidylinositols) in the blood from the first trimester of pregnancy, a correlation with changes in the main clinical markers of PE at the manifestation of pathology (in particular, ALT, AST, alkaline phosphatase, creatinine, sFlt-1/PLGF, proteinuria, and thrombocytopenia) was found.
The values of relative risk for the groups defined by the proposed model based on plasma lipid profile are significantly higher than the values of relative risk for the groups formed on the basis of screening information. This demonstrates the higher predictive ability of plasma lipidome-based models for predicting the development of PE compared with screening, and suggests the prospect of further research related to the creation and validation of diagnostic tests for PE screening based on lipid profiles.
Conclusion
Based on the study findings, it can be concluded that changes in the lipid spectrum of blood plasma in the first trimester of pregnancy, predominantly phosphatidylcholines, lysophosphatidylcholines, phosphatidylethanolamine, triglycerides, and sphingomyelins, are associated with the risk of developing PE in the current pregnancy in the high-risk group of this complication. This allowed us to propose the developed logistic regression models based on first-trimester plasma marker lipid levels as a refinement after first-trimester screening. In the future, these findings can contribute to the improvement of preventive measures for placenta-associated diseases and the timely monitoring of their development.
References
- Duley L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009; 33(3): 130-7. https://dx.doi.org/10.1053/j.semperi.2009.02.010.
- Moutquin J.-M. Classification and heterogeneity of preterm birth. BJOG. 2003; 110(Suppl.): 30-3. https://dx.doi.org/10.1016/s1470-0328(03)00021-1.
- Ilekis J.V., Reddy U.M., Roberts J.M. Preeclampsia--a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. Reprod. Sci. 2007; 14(6): 508-23. https://dx.doi.org/10.1177/1933719107306232.
- Osmond C., Barker D.J. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ. Health Perspect. 2000; 108 l3(Suppl 3): 545-53. https://dx.doi.org/10.1289/ehp.00108s3545.
- Chen C.W., Jaffe I.Z., Karumanchi S.A. Pre-eclampsia and cardiovascular disease. Cardiovasc. Res. 2014; 101(4): 579-86. https://dx.doi.org/10.1093/cvr/cvu018.
- Ahmed R., Dunford J., Mehran R., Robson S., Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J. Am. Coll. Cardiol. 2014; 63(18): 1815-22. https://dx.doi.org/10.1016/j.jacc.2014.02.529.
- Mosca L., Benjamin E.J., Berra K., Bezanson J.L., Dolor R.J., Lloyd-Jones D.M. et al.; American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women – 2011 update: a guideline from the American Heart Association. J. Am. Coll. Cardiol. 2011; 57(12): 1404-23. https://dx.doi.org/10.1016/j.jacc.2011.02.005.
- Leslie M.S., Briggs L.A. Preeclampsia and the risk of future vascular disease and mortality: a review. J. Midwifery Womens Health. 2016; 61(3): 315-24. https://dx.doi.org/10.1111/jmwh.12469.
- Al-Nasiry S., Ghossein-Doha C., Polman S.E.J., Lemmens S., Scholten R.R., Heidema W.M. et al. Metabolic syndrome after pregnancies complicated by pre-eclampsia or small-for-gestational-age: a retrospective cohort. BJOG. 2015; 122(13): 1818-23. https://dx.doi.org/10.1111/1471-0528.13117.
- Muttukrishna S., Knight P.G., Groome N.P., Redman C.W., Ledger W.L. Activin A and inhibin A as possible endocrine markers for pre-eclampsia. Lancet. 1997; 349(9061): 1285-8. https://dx.doi.org/10.1016/S0140-6736(96)09264-1.
- Birdsall M., Ledger W., Groome N., Abdalla H., Muttukrishna S. Inhibin A and activin A in the first trimester of human pregnancy J. Clin. Endocrinol. Metab. 1997; 82(5): 1557-60. https://dx.doi.org/10.1210/jcem.82.5.3934.
- Aitken D.A., Wallace E.M., Crossley J.A., Swanston I.A., van Pareren Y., van Maarle M. et al. Dimeric inhibin A as a marker for Down's syndrome in early pregnancy. N. Engl. J. Med. 1996; 334(19): 1231-6. https://dx.doi.org/10.1056/NEJM199605093341904.
- Wu P., van den Berg C., Alfirevic Z., O'Brien S., Rothlisberger M., Baker P.N. et al. Early pregnancy biomarkers in pre-eclampsia: a systematic review and meta-analysis. Int. J. Mol. Sci. 2015; 16(9): 23035-56. https://dx.doi.org/10.3390/ijms160923035.
- Tornehave D., Folkersen J., Teisner B., Chemnitz J. Immunohistochemical aspects of immunological cross-reaction and masking of epitopes for localization studies on pregnancy-associated plasma protein A. Histochem. J. 1986; 18(4): 184-8. https://dx.doi.org/10.1007/BF01676119.
- Smith G.C.S., Stenhouse E.J., Crossley J.A., Aitken D.A., Cameron A.D., Connor J.M. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J. Clin. Endocrinol. Metab. 2002; 87(4): 1762-7. https://dx.doi.org/10.1210/jcem.87.4.8430.
- Ranta J.K., Raatikainen K., Romppanen J., Pulkki K., Heinonen S. Decreased PAPP-A is associated with preeclampsia, premature delivery and small for gestational age infants but not with placental abruption Eur. J. Obstet. Gynecol. Reprod. Biol. 2011; 157(1): 48-52. https://dx.doi.org/10.1016/j.ejogrb.2011.03.004.
- Wang H.Y., Zhang Z., Yu S. Expression of PAPPA2 in human fetomaternal interface and involvement in trophoblast invasion and migration. Genet. Mol. Res. 2016; 15(3). https://dx.doi.org/10.4238/gmr.15038075.
- Кан Н.Е., Ломова Н.А., Амирасланов Э.Ю., Чаговец В.В., Тютюнник В.Л., Хачатрян З.В., Стародубцева Н.Л., Кициловская Н.А., Франкевич В.Е. Особенности метаболомного профиля при преэклампсии. Акушерство и гинекология. 2019; 11: 82-8. [Kan N.E., Lomova N.A., Amiraslanov E.Yu., Chagovets V.V., Tyutyunnik V.L., Khachatryan Z.V., Starodubtseva N.L., Kitsilovskaya N.A., Frankevich V.E. Specific features of a metabolomic profile in preeclampsia. Obstetrics and Gynecology. 2019; (11): 82-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.11.82-88.
- Kononikhin A., Zakharova N.V., Sergeeva V.A., Indeykina M.I., Starodubtseva N.L., Bugrova A.E. et al. Differential diagnosis of preeclampsia based on urine peptidome featches revealed by high resolution mass spectrometry. Diagnostics (Basel). 2020; 10(12): 1039. https://dx.doi.org/10.3390/diagnostics10121039.
- Tarca A.L., Romero R., Benshalom-Tirosh N., Than N.G., Gudicha D.W., Done B. et al. The prediction of early preeclampsia: Results from a longitudinal proteomics study. PLoS One. 2019; 14(6): e0217273. https://dx.doi.org/10.1371/journal.pone.0217273.
- Сергеева В.А., Муминова К., Стародубцева Н.Л., Кононихин А.С., Бугрова А.Е., Индейкина М.И., Байбакова В.В., Ходжаева З.С., Кан Н.Е., Франкевич В.Е., Шмаков Р.Г., Николаев Е.Н., Сухих Г.Т. Особенности пептидома мочи при гипертензивных патологиях беременных. Биомедицинская химия. 2017; 63(5): 379-84. [Sergeeva V.A., Muminova K.T., Starodubtseva N.L., Kononikhin A.S., Bugrova A.E., Indeykina M.I., Baibakova V.V., Khodzhaeva Z.S., Kan N.E., Frankevich V.E., Shmakov R.G., Nikolaev E.N., Sukhikh G.T. Features of the urine peptidome under the condition of hypertensive pathologies of pregnancy. Biomeditsinskaya Khimiya. 2017; 63(5): 379-84. (in Russian)].
- Starodubtseva N., Nizyaeva N., Baev O., Bugrova A., Gapaeva M., Muminova K. et al. SERPINA1 peptides in urine as a potential marker of preeclampsia severity. Int. J. Mol. Sci. 2020; 21(3): 914. https://dx.doi.org/10.3390/ijms21030914.
- Booth R.A., Hill S.A., Don-Wauchope A., Santaguida P.L., Oremus M., McKelvie R. et al. Performance of BNP and NT-proBNP for diagnosis of heart failure in primary care patients: a systematic review. Heart Fail. Rev. 2014; 19(4): 439-51. https://dx.doi.org/10.1007/s10741-014-9445-8.
- Verlohren S., Perschel F.H., Thilaganathan B., Dröge L.A., Henrich W., Busjahn A., Khalil A. Angiogenic markers and cardiovascular indices in the prediction of hypertensive disorders of pregnancy. Hypertension. 2017; 69(6): 1192-7. https://dx.doi.org/10.1161/HYPERTENSIONAHA.117.09256.
- Giannubilo S.R., Pasculli A., Tidu E., Biagini A., Boscarato V., Ciavattini A. Relationship between maternal hemodynamics and plasma natriuretic peptide concentrations during pregnancy complicated by preeclampsia and fetal growth restriction. J. Perinatol. 2017; 37(5): 484-7. https://dx.doi.org/10.1038/jp.2016.264.
- El Khouly N.I., Sanad Z.F., Saleh S.A., Shabana A.A., Elhalaby A.F., Badr E.E. Value of first-trimester serum lipid profile in early prediction of preeclampsia and its severity: A prospective cohort study. Hypertens Pregnancy. 2016; 35(1): 73-81. https://dx.doi.org/10.3109/10641955.2015.1115060.
- Korkes H.A., Sass N., Moron A.F., Câmara N.O.S., BonettI T., Cerdeira A.S. et al. Lipidomic assessment of plasma and placenta of women with early-onset preeclampsia. PLoS One. 2014; 9(10): e110747. https://dx.doi.org/10.1371/journal.pone.0110747.
- de Lima V.J., de Andrade C.R., Ruschi G.E., Sass N. Serum lipid levels in pregnancies complicated by preeclampsia. Sao Paulo Med. J. 2011; 129(2): 73-6. https://dx.doi.org/10.1590/s1516-31802011000200004.
- Yu L., Li D., Liao Q.-P., Yang H.-X., Cao B., Fu G. et al. High levels of activin a detected in preeclamptic placenta induce trophoblast cell apoptosis by promoting nodal signaling. J. Clin. Endocrinol. Metab. 2012; 97(8): E1370-9. https://dx.doi.org/10.1210/jc.2011-2729.
- Gofman J.W., Delalla O., Glazier F., Freeman N.K., Lindgren F.T., Nichols A.V. et al. The serum lipoprote in transport system in health, metabolic disorders, atherosclerosis and coronary heart disease. J. Clin. Lipidol. 2011; 1(2): 104-41. https://dx.doi.org/10.1016/j.jacl.2007.03.001.
- Ahmed A.A.M., El Omda F.A.A., Mousa M.S.M. Maternal lipid profile as a risk factor for preeclampsia. Egyp. J. Hosp. Med. 2018; 71(6): 3434-8. https://dx.doi.org/10.12816/0047307.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Преэклампсия. Эклампсия. Отеки, протеинурия и гипертензивные расстройства во время беременности, в родах и послеродовом периоде. 2021. [Ministry of Health of the Russian Federation. Clinical guidelines. Preeclampsia. Eclampsia. Edema, proteinuria, and hypertensive disorders during pregnancy, childbirth, and postpartum. 2021.(in Russian)].
Received 03.10.2023
Accepted 06.10.2023
About the Authors
Ekaterina A. Minaeva, post-graduate student, 1st Obstetric Department of Pregnancy Pathology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, +7(916)464-16-67, ek_minaeva@oparina4.ru, https://orcid.org/0000-0001-8555-6670,117997, Russia, Moscow, Ac. Oparin str., 4.
Natalia L. Starodubtseva, PhD (Bio), Head of the Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, n_starodubtseva@oparina4.ru, https://orcid.org/0000-0001-6650-5915, 117997, Russia, Moscow, Ac. Oparin str., 4.
Roman G. Shmakov, Dr. Med. Sci., Professor, Professor of the Russian Academy of Sciences, Director of the Institute of Obstetrics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia; Main Specialist of the Ministry of Health of Russia in obstetrics,
r_shmakov@oparina4.ru, https://orcid.org/0000-0002-2206-1002, 117997, Russia, Moscow, Ac. Oparin str., 4.
Alisa O. Tokareva, PhD, specialist at the Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, alisa.tokareva@phystech.edu, 4 Ac. Oparin str., 117997, Moscow, Russia.
Vitaliy V. Chagovets, PhD, Head of the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, vvchagovets@gmail.com, 117997, Russia, Moscow, Ac. Oparin str., 4.
Anastasia V. Novoselova, Junior Researcher at the Laboratory of Metabolomics and Bioinformatics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, a_novoselova@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Evgeny N. Kukaev, Ph.D, Senior Researcher at the Laboratory of Clinical Proteomics, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia; V.L. Talrose Institute for Energy Problems of Chemical Physics, N.N. Semenov Federal Center of Chemical Physic,
Russian Academy of Sciences, e_kukaev@oparina4.ru, 117997, Russia, Moscow, Ac. Oparin str., 4.
Vladimir E. Frankevich, Dr. Sci. (Physico-mathematical), Deputy Director of the Institute of Translational Medicine, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia; Siberian State Medical University, Ministry of Health of Russia, v_vfrankevich@oparina4.ru, https://orcid.org/0000-0002-9780-4579, 117997, Russia, Moscow, Ac. Oparin str., 4.



