«Insanity: doing the same thing over and
over again and expecting different results».”
(Безумие – это делать одно и то же снова и снова и при этом ожидать другого результата)
(Альберт Энштейн)
Рак шейки матки (РШМ) остается одной из наиболее распространенных форм злокачественных новообразований у женщин. Это ведущая злокачественная опухоль в структуре женской онкологической заболеваемости и смертности в развивающихся и важнейшая медицинская и социальная проблема во всех экономически развитых странах. Ежегодно в мире диагностируется около 570 тысяч новых случаев РШМ, при этом рост заболеваемости РШМ в мире за последние 10 лет составил 7,8%, а смертности – 13,1% [1]. В Российской Федерации показатели заболеваемости и смертности от РШМ приближаются к средним мировым и составляют, соответственно, 15,76 и 5,18 на 100 тыс. населения (стандартизованные показатели) [2]. За последние десять лет заболеваемость РШМ в России выросла на 25%, а смертность от РШМ – на 4,31%. Важно отметить, что среди молодых женщин, умерших от онкозаболеваний в возрасте 30–34 лет, почти каждая четвертая умерла от РШМ.
Терминальной стадии инвазивного РШМ предшествует развитие предраковых состояний – цервикальных интраэпителиальных неоплазий (CIN) – тканевых изменений морфологического характера c признаками интенсивного патологического размножения клеток и атипией. По степени выраженности патологических морфологических изменений и клинических проявлений выделяют три основные формы CIN: легкую (CIN 1), среднюю (CIN 2) и тяжелую (CIN 3).
Как правило, цервикальный канцерогенез – достаточно медленный процесс, занимающий 5–10 и более лет. Однако в некоторых случаях, под влиянием негативных факторов, этот временной промежуток может существенно сокращаться, вплоть до нескольких месяцев.
Общепризнанным этиологическим фактором возникновения РШМ и предшествующих ему дисплазий является инфицирование цервикальных тканей вирусом папилломы человека (ВПЧ) высокого онкогенного риска. ДНК ВПЧ высокого онкогенного риска (преимущественно 16 и 18 типов) обнаруживают в 50–80% образцов умеренной и тяжелой дисплазии плоского эпителия шейки матки (ШМ) и в 90% – инвазивного РШМ.
При отсутствии отягощающих факторов интраэпителиальные поражения низкой степени (LSIL) в течение 3–5 лет в 30–60% случаев подвергаются регрессии. В то же время у 10–30% женщин, имеющих нормальную цитологическую картину цервикального эпителия и являющихся носителями ДНК ВПЧ высокого онкогенного риска, наблюдается прогрессирование CIN 1 до CIN 3 и карциномы in situ, а у 1,5% – развивается инвазивный РШМ.
На трансформацию латентного носительства ВПЧ в неоплазию ШМ влияют такие факторы, как неблагоприятный гормональный фон, раннее начало половой жизни и частая смена сексуальных партнеров, хронические воспалительные, инфекционные и вирусные заболевания генитального тракта, травматизация тканей ШМ во время абортов, родов и диагностических выскабливаний, курение, иммунодефицит, длительный (более 5 лет) прием оральных контрацептивов [3].
Общемировая статистика в отношении CIN также внушает тревогу. Ежегодно в мире диагностируется около 30 млн. новых случаев CIN 1 и более 10 млн. случаев CIN 2–3 [4]. Более благополучная ситуация в отношении рака и предрака ШМ отмечается сегодня в США, развитых странах Европы и Азии. Однако даже там имеет место отчетливая тенденция к росту диагностированных CIN и РШМ. Так, например, в Швейцарии, стране с высоким уровнем медицинского обслуживания, налаженным скринингом РШМ и одними из самых низких в мире показателями заболеваемости и смертности от РШМ, за период с 2000 г. по 2014 г. частота обнаружения CIN всех трех степеней (CIN 1, CIN 2, CIN 3), выявляемых при конизации, выросла приблизительно в 2,5 раза, а общее число случаев РШМ – почти на 40% [5]. Таким образом, количество конизаций, выполняемых в центральной Швейцарии ежегодно, увеличилось более чем в два раза, однако при этом предраковые поражения ШМ обнаруживались только в 50% случаев, а половина всех конизаций выполнялась в отсутствие предрака, то есть без должных на то оснований. Такая практика лечения, особенно у молодых женщин с нереализованной или не до конца реализованной репродуктивной функцией, безусловно, не может не вызывать беспокойство. Вместе с тем число случаев РШМ, выявленных в ходе конизации, составляло менее половины от всех случаев диагностированного РШМ, а число случаев РШМ, обнаруженного на поздней стадии, увеличилось. В качестве возможных причин большого числа случаев РШМ, не диагностируемого при конизации, авторы исследования называют наличие сбоев в существующем алгоритме диагностики, а также неучастие части женской популяции из группы риска в скрининговых программах. В итоге делается заключение о трудностях реализации организованных скрининговых программ в Швейцарии – стране с федеративным государственным устройством и обсуждаются пути решения этой задачи, в частности необходимость оптимизации и расширения возможностей скрининга, пересмотр руководств по его проведению в соответствующих возрастных группах, а также привлечение дополнительной группы пациентов, находящихся в группе риска по РШМ.
Несмотря на огромное разнообразие клинических форм папилломавирусной инфекции, различают два основных ее варианта: 1) продуктивная, при которой ДНК вируса находится в инфицированной клетке в свободной эписомальной форме и 2) интегративная, или трансформирующая, при которой ДНК вируса встраивается в геном инфицированной клетки, утрачивая свою индивидуальность [6] (рис. 1).
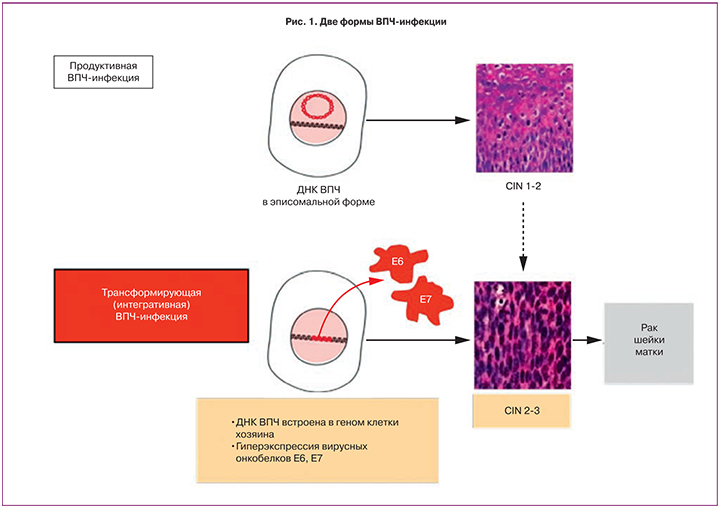
При продуктивной инфекции после деления инфицированной клетки на две дочерние одна остается в базальном слое и поддерживает состояние вирус-инфицированности, а другая – мигрирует к поверхности эпителия, входит в процесс дифференцировки и способствует репродукции вирусных частиц, в результате чего происходит повторное самоинфицирование или инфицирование полового партнера. Продуктивная инфекция является обратимой и при нормальном иммунитете заканчивается элиминацией вируса из организма. При неблагоприятных условиях продуктивная инфекция вызывает развитие доброкачественных образований и цервикальных дисплазий слабой и средней степени.
В отличие от продуктивной инфекции интеграция ДНК ВПЧ высокого онкогенного риска в геном клетки хозяина приводит к глобальным изменениям клеточного метаболизма и является первым шагом к ее опухолевому перерождению. Клетка с интегрированной вирусной ДНК начинает активно синтезировать вирусные онкобелки Е6 и Е7. Запускается конверсия эстрогенов в агрессивный метаболит 16α-гидроксиэстрон (16α-OHE1), что обеспечивает быстрое деление инфицированных клеток эпителия. В таких клетках все структуры и функции ориентированы на усиленную продукцию онкобелков ВПЧ и проявление их туморогенной активности. Такие клетки выходят из-под иммунного контроля организма, избегают апоптоза и со временем могут перейти в злокачественное состояние [7].
Известно, что важнейшую роль в цервикальном канцерогенезе играют эпигенетические нарушения, которые приводят к инактивации опухоль-супрессорных генов и, как следствие, к повышению риска малигнизации ВПЧ-инфицированных клеток ШМ [6, 7]. Экспериментально установлено, что уровень аномальных эпигенетических модификаций, в частности уровень промоторного ДНК-метилирования противоопухолевых генов, неуклонно растет по мере прогрессии CIN [8] и характерен для интегративной (трансформирующей) формы ВПЧ-инфекции [6]. При этом качественный скачок роста эпигенетических аномалий отмечается при переходе патологического неопластического процесса от стадии LSIL к стадии HSIL и индуцируется под действием вирусных онкобелков, активно продуцирующихся в клетке с интегрированной вирусной ДНК. Доказано, что вирусные онкобелки Е6 и Е7 в условиях in vitro и in vivo способны прямо или опосредованно активировать ферменты эпигенетических модификаций – ДНК-метилтрансферазу и гистондеацетилазу и, таким образом, индуцировать неконтролируемую клеточную пролиферацию и ослабление иммунной защиты [7, 9].
Необходимо отметить, что, вопреки распространенной точке зрения, интеграция ДНК ВПЧ высокого онкогенного риска в геном клетки хозяина нередко оказывается очень ранним событием в цервикальном канцерогенезе. Есть данные, что от 30 [10] до 50% [11] от всех случаев CIN 1 сопровождаются интеграцией ДНК высокоонкогенных типов (16/18) ВПЧ в геном хозяина. Таким образом, недооценивать значение ранних стадий ВПЧ-обусловленных диспластических процессов с точки зрения возможности их онкотрансформации ни в коем случае нельзя. Тем более что, согласно статистике, в последние годы наметилась тенденция к снижению доли CIN 1–2, подвергающихся спонтанной регрессии. В качестве возможных причин этого явления называются нарушение биоценоза влагалища, сопутствующие инфекции и снижение местного мукозального иммунитета.
Согласно современным взглядам на патогенез цервикальных дисплазий и РШМ, продуктивные и интегративные формы ВПЧ-инфекции возникают и развиваются в разных типах клеток цервикального эпителия. Продуктивная инфекция возникает в базальных клетках метапластического плоского эпителия зоны трансформации и примыкающего к ней эктоцервикса. В то время как самым уязвимым местом для возникновения интегративной, или трансформирующей, формы ВПЧ-инфекции и приводящих к раку дисплазий является переходная зона и зона стыка между многослойным плоским эпителием и однослойным цилиндрическим (железистым) эпителием шейки матки. Есть данные, что повышенная предрасположенность данной области к развитию предрака и РШМ связана с преобладанием в ней мультипотентных стволовых (резервных) клеток, имеющих высокий потенциал к последующей опухолевой трансформации при инфицировании ВПЧ высокого онкогенного риска [12, 13]. Накоплено большое количество экспериментальных данных, доказывающих, что инфицирование стволовых клеток зоны стыка высокоонкогенными типами ВПЧ с последующей интеграцией вирусной ДНК в геном клетки хозяина и гиперэкспрессией вирусных онкогенов является пусковым механизмом цервикального канцерогенеза [14–16]. При неблагоприятных условиях (изменение клеточного микроокружения на провоспалительное) вирус-инфицированные стволовые клетки зоны стыка приобретают фенотип туморогенных опухолевых стволовых клеток и весь клеточный метаболизм перестраивается в направлении онкотрансформации. Такое перепрограммирование клеточного генома осуществляется посредством эпигенетических механизмов, главным из которых является аномальное ДНК-метилирование.
Результаты многочисленных экспериментальных и клинических исследований показали, что уникальное соединение 3,3’-дииндолилметан (ДИМ) способно блокировать множественные молекулярные механизмы, приводящие к патологической клеточной пролиферации и опухолевой трансформации в гормон-зависимых тканях, в том числе в ВПЧ-инфицированных клетках цервикального эпителия [17, 18]. Множественная противооопухолевая активность ДИМ в литературе получила название «терапевтического чуда» [19] (рис. 2).
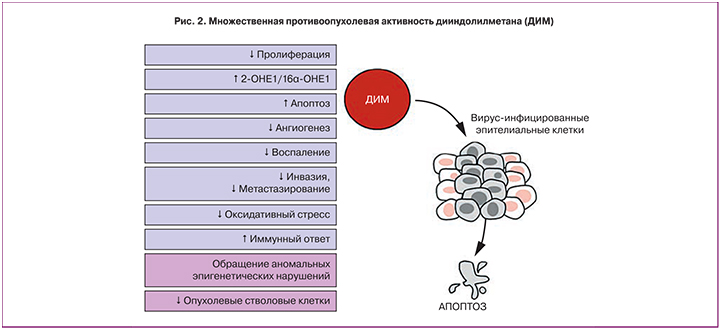
Мультитаргетная противоопухолевая активность ДИМ в отношении ВПЧ-обусловленных заболеваний ШМ была подтверждена в многочисленных экспериментах in vivo и в клинических исследованиях [18, 20, 21]. Было показано, что ДИМ эффективно блокирует основные молекулярные механизмы, опосредующие опухолевые процессы в цервикальном эпителии. Как и его метаболический предшественник индол-3-карбинол, ДИМ нормализует обмен эстрогенов путем индукции активности цитохрома CYP1A1, ответственного за преимущественное образование функционального метаболита 2-гидроксиэстрона (2-OHE1), а также избирательно ингибирует экспрессию онкогена Е7 ВПЧ [20, 22]. ДИМ проявляет выраженную проапоптотическую активность в отношении вирус-инфицированных эпителиальных клеток ШМ [23], а также подавляет местное воспаление за счет нейтрализации ядерного фактора транскрипции NF-кB и других провоспалительных медиаторов (NO, PGE2, TNF-α, IL-6, and IL-1β), ингибирует патологический неоангиогенез и метастатическую клеточную активность [17]. Есть сведения об иммуномодулирующих свойствах ДИМ, опосредованных стимуляцией IFNγ-зависимых сигнальных каскадов [20], а также его противоопухолевой эпигенетической активности, обусловленной ДНК-деметилированием «молчащих» опухоль-супрессорных генов и приводящим к изменению конформации хроматина ингибированием фермента гистондеацетилазы [24–26]. Наконец, в недавних исследованиях была показана избирательная активность ДИМ в отношении опухолевых стволовых клеток, являющихся, согласно современным представлениям, главной причиной рецидивирования и метастазирования злокачественных опухолей [27–29].
Терапевтическая активность и хорошая переносимость при пероральном приеме ДИМ в составе формуляции BioResponse-DIM (BR-DIM, LLC, Boulder, CO) была показана в зарубежных рандомизированных клинических исследованиях на пациентах с цервикальными дисплазиями [21, 30]. Однако результаты этих исследований нельзя считать полностью удовлетворительными. В некоторых из них имелись очевидные недостатки дизайна, а также не наблюдалось статистической значимости различий конечной эффективности в опытной и контрольной группах пациентов, что, вероятно, было обусловлено низкой биодоступностью активного вещества в исходной формуляции и, как следствие, недостаточной конечной концентрацией ДИМ в тканях ШМ.
С учетом противовирусных и противоопухолевых свойств ДИМ, на основе данного вещества был создан отечественный лекарственный препарат Цервикон-ДИМ в форме вагинальных суппозиториев [31]. Доклинические испытания показали, что Цервикон-ДИМ является безопасным и эффективным средством, нетоксичным в отношении жизненно важных органов и систем организма. Его применение не сопровождается местным раздражением тканей и другими выраженными побочными эффектами. Препарат отличает уникальная способность действовать на ВПЧ-инфицированные эпителиальные клетки ШМ с аномально измененным метаболизмом (находящиеся в состоянии клеточного стресса) независимо от места их локализации и степени опухолевой трансформации. Действуя локально, Цервикон-ДИМ проникает внутрь вирус-инфицированных клеток и через активацию молекулярно-генетических механизмов запускает процесс их физиологической гибели – апоптоз.
Последующие клинические исследования по изучению эффективности и безопасности Цервикон-ДИМ при цервикальных дисплазиях, проведенные в соответствии с международными стандартами Надлежащей клинической практики GCP, также прошли успешно.
В двойное слепое рандомизированное плацебо-контролируемое клиническое исследование II фазы по изучению эффективности и безопасности Цервикон-ДИМ были включены 78 пациенток в возрасте 18–39 лет с гистологически верифицированной цервикальной интраэпителиальной неоплазией 1–2 степени (CIN 1–2) [18, 32, 33]. Пациентки первой и второй экспериментальных групп получали Цервикон-ДИМ интравагинально соответственно в дозе 100 мг ДИМ в сутки (1 суппозиторий с плацебо и 1 суппозиторий исследуемого препарата в сутки) и 200 мг ДИМ в сутки (1 суппозиторий исследуемого препарата 2 раза в сутки), пациентки третьей группы получали плацебо (1 суппозиторий с плацебо 2 раза в сутки).
Критерием эффективности служила полная регрессия CIN при гистологическом обследовании биоптатов пораженных участков ШМ. Максимальный срок активной терапии составлял 180 дней. Исследование предусматривало четыре контрольных визита: в начале исследования, через 30 дней (один месяц), через 90 дней (три месяца) и через 180 дней (шесть месяцев) от начала исследования. На визитах через три и шесть месяцев после начала исследования подсчитывалось число пациенток с гистологически подтвержденным отсутствием CIN. Сохранение фокусов CIN в биоптатах считалось отрицательным ответом на лечение.
Было показано, что эффективность интравагинального применения препарата Цервикон-ДИМ в дозе 100 мг ДИМ в сутки была статистически значимо выше, чем в группе плацебо, при терапии в течение шести месяцев (90,5% и 61,1%; р=0,036). В то же время эффективность терапии препаратом Цервикон-ДИМ в дозе 200 мг ДИМ в сутки была статистически значимо выше, чем в группе плацебо, как при трехмесячном (85,0% и 52,6%; р=0,032), так и при шестимесячном курсах лечения (100% и 61,1%; р=0,0361). Серьезные побочные реакции в группах не отмечались [18, 33].
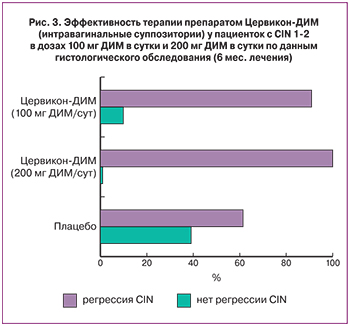 Таким образом, при CIN 1–2 интравагинальное применение препарата Цервикон-ДИМ в дозах 100 мг ДИМ в сутки и 200 мг ДИМ в сутки в течение трех месяцев вызывало клинически выраженную регрессию CIN 1–2, однако эффективность терапии была статистически значимо выше по сравнению с контролем только в группе приема препарата в дозе 200 мг ДИМ в сутки. При продлении курса терапии до шести месяцев эффективность лечения препаратом Цервикон-ДИМ была значимо выше по сравнению с плацебо как при применении дозы 100 мг ДИМ в сутки, так и дозы 200 мг ДИМ в сутки. При этом регрессия CIN наблюдалась практически у всех у пациенток, не давших ответа на лечение в течение трех месяцев терапии (рис. 3). Это означает, что после 6-месячного курса терапии у пациенток с CIN 1–2 клиническая эффективность интравагинального применения Цервикон-ДИМ достоверно отмечалась при обеих дозах его введения: 100 мг ДИМ в сутки и 200 мг ДИМ в сутки.
Таким образом, при CIN 1–2 интравагинальное применение препарата Цервикон-ДИМ в дозах 100 мг ДИМ в сутки и 200 мг ДИМ в сутки в течение трех месяцев вызывало клинически выраженную регрессию CIN 1–2, однако эффективность терапии была статистически значимо выше по сравнению с контролем только в группе приема препарата в дозе 200 мг ДИМ в сутки. При продлении курса терапии до шести месяцев эффективность лечения препаратом Цервикон-ДИМ была значимо выше по сравнению с плацебо как при применении дозы 100 мг ДИМ в сутки, так и дозы 200 мг ДИМ в сутки. При этом регрессия CIN наблюдалась практически у всех у пациенток, не давших ответа на лечение в течение трех месяцев терапии (рис. 3). Это означает, что после 6-месячного курса терапии у пациенток с CIN 1–2 клиническая эффективность интравагинального применения Цервикон-ДИМ достоверно отмечалась при обеих дозах его введения: 100 мг ДИМ в сутки и 200 мг ДИМ в сутки.
Из полученных данных следует, что дозировка препарата Цервикон-ДИМ 200 мг ДИМ в сутки является более предпочтительной. При этом длительность терапии, равная как трем, так и шести месяцам, является допустимой. Однако поскольку для подавляющего большинства пациентов увеличение длительности терапии препаратом в дозе 200 мг ДИМ в сутки не приводило к существенному повышению эффективности при сохранении риска возможных побочных явлений, при клиническом исследовании III фазы был рекомендован режим терапии препаратом Цервикон-ДИМ в дозировке 200 мг ДИМ в сутки в течение трех месяцев [18, 33].
Двойное слепое рандомизированное плацебо-контролируемое многоцентровое клиническое исследование III фазы по изучению эффективности и безопасности препарата Цервикон-ДИМ в лечении цервикальной интраэпителиальной неоплазии проводилось в 17 российских клинических центрах [34]. В исследование было включено 160 женщин в возрасте от 18–45 лет с гистологически верифицированным диагнозом CIN 1–2 (размер видимого участка поражения при кольпоскопии не менее 1 см2, не менее трех биоптатов из наиболее измененного участка), рандомизированные в две группы. Согласно протоколу, пациентки основной группы получали Цервикон-ДИМ в дозе 200 мг ДИМ в сутки (по одному вагинальному суппозиторию два раза в сутки) в течение трех месяцев, пациентки контрольной группы получали плацебо по той же схеме.
Эффективность терапии оценивалась как доля пациенток с полной или частичной регрессией CIN 1, СIN 2 через 3 месяца после начала применения препарата Цервикон-ДИМ на основании результатов гистологического исследования биоптатов ШМ. Считалось, что произошла частичная регрессия CIN в случае, если CIN 2 перешла в CIN 1.
Опытная и контрольная группы были хорошо сбалансированы по всем основным параметрам. Результаты предварительного обследования показали, что все участницы были инфицированы ВПЧ, из высокоонкогенных типов ВПЧ превалировали 16-й (28%) и 18-й (8%). По данным кольпоскопии, на момент включения в исследование у всех пациенток отмечались изменения, характерные для ВПЧ-ассоциированных состояний. По результатам цитологического исследования патологические изменения на стадии скрининга отмечались у 58% пациенток в группе Цервикон-ДИМ и у 62% пациенток в группе плацебо. Доля пациенток с CIN 1 и CIN 2 на момент включения в исследование составляла соответственно в группе Цервикон-ДИМ 58% и 42%, в группе плацебо – 63% и 37%. Значимых различий в характеристиках исходного состояния – данных гинекологического анамнеза (возраст менархе, длительность менструаций, интервал между менструациями, возраст начала половой жизни) выявлено не было. После завершения скрининга из исследования выбыло: из группы плацебо – 2 пациентки, из группы Цервикон-ДИМ – 7 пациенток.
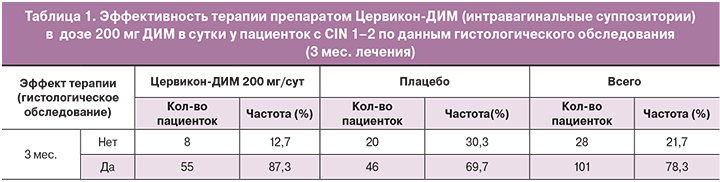
По итогам исследования препарат Цервикон-ДИМ продемонстрировал достоверное преимущество эффективности по сравнению с плацебо: доля пациенток с полной или частичной регрессией CIN 1–2 через три месяца в основной группе составила 87,3% (р=0,013). Значимые различия между группами по количеству пациенток, у которых было отмечено наличие нежелательных явлений, отсутствовали (р=0,101) (табл. 1).
В ходе исследования была отмечена хорошая переносимость и высокий уровень безопасности препарата Цервикон-ДИМ. Интравагинальный способ введения препарата позволяет максимально увеличить концентрацию активного вещества в инфицированных тканях шейки матки и свести к минимуму его отрицательное системное действие. Ранее было показано, что 72–73% от введенной дозы ДИМ распределяется в тканях влагалища и только 3–4% обнаруживается в системном кровотоке (данные доклинических исследований).
Авторами исследования был сделан вывод о высокой эффективности препарата Цервикон-ДИМ в терапии преинвазивных заболеваний шейки матки (CIN 1–2). Отмечалось, что курсовое лечение данным препаратом дает возможность избежать деструктивных хирургических вмешательств, особенно у молодых нерожавших женщин, сохраняя анатомо-функциональную целостность шейки матки и архитектонику цервикального канала.
На основании результатов проведенных доклинических и клинических исследований Цервикон-ДИМ (дииндолилметан) был официально зарегистрирован как лекарственное средство и вошел в фармакотерапевтическую группу «прочие противоопухолевые препараты».
На сегодняшний день Цервикон-ДИМ – это единственный лекарственный препарат, непосредственно воздействующий на патогенез CIN. Его отличает:
- новый механизм действия, отличный от противовирусных препаратов и иммуномодуляторов;
- прямое показание к применению для лечения дисплазии шейки матки;
- эффективность в отношении регрессии цервикальных дисплазий, подтвержденная гистологическим методом исследования;
- локальное действие и отсутствие системного эффекта.
Цервикон-ДИМ открывает новые перспективы в консервативном лечении дисплазий шейки матки у женщин репродуктивного возраста и значительно расширяет возможности вторичной профилактики рака шейки матки.



