Одним из основных заболеваний шейки матки, опасных для жизни женщины, является рак шейки матки (РШМ), распространенность которого ежегодно увеличивается. Об этом свидетельствуют данные показателей заболеваемости в России: среднегодовой темп прироста РШМ составил в 2018 г. 2,19%, за период с 2008 по 2018 гг. – 24,93% [1]. По данным Всемирной Организации Здравоохранения (2018), зарегистрировано 569 847 новых случаев заболевания РШМ и 311 365 летальных исходов. Вместе с тем РШМ в настоящее время является одним из предотвратимых видов рака.
Цервикальные интраэпителиальные неоплазии умеренной и тяжелой степени (CIN2–3), которые относятся к предраковым поражениям шейки матки, могут предшествовать развитию РШМ на протяжении нескольких лет и даже десятилетий [2]. За столь длительный период времени цервикальные поражения могут быть выявлены и излечены до развития инвазивного рака. Вместе с тем нередко процесс перехода в РШМ протекает достаточно стремительно, не укладываясь в четкую структуру поэтапного развития канцерогенеза. В этой связи особый интерес в настоящее время вызывают вопросы, связанные с лечением предраковых заболеваний шейки матки. Согласно клиническим рекомендациям 2020 г., лечение CIN2+ предусматривает проведение хирургического лечения: петлевую эксцизию шейки матки или конизацию в зависимости от типа зоны трансформации (ЗТ) с последующим выскабливанием цервикального канала [3]. В зарубежной литературе эту процедуру обычно называют двумя терминами: LEEP – loop electrosurgical excision procedure (чаще используется в США); LLETZ – large loop excision of transformation zone (чаще используется в Великобритании).
При проведении эксцизии необходимо иссечь всю ЗТ с переходной зоной и частью вышележащих эндоцервикальных крипт. При гистологическом подтверждении диагноза и отсутствии опухолевых клеток в краях резекции и в соскобе из оставшейся части цервикального канала проведенный объем хирургического вмешательства считается адекватным. Если в краях резекции шейки матки или в соскобе из оставшейся части цервикального канала обнаруживаются плоскоклеточные интраэпителиальные поражения высокой степени (HSIL), рекомендуются цитологический, кольпоскопический контроль и исследование на вирус папилломы человека (ВПЧ) через 2–4 месяца. При наличии аномальных результатов цитологии, и/или аномальной кольпоскопической картины, и/или при позитивном ВПЧ с сохранением вирусной нагрузки показана повторная конизация [4].
В клинической практике петлевая электроэксцизия зарекомендовала себя как наиболее эффективный метод лечения CIN, позволяющий провести процедуру, сохранив функциональную целостность шейки матки. Лечение CIN2+ методом LEEP является наиболее эффективным, и пациенты, как правило, не нуждаются в дальнейшем лечении. Самое главное достоинство данной методики – возможность гистологической оценки всего удаленного образца ткани после выполнения процедуры [5]. Вместе с тем, по данным различных авторов, у 6–20% пациентов после проведенного лечения развивается рецидив заболевания [6]. Так, по данным Американской Ассоциации по кольпоскопии и цервикальной патологии (ASCCP), у 6% женщин с выявленным CIN2+ диагностирован рецидив CIN3 в течение 5 лет после проведенной терапии, у 16,5% – выявлен рецидив CIN2.
Известно, что ВПЧ – ключевой фактор развития CIN. При развитии инфекции возможно латентное течение с последующей спонтанной самоэлиминацией ВПЧ; однако длительная его персистенция увеличивает риск прогрессирования CIN и уменьшает вероятность обратного развития заболевания. При этом у женщин с положительным тестом на ВПЧ статистически чаще выявляются рецидивы CIN. Так, в исследовании Heymans J. at al. (2016) женщин с выявленным ВПЧ 16 генотипа отнесли к группе высокого риска по развитию рецидива заболевания [7]. Эти данные согласуются с результатами исследований других авторов. Так, Maria Teresa Bruno et al. (2019) провели анализ 182 случаев CIN3/CIS (carcinoma in situ) с положительным тестом на ВПЧ. У 104 пациенток (57,1%) был выявлен ВПЧ 16 генотипа, у 78 (42,8%) – другие генотипы ВПЧ. После проведения LEEP у 144 (79,1%) пациенток в течение 6 месяцев наблюдения тест на ВПЧ был отрицательным, у 9 (4,9%) обнаружен ВПЧ высокого онкогенного риска, у 29 (15,9%) – ВПЧ 16 генотипа, выявлявшийся ранее. У 20 пациенток (10,9%) после LEEP по результатам гистологического исследования был обнаружен положительный край резекции, у 9 из них – ВПЧ 16 генотипа, у 6 – другие высокоонкогенные типы ВПЧ. Следует отметить, что во всех 9 случаях (100%) при положительном крае резекции и наличии ВПЧ 16 генотипа в процессе наблюдения диагностировались рецидивы CIN2+; в то время как из 6 женщин с наличием ВПЧ других высокоонкогенных типов рецидив CIN2+ был диагностирован лишь в одном случае (16,6%). Вместе с тем рецидивы CIN2+ были выявлены у 8 (40%) женщин с отрицательным краем резекции при позитивном ВПЧ 16 генотипа [8]. Результаты исследования Aiping Fan et al. (2018) показали, что среди 172 пациенток, которым провели LEEP по поводу CIN2+, у 14 (8,1%) впоследствии был диагностирован рецидив заболевания. При этом у 51 пациентки (29,7%) после проведенного лечения был выявлен ВПЧ: в 41 случае – 16, 18 генотипов, в 10 случаях – других типов. Из 14 случаев рецидива CIN всего в одном наблюдении не был обнаружен ВПЧ [9]. По данным исследования Kreimer A.R et al. (2019), дополняющего вышеприведенные выводы, выявление ВПЧ 16 генотипа в течение 6 месяцев после проведения LEEP связано с увеличением абсолютного риска развития CIN2+ на 37%, что вдвое превышает риск при носительстве ВПЧ 18 генотипа (18,5%) и в 3 раза – при других типах ВПЧ высокого онкогенного риска (10,8%) [10]. Таким образом, риск рецидива CIN – минимальный, если отсутствует дополнительное воздействие ВПЧ.
Полученные данные свидетельствуют о том, что наличие ВПЧ 16 генотипа следует рассматривать как значимый фактор риска развития рецидива CIN2+, поскольку рецидивы наблюдались даже у женщин при отрицательном крае резекции. Кроме того, персистенция ВПЧ 16 генотипа связана со значительно большим риском развития рецидивов CIN2+ после проведения LEEP по сравнению с персистенцией других высокоонкогенных типов ВПЧ.
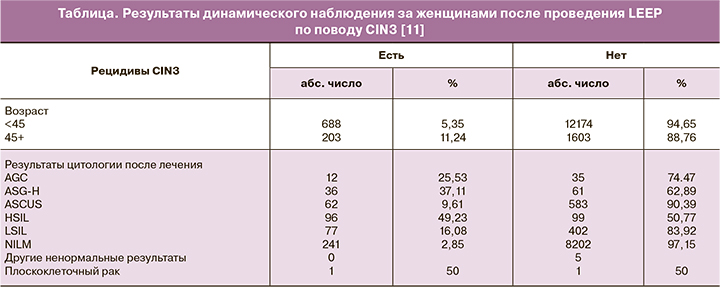
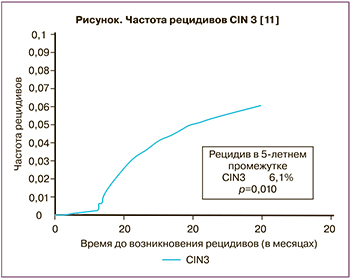
Анализ данных литературы, посвященный контролю за состоянием шейки матки после хирургического вмешательства, показал, что чрезвычайно важным является динамическое наблюдение за данным контингентом женщин. Так, в рамках исследования Brenna E. Swift et al. (2020) были проанализированы результаты 14 668 женщин, которым провели LEEP по поводу CIN3. У 891 пациентки (6,1%) был выявлен рецидив заболевания. Анализ показал, что, по данным цитологического исследования, в 49,5% наблюдений диагностирован HSIL, в 37,1% случаев – атипичные плоскоклеточные клетки, не исключающие HSIL (ASC-H), у 22,6% пациенток – атипичные железистые клетки (AGC), у 15,9% – плоскоклеточные поражения плоского эпителия низкой степени (LSIL) и в 9,8% случаев – атипичные клетки плоского эпителия неопределенного происхождения (ASCUS) (таблица). При этом наиболее значимыми факторами риска являлись: возраст женщины более 45 лет, при котором риск рецидива составил 11,2% против 5,6% у женщин моложе 45 лет, и наличие ВПЧ 16, 18, 45 генотипов [11]. Авторами сделан вывод о необходимости динамического наблюдения за пациентами после проведения LEEP: риск развития рецидива заболевания при выполнении трех цитологических исследований составил 3,4%, в то время как при одном – 7,1% (рисунок).
 В клинической практике для своевременного выявления и лечения рецидивов CIN важна оценка факторов риска, которые могут привести к развитию рецидивов заболевания. В 2019 г. были опубликованы результаты исследования Fernandez-Montol M.E. et al., согласно которым к основным предикторам развития рецидивов CIN следует относить [12]:
В клинической практике для своевременного выявления и лечения рецидивов CIN важна оценка факторов риска, которые могут привести к развитию рецидивов заболевания. В 2019 г. были опубликованы результаты исследования Fernandez-Montol M.E. et al., согласно которым к основным предикторам развития рецидивов CIN следует относить [12]:
1) возраст женщин. Пациентки старше 35 лет находятся в группе повышенного риска, так как установлено, что статистически частота встречаемости рецидивов в этом возрасте выше;
2) положительный тест на ВПЧ. Вероятность развития рецидива CIN у женщин с персистенцией высокоонкогенных типов ВПЧ после LLETZ приблизительно в 32 раза выше по сравнению с пациентками с отрицательным результатом теста на ВПЧ;
3) при положительном крае резекции возможно развитие рецидива даже при отрицательном тесте на ВПЧ. При выявлении высокоонкогенных типов ВПЧ риск развития рецидива дополнительно повышается примерно в 30,5 раза;
4) отсутствие наблюдения после проведенного лечения. В настоящее время до 50% случаев РШМ диагностируется у пациенток, которым не проводилось динамическое наблюдение;
5) недостаточная глубина воздействия при проведении LEEP. При эксцизии необходимо иссечь всю ЗТ с переходной зоной и частью вышележащих эндоцервикальных крипт. При ЗТ 1 типа глубина иссечения должна быть не менее 7 мм. При ЗТ 2 типа глубина иссечения увеличивается до 10 мм. При затруднении полной визуализации стыка многослойного плоского эпителия и цилиндрического эпителия и при наличии эндоцервикального компонента глубина иссечения не должна быть менее 15 мм [3];
6) наличие воспалительных заболеваний нижнего отдела половых органов. Доказано, что сопутствующие CIN цервициты и вульвовагиниты могут служить источником диагностических ошибок при интерпретации кольпоскопической картины шейки матки, результатов цитологического и гистологического методов исследования. Неадекватная оценка состояния шейки матки зачастую приводит к необоснованным в последующем манипуляциям [13]. Учитывая то, что в настоящее время все чаще в клинической практике мы сталкиваемся с микст-инфекцией, чрезвычайно важной является подготовка женщин к хирургическим методам лечения. Совершенно очевидным является тот факт, что адекватная противовоспалительная терапия определяет возможность в короткие сроки эффективно подготовить пациентку к проведению прицельной биопсии и/или эксцизии шейки матки. Таким образом, своевременная терапия цервицитов и вульвовагинитов позволяет избежать «ложных» результатов обследования пациентов и, если это необходимо, провести соответствующее лечение. При этих состояниях повышается восприимчивость к инфекциям, передающимся половым путем, выявляются высокие титры ВПЧ и снижается вероятность его самоэлиминации, значительно сокращается возможность регресса CIN [14, 15]. В случае выявления оппортунистических микст-инфекций (бактериальный вагиноз, вульвовагинальный кандидоз, аэробный вагинит) принципиально важным является назначение местного лечения комбинированными препаратами широкого спектра действия.
Одним из таких препаратов является широко используемый в клинической практике «Тержинан». Препарат оказывает противовоспалительное, противомикробное, противопротозойное и противогрибковое действия, обеспечивая постоянство pH среды и целостность слизистых оболочек влагалища. В состав препарата входят: тернидазол 200 мг – противогрибковое средство из группы производных имидазола, активный также в отношении анаэробных бактерий, в частности Gardnerella spp.; неомицина сульфат 100 мг – антибиотик широкого спектра действия из группы аминогликозидов, действующий бактерицидно в отношении грамположительных и грамотрицательных микроорганизмов; нистатин 100 000 МЕ – противогрибковый антибиотик из группы полиенов, высокоэффективный в отношении дрожжеподобных грибов рода Candida; преднизолон 3 мг – аналог гидрокортизона, оказывающий выраженное противовоспалительное, противоаллергическое, противоэкссудативное действия.
В исследовании Е.И. Боровковой и соавт. (2017) показано, что при применении препарата «Тержинан» отмечается положительная динамика изменений состава микробиоценоза влагалища; при этом доказана нормализация синтеза иммуноглобулинов вне зависимости от наличия ВПЧ после окончания курса терапии [16]. В рамках исследования были проанализированы данные 160 пациенток репродуктивного возраста (25–45 лет), из них у 47 (29,4%) был выявлен ВПЧ. В 139 (86,8%) случаях выявлены значительные изменения состава микробиоценоза влагалища: повышение количества бифидо- и лактобактерий на фоне снижения количества клостридий, пептострептококков, стафилококков, коринебактерий, актиномицетов и энтерококков.
Также при сравнении состояния локального иммунитета показано, что у пациенток до начала терапии препаратом «Тержинан» в цервикальной слизи преобладали IgA, секреторный IgA и IgG. После лечения отмечено увеличение количества IgG, снижение концентрации IgA и стабильные концентрации IgM и секреторного IgA. Результаты иммунологического исследования показали, что у пациенток без ВПЧ уровень Ig до и после проведенной терапии находился в пределах нормативных значений. У женщин с аэробным вагинитом и папилломавирусной инфекцией отмечалось незначительное снижение показателей местного иммунитета, без достоверной разницы результатов до и после проведенного лечения.
Согласно утвержденной инструкции, «Тержинан» может быть использован как с лечебной целью при вульвовагинальном кандидозе, бактериальных и смешанных вагинитах, так и с профилактической целью перед проведением гинекологических операций, перед родами или абортом, до и после деструктивных или эксцизионных методов лечения шейки матки, перед проведением гистерографии [13]. Применение комбинированного препарата «Тержинан» для подготовки пациенток к прицельной биопсии шейки матки и эксцизионным методам лечения в два раза сокращает сроки эпителизации после коагулирующего воздействия на шейку матки и позволяет снизить число сомнительных результатов гистологического исследования.
Данные исследования М.Н. Коставы (2002) показали, что у 68 пациенток, получавших перед биопсией шейки матки препарат «Тержинан», отсутствовали сомнительные в плане наличия CIN данные по сравнению со 2-й группой пациенток с аналогичными поражениями шейки матки, принимавших другие лекарственные средства местного действия. Кроме того, при применении препарата «Тержинан» ни в одном случае не понадобилось проведения дополнительного противовоспалительного лечения и повторной биопсии шейки матки, а сроки эпителизации после коагулирующего воздействия составили 3–4 недели по сравнению с результатами второй группы, где срок эпителизации составил 8–11 недель [17]. Так, использование «Тержинана» способствует физиологическому течению послеоперационного периода, снижает число повторных биопсий и частоту развития осложнений.
Согласно результатам исследования Ю.А. Дубоссарской и соавт., в 71–98% случаев препарат «Тержинан» не нарушает качественный и количественный состав микрофлоры влагалища, что позволяет применять препарат без сопутствующего назначения пробиотиков [18].
Таким образом, «Тержинан» не вызывает выраженного раздражающего действия на слизистую оболочку влагалища, удобен в применении, не только эффективно угнетает рост патогенной микрофлоры, но и способствует нормализации состава микробиоты, а широкий спектр его действия с высокой эффективностью и безопасностью применения позволяет рекомендовать его для лечения смешанных инфекций [19, 20].
Учитывая то, что нарушения микробиоценоза влагалища являются одной из ключевых причин персистенции ВПЧ, своевременное назначение противовоспалительной терапии с учетом выявленных возбудителей позволит в известной мере снизить число хирургических вмешательств на шейке матки.
Высокие показатели смертности, тенденция к «омоложению» РШМ, определенные сложности с выявлением заболевания на ранних стадиях определяют необходимость проведения первичного скрининга как научно обоснованного подхода к решению данной проблемы [21].
По данным ВОЗ (2019), 51% стран включили вакцинацию против ВПЧ в национальные программы иммунизации. В мире в настоящее время зарегистрированы три вакцины против ВПЧ: двухвалентная вакцина против ВПЧ 16 и 18 генотипов (Cervarix), четырехвалентная вакцина против ВПЧ 6, 11, 16 и 18 генотипов (Gardasil) и девятивалентная вакцина против типов ВПЧ, входящих в четырехвалентную вакцину, и дополнительно против 31, 33, 45, 52 и 58 генотипов ВПЧ (Gardasil 9). В нашей стране зарегистрированы двух- и четырехвалентные вакцины [22]. В результате многочисленных исследований было доказано, что вырабатываемый вакциной иммунитет осуществляется опосредованно путем индуцирования вирусоподобными частицами L1 образования в плазме крови высоких концентраций IgG – основного иммуноглобулина цервикального секрета. Антитела накапливаются в достаточно больших концентрациях для того, чтобы инактивировать вирусные частицы, тем самым предотвращая развитие инфекционного процесса [21].
Проведение вакцинации четырехвалентной вакциной показано в возрасте от 9 до 45 лет, однако самые высокие показатели иммунного ответа были зарегистрированы в возрасте 9–15 лет (до наступления возраста половой активности) [23]. Важно отметить, что вакцина против ВПЧ является профилактической, и желательно, чтобы вакцинация была проведена в раннем подростковом возрасте до заражения ВПЧ. Ретроспективный анализ применения двух- и четырехвалентной вакцин показал, что у женщин, инфицированных ВПЧ, при последующем наблюдении частота выявлений рецидивов CIN была значительно ниже по сравнению с невакцинированными пациентами [24]. Вместе с тем эти данные в известной мере противоречивы. Так, по данным исследования Hildesheim A. et al. (2016), были оценены результаты наблюдения за 311 женщинами (142 из них были вакцинированы), которым была проведена LEEP по поводу CIN. После проведенной эксцизии у 34% женщин были выявлены невакцинальные типы ВПЧ. В 1,6% случаев наблюдались рецидивы CIN. Таким образом, авторам не удалось найти убедительных доказательств эффективности вакцины против возникновения рецидивов CIN [25]. Вместе с тем результаты исследования Joura E.A. et al. (2012) показали, что из 1350 женщин, прошедших лечение по поводу CIN2–3, у 587 вакцинированных пациентов рецидивы заболевания выявлялись значительно реже, чем у 763 человек, получивших плацебо. Впоследствии была оценена эффективность вакцины против рецидива CIN после проведенного лечения, которая составила 64,9% при CIN2 и 48,3% – при CIN1 [26]. Эти данные согласуются с результатами, опубликованными в работах других авторов. Так, в исследовании Garland S.M. et al. (2016) были проанализированы данные 18 644 пациенток, которым в течение 4 лет проводили динамический контроль за состоянием шейки матки. У 454 женщин была диагностирована CIN2+ и проведено эксцизионное лечение. Из них 190 пациенток были вакцинированы против ВПЧ. Корреляционный анализ показал, что среди вакцинированных женщин рецидивы CIN2+ встречались на 88,2% реже, чем у невакцинированных. Подобная корреляция не так четко прослеживалась в случаях с рецидивами CIN2 (42,6%) [27].
В 2020 г. опубликована обзорная статья Katie Lichter et al., в которой с помощью электронных баз Cochrane, EMBASE, MEDLINE, Scopus и ClinicalTrials.gov были проанализированы 6 статей (исследования были проведены в Италии, Коста-Рике, США, Республике Корея). Статьи посвящены роли вакцинопрофилактики против ВПЧ в снижении частоты рецидивов CIN [28]. В трех исследованиях сообщалось о значительном снижении риска рецидива CIN2+, авторы двух работ сообщили лишь о незначительном снижении риска рецидива среди женщин, прошедших вакцинацию. И в одном исследовании не выявили корреляции между проведением вакцинопрофилактики и снижением риска развития рецидива CIN после проведенного хирургического лечения.
При изучении частоты рецидивов CIN различной степени тяжести в течение 6–48 месяцев наблюдения среди женщин, прошедших вакцинацию, по сравнению с теми, кому было введено плацебо, было выявлено значительное снижение распространения рецидивов CIN. Всего было зарегистрировано 243 случая CIN: 86 случаев у пациентов, которые получили вакцинацию (6,3%), и 157 у женщин, которые не вакцинировались против ВПЧ (9,7%). В целом проведение вакцинации коррелировало с 33% снижением риска CIN1+ в вакцинированной группе. Во всех исследованиях уделялось внимание риску рецидива CIN у вакцинированных и не вакцинированных против ВПЧ пациентов. Два исследования сообщали о рецидивах CIN 3 в течение 6–48 месяцев наблюдения после хирургического лечения. Среди 1137 женщин 496 пациенток были вакцинированы, 641 получили плацебо. Всего наблюдалось 17 случаев (2,8%) рецидивов CIN3 в обоих исследованиях. Из них 3 случая были зарегистрированы в вакцинированной группе (0,6%), 14 случаев – в группе, где вакцинация не проводилась (2,2%). В целом было отмечено снижение риска развития рецидивов CIN3 на 68% в вакцинированной группе.
По поводу эффективности вакцинации было выдвинуто несколько гипотез. Во-первых, вакцинация может обеспечивать перекрестную защиту против разных типов ВПЧ, которые ранее не выявлялись у пациентов. Таким образом, вакцинация может обеспечить защиту от контакта с новыми типами ВПЧ после проведенного лечения [29]. Вторая гипотеза касается изменений иммунитета, вызванных хирургическим вмешательством, которые могут создавать новую иммунную микросреду слизистой шейки матки без признаков поражения ВПЧ, что определяет максимальную эффективность профилактической вакцины. Эта теория была подтверждена иммунологическими исследованиями ряда авторов [30, 31]. Исходя из этого, можно сделать вывод о том, что после проведения эксцизионных методов лечения целесообразно проводить вакцинацию против ВПЧ.
Анализируя все приведенные ранее результаты, можно сделать вывод о том, что существует потенциальная польза вакцинации для снижения риска рецидивов CIN, однако для определения ее эффективности требуются дальнейшие исследования в этом направлении.
Заключение
Таким образом, несмотря на высокую эффективность применения LEEP при лечении CIN2+, ведение женщин с данной патологией требует комплексного подхода, направленного на предотвращение возможного рецидива заболевания. К предикторам рецидивов следует относить: возраст женщины старше 35 лет, носительство и персистенцию ВПЧ, положительный край резекции после проведенной эксцизии, отсутствие последующего контроля за состоянием шейки матки. При ведении пациенток с CIN необходимо учитывать эти факторы риска в процессе динамического наблюдения для своевременного выявления заболевания, проведения профилактических и лечебных мероприятий. Важно также подчеркнуть, что в настоящее время вакцинацию против ВПЧ следует рассматривать как один из эффективных методов профилактики развития рецидивов CIN.



