Monitoring of COVID-19 vaccination in pregnant women of Siberia and the Russian Far East
Objective: To evaluate the dynamics of vaccination against the new coronavirus infection COVID-19 in pregnant women in the Far Eastern and Siberian Federal Districts from October 29, 2021 to December 24, 2021. Materials and methods: A total of 127787 pregnant women were monitored for vaccination from October 29, 2021 to December 24, 2021 in 11 regions of the Far Eastern Federal District and 10 regions of the Siberian Federal District. The findings were presented by the chief obstetricians-gynecologists of the regions. Statistical processing of the obtained data was carried out using the software package Microsoft Excel 2007. The level of null hypothesis testing was considered to be statistically significant at p<0.05. Results: According to the presented data, 126897 pregnant women were registered in the regions of the Far East and Siberia as of October 29, 2021, and 127787 women as of December 24, 2021. The results of the study showed an extremely low percentage of preconception specific prevention in pregnant women in the Far East and Siberia as of October 29, 2021. This indicator increased within two months by more than 2.26 times, namely from 4.2% to 9.5%. During monitoring, the proportion of vaccinated women before 22 weeks gestation increased by 2.1 times, from 0.7 to 1.5%; after 22 weeks gestation, it increased by 3.7 times, from 1.5 to 5.5%. The proportion of pregnant women who were ill with COVID-19 or vaccinated in the regions of the Far Eastern and Siberian Federal Districts increased from 13.0% to 26.3% during the monitoring period (p<0.001). There were no serious adverse events during COVID-19 vaccination with the Gam-COVID-Vac vaccine (Sputnik V) in 9667 pregnant women. Conclusion: Despite the absence of serious adverse events during COVID-19 vaccination with the Gam-COVID-Vac (Sputnik V) vaccine in pregnant women, it is necessary to conduct further detailed studies of the safety of vaccination during pregnancy, and also to develop a set of organizational measures aimed at increasing compliance with vaccination against COVID-19 at the period of pregnancy planning.Artymuk N.V., Belokrinitskaya T.E., Parfenova Ya.A., Frolova N.I.
Keywords
The physiological immunological changes that occur during pregnancy may potentially affect the susceptibility and severity of novel coronavirus infection COVID-19 [1–5]. There are conflicting data regarding the susceptibility of pregnant women to this infection [1, 3, 4]. However, pregnancy has been proven to be a risk factor for severe disease associated with COVID-19 [1-9]. Pregnant women with this infection have been found to be hospitalized in intensive care units more frequently; they are more likely to require invasive mechanical ventilation, extracorporeal membrane oxygenation and their mortality is higher than one of reproductive-aged non-pregnant women [1–9]. Moreover, it was found that COVID-19 in pregnant women is characterized by the sudden development of a critical condition during a stable course of the disease [7].
Vaccination has been proven to be the only reliable method for specific prevention of an infectious disease [10]. Many professional medical communities currently recommend vaccination against COVID-19 to pregnant women and nursing mothers, regardless of their gestation and type of vaccine [11–15].
According to the published data on the effect of COVID-19 vaccines on obstetric and perinatal outcomes, there is no evidence that these vaccines pose a risk to pregnant women or their offspring [1]. The study of reproductive toxicity in animals has not found any negative effect on the course of pregnancy and intrauterine development of the fetus [16, 17]. For example, all three COVID-19 vaccines registered in the USA are currently approved by CDC for use in pregnant women and nursing mothers: two mRNA vaccines (Pfizer-BioNTech, New York, and Moderna, Cambridge, Massachusetts) and one adenovirus vector vaccine (Johnson & Johnson-Janssen, Belgium) [1]. The CDC (2021) indicates that any of the currently approved vaccines can be administered to pregnant women or nursing mothers without any preference for the type of vaccine [10, 13]. ACOG (2020) does not indicate any preference regarding the type of vaccine or the time of vaccination during pregnancy either [14]. RCOG (2021) recommends mRNA vaccine for pregnant women because there is more information about the safety of mRNA vaccine in comparison with adenovirus vaccine [11].
In the Russian Federation, only one combined vector vaccine, Gam-COVID-Vac (Sputnik V), can currently be used during pregnancy and lactation. According to the instructions for the vaccine Gam-COVID-Vac dated by June 25, 2021, the medication can be given to pregnant women after 22 weeks gestation in cases when the expected benefit to the mother outweighs the risk to the fetus [18]. However, pregnant women started to be vaccinated against COVID-19 actively only from the end of October 2021 due to the obvious increase in severe maternal morbidity and mortality from this infectious disease.
The aim of the study was to evaluate the dynamics of vaccination against the new coronavirus infection COVID-19 in pregnant women in the Far Eastern and Siberian Federal Districts from October 29, 2021 to December 24, 2021.
Materials and methods
A total of 127787 pregnant women were monitored for vaccination from October 29, 2021 to December 24, 2021 in 11 regions of the Far Eastern Federal District and 10 regions of the Siberian Federal District. The findings were presented by the chief obstetricians-gynecologists of the regions.
Statistical analysis
Statistical processing of the obtained data was carried out using the software package Microsoft Excel 2007. The level of null hypothesis testing was considered to be statistically significant at p<0.05.
Results
According to the presented data, 126897 pregnant women were registered in the regions of the Far East and Siberia as of October 29, 2021, and 127787 women as of December 24, 2021. The proportion of pregnant women who had COVID-19 and vaccine against COVID-19 in the Far Eastern and Siberian Federal Districts is shown in Figure.
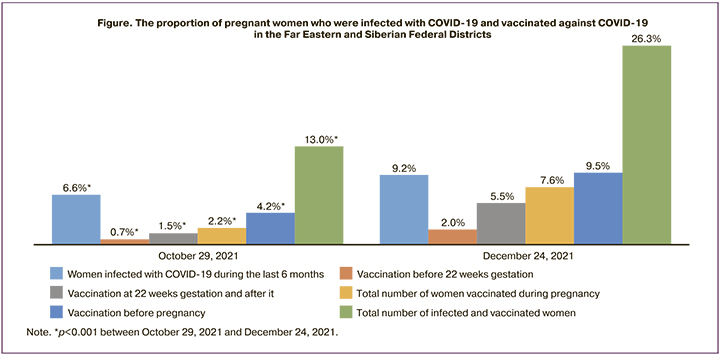
The results of the study showed that during the last six months 8361 (6.6%) pregnant women were infected with COVID-19 as of October 29, 2021. The number of pregnant women who had COVID-19 and who were vaccinated was 16,530 (13.0%) in the Far Eastern and Siberian Federal Districts as of October 29, 2021, including 5147 (5.9%) women in the Siberian Federal District and 3214 (8.0%) women in the Far Eastern Federal District (p<0.001). At the preconception stage, only 4.2% of women were vaccinated against COVID-19, namely 4.6% in Siberia and 3.3% in Far Eastern Federal District (p<0.001). Before 22 weeks gestation, 880 (0.7%) women were vaccinated in the Siberian Federal District; after 22 weeks gestation there were 1931 (1.5%) such women; there were 1.7% and 1.2% of women in the Far Eastern Federal District, respectively (p<0.001).
The number of pregnant women who had COVID-19 and who were vaccinated was 16530 (13.0%) in the Far Eastern and Siberian Federal Districts as of October 29, 2021. This indicator had no statistically significant differences between the districts and amounted to 11238 (12.9%) in Siberia and 5292 (13.2%) in the Far East (p=0.229).
The proportion of pregnant women who were infected with COVID-19 increased to 11757 (9.2%) (p<0.001) as of December 24, 2021 in the Far East and Siberia, while the proportion of pregnant women who had COVID-19 was significantly higher in the Far East than in Siberia, 5252 (13.2%) vs 6505 (7.4%) (p<0.001). During the two-month follow-up period, there was a statistically significant increase in the proportion of vaccinated pregnant women in both districts. Before 22 weeks gestation, 2575 (2.0%) pregnant women were vaccinated in the Siberian Federal District and Far Eastern Federal District, after 22 weeks gestation there were 7092 (5.5%) vaccinated women (p<0.001).
Thus, a total of 9667 (7.6%) pregnant women were vaccinated in Siberian and Far Eastern Federal Districts: the indicator was higher in Siberia than in the Far East, 6872 (7.8%) vs. 2795 (7.1%), respectively (p<0.001). The number of women who had COVID-19 and were vaccinated within 6 months was 33561 (26.3%) in the Far East and Siberia as of December 24, 2021; while the proportion of women who had COVID-19 and were vaccinated in the Far East was significantly higher than in Siberia mainly due to a larger proportion of those infected with COVID-19 and amounted to 13677 (34.6%) vs 19884 (22.5%) (p<0.001).
Discussion
Vaccination during pregnancy has been recognized to be one of the most effective and promising strategies for preventing severe maternal morbidity and mortality from infectious causes. Due to the obtained data on the effectiveness and safety of the vaccine prophylaxis of new coronavirus infection COVID-19 during pregnancy, an increasing number of countries include it in the priority vaccination programs [12–15, 18, 19].
The results of our study showed the absence of severe adverse effects during vaccination against COVID-19 in 9667 pregnant women; 2575 of them were vaccinated before 22 weeks gestation and 7092 women were vaccinated after 22 weeks gestation. However, the main cause of refusal to be vaccinated was the fear of the adverse effect of the vaccine on the course and outcome of pregnancy.
Moreover, there is no evidence that currently available vaccines pose any danger to pregnant women or the fetus and may have a negative influence on obstetric and perinatal outcomes; therefore, foreign national associations recommend vaccination against COVID-19 to pregnant women and nursing mothers [12–15]. The use of mRNA vaccine in pregnant women demonstrated a similar reactogenicity profile as in non-pregnant women, but in pregnant women the frequency of pain at the injection site was slightly higher, and the frequency of headache, myalgia, chills and fever was slightly lower than in non-pregnant women [20]. Among 827 women whose pregnancy was registered in the V-safe registry, the frequency of adverse pregnancy outcomes, including miscarriage, premature birth, low birth weight and congenital anomalies, was identical to the parameters published previously; there were no cases of neonatal deaths [20]. The data on the safety of Johnson & Johnson-Janssen (adenovirus vector) vaccine against COVID-19 are limited because it was approved for use later. There were six cases of cerebral venous sinus thrombosis with thrombocytopenia among vaccine recipients after the FDA permitted the urgent use of the Johnson & Johnson-Janssen vaccine. Similar cases of thrombosis with thrombocytopenia syndrome (TTS) were reported after vaccination with the Oxford AstraZeneca adenovirus vaccine (Great Britain, Sweden) [21]. Most cases of the disease were identified among women aged 18 to 49 years, its frequency is 7.0 cases per one million doses of the vaccine [22]. Despite the fact that the risk of thrombosis increases during pregnancy and after childbirth, the mechanism of TTS differs from thrombosis associated with pregnancy and it can be assumed that there are no particular concerns for the use of this vaccine in pregnant women [23].
Vaccination of pregnant women has been allowed in Russia since June 2021 with the Gam-COVID-Vac vaccine (Sputnik V). Previously, preclinical studies did not reveal any negative effect of the Gam-COVID-Vac vaccine on the course of pregnancy and the development of offspring in animals [24, 25]. Currently, the use of this vaccine is recommended when pregnancy is planned, as it was proven that it has no effect on female and male fertility [26, 27]. Academician G.T. Sukhikh et al. (2021) collected and analyzed the data from 26 regions of the Russian Federation on 773 women vaccinated against COVID-19 during pregnancy. According to the results of the study, the frequency of miscarriages and fetal malformations in this group was consistent with the population data [17].
The results of our study demonstrated an extremely low percentage of preconceptional specific prevention in pregnant women as of October 29, 2021 in the Siberian and Far Eastern Federal Districts, namely 4.2%, which increased by more than 2.26 times up to 9.5% of cases within two months. Other authors have previously indicated a lower rate of vaccination among pregnant women in comparison with non-pregnant women of reproductive age [12]. The significant positive dynamics of vaccination in pregnant women was registered in most regions of the Far Eastern and Siberian Federal Districts. The proportion of vaccinated women before 22 weeks gestation increased by 2.1 times, namely from 0.7 to 1.5%, and after 22 weeks gestation by 3.7 times, from 1.5 to 5.5% of all registered pregnant women. The proportion of pregnant women who were infected with COVID-19 or vaccinated in the regions of the Far Eastern and Siberian Federal Districts increased from 13.0% to 26.3% from October 29, 2021 to December 24, 2021.
Conclusion
The number of pregnant women in the regions of the Siberian and Far Eastern Federal Districts who were infected with the novel coronavirus infection COVID-19 during the last 6 months, or were vaccinated, is extremely small and amounted to only 26.3% as of December 24, 2021. The proportion of vaccinated pregnant women after 22 weeks gestation increased by 3.7 times within two months, however, the registered positive dynamics of vaccination of pregnant women was insufficient to form collective immunity and reduce maternal mortality from the COVID-19 infection. There were no serious adverse events during COVID-19 vaccination with the Gam-COVID-Vac vaccine (Sputnik V) in 9667 pregnant women. Despite the absence of serious adverse events during COVID-19 vaccination with the Gam-COVID-Vac (Sputnik V) vaccine in pregnant women, it is necessary to conduct further detailed studies on the safety of vaccination during pregnancy, and also to develop a set of organizational measures aimed at increasing compliance with vaccination against COVID-19 at the period of pregnancy planning.
References
- Jamieson D.J., Rasmussen S.A. An update on COVID-19 and pregnancy. Am. J. Obstet. Gynecol. 2022; 226(2):177-86. https://dx.doi.org/10.1016/j.ajog.2021.08.054.
- Dashraath P., Wong J.L.J., Lim M.X.K., Lim L.M., Li S., Biswas A. et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020; 222(6): 521-31. https://dx.doi.org/10.1016/j.ajog.2020.03.021.
- Белокриницкая Т.Е., Артымук Н.В., Филиппов О.С., Фролова Н.И. Клиническое течение, материнские и перинатальные исходы новой коронавирусной инфекции COVID-19 у беременных Сибири и Дальнего Востока. Акушерство и гинекология. 2021; 2: 48-54. [Belokrinitskaya T.E., Artymuk N.V., Filippov O.S., Frolova N.I. Clinical course, maternal and perinatal outcomes of a new coronavirus infection COVID-19 in pregnant women in Siberia and the Far East. Akusherstvo i ginekologiya/ Obstetrics and Gynecology. 2021; 2: 48-54. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.2.48-54.
- Artymuk N.V., Belokrinitskaya T.E., Filippov O.S., Frolova N.I., Surina M.N. Perinatal outcomes in pregnant women with COVID-19 in Siberia and the Russian Far East. J. Matern. Fetal Neonatal Med. 2021; Feb 2: 1-4. https://dx.doi.org/10.1080/14767058.2021.1881954.
- Westgren M., Pettersson K., Hagberg H., Acharya G. Severe maternal morbidity and mortality associated with COVID-19: The risk should not be downplayed. Acta Obstet. Gynecol. Scand. 2020; 99(7): 815-6. https://dx.doi.org/10.1111/aogs.13900.
- Collin J., Byström E., Carnahan A., Ahrne M. Public Health Agency of Sweden's Brief Report: Pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet. Gynecol. Scand. 2020; 99(7): 819-22. https://dx.doi.org/10.1111/aogs.13901.
- Vallejo V., Ilagan J.G. A Postpartum death due to coronavirus disease 2019 (COVID-19) in the United States. Obstet. Gynecol. 2020; 136(1): 52-5. https://dx.doi.org/10.1097/AOG.0000000000003950.
- Jafari M., Pormohammad A., Sheikh Neshin S.A., Ghorbani S., Bose D. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: A systematic review and meta-analysis. Rev. Med. Virol. 2021; 31(5): 1-16. https://dx.doi.org/10.1002/rmv.2208.
- Di Toro F., Gjoka M., Di Lorenzo G., De Santo D., De Seta F. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2021; 27(1): 36-46. https://dx.doi.org/10.1016/j.cmi.2020.10.007.
- Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. 2021. Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html#pregnant Accessed October 15, 2021.
- COVID RCOG 19 vaccines, pregnancy and breastfeeding. 2021. Available at: https://www.rcog.org.uk/en/guidelines-research-services/coronavirus-covid-19-pregnancy-and-womens-health/covid-19-vaccines-and-pregnancy/covid-19-vaccines-pregnancy-and-breastfeeding/ Accessed October 15, 2021.
- Razzaghi H., Meghani M., Pingali C., Crane B., Naleway A., Weintraub E. et al. COVID-19 vaccination coverage among pregnant women during pregnancy—eight integrated health care organizations, United States, December 14, 2020–May 8, 2021. MMWR Morb. Mortal Wkly Rep. 2021; 70(24): 895-9. https://dx.doi.org/10.15585/mmwr.mm7024e2.
- Centers for Disease Control and Prevention. V-safe COVID-19 vaccine pregnancy registry. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html Accessed October 15, 2021.
- American College of Obstetricians and Gynecologists COVID-19 vaccination considerations for obstetric–gynecologic care. Practice advisory. 2020. Available at: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care Accessed October 15, 2021.
- RANZCOG, COVID-19 Vaccination in Pregnant and Breastfeeding Women. Updated Wednesday 10 March 2021. Available at: https://ranzcog.edu.au/statementsguidelines/covid-19-statement/covid-19-vaccination-information
- Rasmussen S.A., Kelley C.F., Horton J.P., Jamieson D.J. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet. Gynecol. 2021; 137(3): 408-14. https://dx.doi.org/10.1097/AOG.0000000000004290.
- Сухих Г.Т., Долгушина Н.В., Шмаков Р.Г., Климов В.А., Яроцкая Е.Л. Исходы беременности у пациенток, вакцинированных от COVID-19 во время беременности: предварительные данные. Акушерство и гинекология. 2021; 11: 5-8. [Sukhih G.T., Dolgushina N.V., Shmakov R.G., Klimov V.A., Yarotskaya E.L. Pregnancy outcomes in patients vaccinated against COVID-19 during pregnancy: preliminary data. Akusherstvo i ginekologiya/ Obstetrics and Gynecology. 2021; 11: 5-8 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.5-8.
- Министерство здравоохранения Российской Федерации. Временные методические рекомендации «Организация оказания медицинской помощи беременным, роженицам, родильницам и новорожденным при новой коронавирусной инфекции COVID-19». Версия 4 (01.07.2021). Доступно по: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/057/333/original/05072021_MR_Preg_v4.pdf Ссылка активна на 08.01.2022. [Ministry of Health of the Russian Federation. Temporary methodological recommendations "Organization of medical care for pregnant women, women in labor, maternity and newborns with a new coronavirus infection COVID-19". Version 4 (01.07.2021) (in Russian)]. Available at: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/057/333/original/05072021_MR_Preg_v4.pdf Accessed 08.01.2022.
- Blumberg D., Sridhar A., Lakshminrusimha S., Higgins R.D., Saade G. COVID-19 vaccine considerations during pregnancy and lactation. Am. J. Perinatol. 2021; 38(6): 523-8. https://dx.doi.org/10.1055/s-0041-1726390.
- Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Odoyebo T., Panagiotakopoulos L. et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N. Engl. J. Med. 2021; 384(24): 2273-82. https://dx.doi.org/10.1056/NEJMoa2104983.
- MacNeil J.R., Su J.R., Broder K.R., Guh A.Y., Gargano J.W., Wallace M. et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb. Mortal Wkly Rep. 2021; 70(17): 651-6. https://dx.doi.org/10.15585/mmwr.mm7017e4.
- MacIntyre C.R., Veness B., Berger D., Hamad N., Bari N. Thrombosis with thrombocytopenia syndrome (TTS) following AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19 vaccination - a risk-benefit analysis for people < 60 years in Australia. Vaccine. 2021; 39(34): 4784-7. https://dx.doi.org/10.1016/j.vaccine.2021.07.013.
- American College of Obstetricians and Gynecologists COVID-19 vaccination considerations for obstetric–gynecologic care. Practice advisory. 2020. Available at: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care Accessed October 15, 2021.
- Шипицына Е.В., Ширшова Н.Ю., Коган И.Ю. Вакцинация во время беременности: настоящее и будущее. Акушерство и гинекология. 2021; 11: 9-16. [Shipitsyna E.V., Shirshova N.Y., Kogan I.Y. Vaccination during pregnancy: present and future. Akusherstvo i ginekologiya/ Obstetrics and Gynecology. 2021; 11: 9-16 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.11.9-16.
- Министерство здравоохранения Российской Федерации. Временные методические рекомендации «Организация оказания медицинской помощи беременным, роженицам, родильницам и новорожденным при новой коронавирусной инфекции COVID-19». Версия 5 (28.12.2021) Доступно по: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/059/052/original/BMP_preg_5.pdf Ссылка активна на: 08.01.2022. [Ministry of Health of the Russian Federation. Temporary methodological recommendations "Organization of medical care for pregnant women, women in labor, maternity women and newborns with a new coronavirus infection COVID-19". Version 5 (28.12.2021) (in Russian)]. Available at: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/059/052/original/BMP_preg_5.pdf Accessed 08.01.2022.
- Долгушина Н.В., Драпкина Ю.С., Кречетова Л.В., Иванец Т.Ю., Менжинская И.В., Гус А.И., Байрамова Г.Р., Сухих Г.Т. Вакцина Гам-КОВИД-Вак (Спутник V) не оказывает негативного влияния на овариальный резерв у женщин репродуктивного возраста. Акушерство и гинекология. 2021; 7: 81-6. [Dolgushina N.V., Drapkina Yu. S., Krechetova L.V., Ivanets T. Yu., Menzhinskaya I.V., Gus A.I., Bayramova G.R., Sukhikh G.T. The Gam-COVID-Vac vaccine (Sputnik V) does not adversely affect the ovarian reserve in women of reproductive age. Akusherstvo i ginekologiya/ Obstetrics and Gynecology. 2021; 7: 81-6. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.7.81-86.
- Драпкина Ю.С., Долгушина Н.В., Шатылко Т.В., Николаева М.А., Менжинская И.В., Иванец Т.Ю., Кречетова Л.В., Красный А.М., Гамидов С.И., Байрамова Г.Р., Сухих Г.Т. Вакцина Гам-КОВИД-Вак (Спутник V) не оказывает негативного влияния на сперматогенез у мужчин. Акушерство и гинекология. 2021; 7: 88-94. [Drapkina Yu.S., Dolgushina N.V., Shatylko T.V., Nikolaeva M.A., Menzhinskaya I.V., Ivanets T.Yu., Krechetova L.V., Krasnyi A.M.,Gamidov S.I., Bayramova G.R., Sukhikh G.T. The Gam-COVID-Vacvaccine (Sputnik V) does not adversely affect spermatogenesis in men. Akusherstvo i ginekologiya/ Obstetrics and Gynecology. 2021; 7: 88-94. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.7.88-94.
Received 02.03.2022
Accepted 24.03.2022
About the Authors
Natalya V. Artymuk, MD, PhD, Professor, Head of the G.A. Ushakova Department of Obstetrics and Gynecology, Kemerovo State Medical University, Ministry of Healthof Russia, +7(3842)73-48-56, artymuk@gmail.com, https://orcid.org/0000-0001-7014-6492, 22a Voroshilova str., Kemerovo, 650056, Russian Federation.
Tatiana E. Belokrinitskaya, MD, PhD, Professor, Head of the Department of Obstetrics and Gynecology, Chita State Medical Academy, Ministry of Health of Russia, +7(3022)32-30-58, tanbell24@mail.ru, https://orcid.org/0000-0002-5447-4223, 39a Gorky str., Chita, 672000, Russian Federation.
Yana A. Parfenova, MD, postgraduate student of the G.A. Ushakova Department of Obstetrics and Gynecology, Kemerovo State Medical University, Ministry of Health
of Russia, +7(904)579-15-08, yanachka titova@list.ru, https://orcid.org/0000-0003-2378-9078, 22a Voroshilova str., Kemerovo, 650056, Russian Federation.
Nataly I. Frolova, MD, PhD, Associate Professor at the Department of Obstetrics and Gynecology, Pediatric Faculty, Faculty of Continuing Education and Professional Retraining, Chita State Medical Academy, Ministry of Health of Russia, taasyaa@mail.ru,
https://orcid.org/0000-0002-7433-6012, 39a Gorky str., Chita, 672000, Russian Federation.
Corresponding author: Natalya V. Artymuk, artymuk@gmail.com
Authors’ contributions: Artymuk N.V., Belokrinitskaya T.E. – developing the concept and design of the study, writing the text; Artymuk N.V., Belokrinitskaya T.E., Parfenova Ya.A. – collecting and processing the material; Parfenova Ya.A., Frolova N.I. – statistical data processing; Belokrinitskaya T.E., Artymuk N.V. – editing the text of the manuscript.
Conflicts of interest: The authors declare that they have no competing interests.
Funding: The study was performed without external funding.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Artymuk N.V., Belokrinitskaya T.E., Parfenova Ya.A., Frolova N.I.
Monitoring of COVID-19 vaccination in pregnant women of Siberia and the Russian Far East.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 5: 53-58 (in Russian)
https://dx.doi.org/10.18565/aig.2022.5.53-58



