За последние 40 лет наблюдается непрерывный прогресс в репродуктивной медицине, сопровождающийся возникновением новых и более совершенных методик вспомогательных репродуктивных технологий. Тем не менее частота наступления беременности (ЧНБ) в развитых странах мира достигла своего плато и не меняется кардинально на протяжении последнего десятилетия. Анализ регистров Российской ассоциации репродукции яеловека (РАРЧ) за период 2012-2018 гг., представленный на рисунке, подтверждает этот факт.
В соответствии с данными регистра РАРЧ за 2018 г., ЧНБ после переноса эмбрионов на стадии бластоцисты, по сравнению с переносом на стадии дробящегося эмбриона, оказалась более высокой и составила соответственно в свежих циклах экстракорпорального оплодотворения (ЭКО) и интрацитоплазматической инъекции сперматозоида (ИКСИ) — 38,6 и 28,1%, в циклах с размороженными эмбрионами — 41,7 и 32,4%, в циклах с донорскими ооцитами — 46,0 и 30,2%, в циклах с преимплантационным генетическим тестированием (ПГТ)/преимплантационным генетическим скринингом (ПГС) — 47,5 и 22,6% и в программах суррогатного материнства — 45,6 и 41,3%.
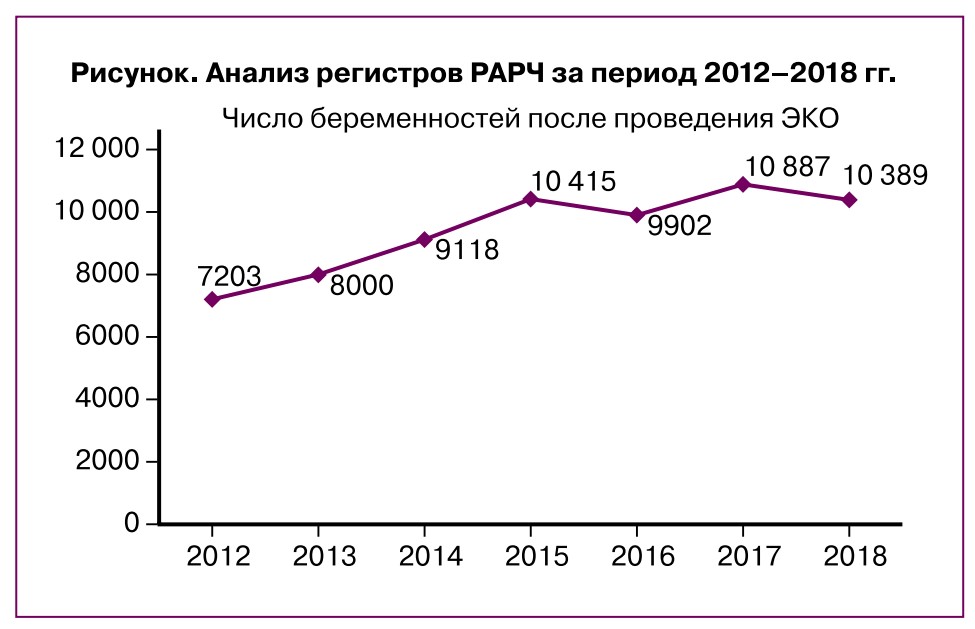
Сравнив аналогичные данные регистра РАРЧ за 2013 г., мы не обнаружили значительного повышения ЧНБ на цикл ЭКО; она составила в расчете на цикл лечения 33,1% (2012 г. — 33,0%), на пункцию — 34,2% (2012 г. — 34,3%), на перенос эмбрионов — 38,5% (2012 г. — 38,5%). В программе ИКСИ эти показатели составили соответственно 29,7, 30,87, 36,9% (2012 г. — 29,6, 30,4, 36,1%). ЧНБ в программе донорства ооцитов составила в расчете на цикл 38,2% (2012 г. — 40,6%), на перенос эмбрионов — 43,8% (2012 г. — 44,1%). Эти же показатели в программе переноса размороженных эмбрионов в расчете на цикл составили 29,6% (2012 г. — 31,3%), на перенос эмбрионов — 33,4% (2012 г. — 33,2%); в программе ПГТ/ПГС на цикл — 20,5% (2012 г. — 28,8%), на перенос эмбрионов — 31,8% (2012 г. — 36,9%).
Таким образом, ни широкое использование в рутинной клинической практике методов ПГТ, ни модификации протоколов контролируемой овариальной стимуляции и эмбриологических пособий не привели к значительным изменениям по основным показателям эффективности программ ЭКО: ЧНБ и частоте живорождения.
Кроме того, многие вопросы, касающиеся генеза ряда нарушений репродуктивной системы, остаются без ответа. Специалисты продолжают задаваться вопросом о природе идиопатического бесплодия, почему формально здоровые пары не могут достичь беременности? Неясно, почему молодые женщины без видимых причин имеют низкие показатели овариального резерва, почему в повторяющихся циклах ЭКО имеют место низкое качество ооцитов и так называемый «арест раннего эмбриогенеза», когда эмбрионы перестают развиваться на 2-3-й день культивирования. И наконец, существует ли межцикловая вариабельность качества рекрутированных фолликулов и от чего это зависит?
Ответы на эти и ряд других вопросов лежат, скорее всего, не на поверхности, в плоскости гормональнозависимой фазы фолликулогенеза, а в определении характера и особенностей внутрияичникового фолликулогенеза, механизмов перехода фолликулов от одной стадии развития к другой. Ряд дефектов оогенеза формируется в отсутствие гонадотропной регуляции и, вероятно, полностью зависит от внутренних сигнальных механизмов, сложной сети передачи сигналов между ооцитом и его окружением [1].
Для идентификации механизмов формирования «поломок» в ооцитах и дальнейшего поиска их преодоления принципиально важным становится изучение механизмов их первичной селекции [2].
Теории рекрутирования пула фолликулов и перехода их в гонадотропин-зависимую фазу
Успешное применение модифицированных протоколов овариальной стимуляции и возможность стимуляции яичников в любой день менструального цикла, в режимах так называемых random-start протоколов, создали определенный научный парадокс [3]. Ведь, следуя классическим, сформулированным в прошлом столетии представлениям о функции яичников, это невозможно, т.к. считается, что когорта фолликулов, выходящих в гонадотропин-зависимую фазу, рекрутируется один раз в течение цикла, к концу лютеиновой фазы предыдущего цикла. Один фолликул становится доминантным и овулирует, другие подвергаются атрезии. Считается, что стимуляция с начала фолликулярной фазы за счет повышения концентрации ФСГ «спасает» фолликулы, которые должны были бы атрезироваться, тем самым обеспечивает мультифолликулярный рост [4]. Это классика стимуляции функции яичников, существующая десятилетиями. Клиническая практика последних лет опровергает классические представления о физиологии фолликулогенеза.
На данный момент были предложены три различные теории рекрутирования фолликулов [4].
- Теория непрерывного рекрутинга. Исследования, проведенные изначально на млекопитающих, в первую очередь на крупном рогатом скоте, привели к выводу о непрерывно происходящем рекрутинге фолликулов, вне зависимости от уровней ФСГ и ЛГ [4].
- Теория единичного эпизода рекрутирования за цикл, в соответствии с которой когорта из антральных фолликулов диаметром 2—5 мм лишь единожды за менструальный цикл (или интеровуляторный интервал) отбирается для дальнейшего развития или апоптоза [4].
- Теория «волнового» рекрутинга. Сторонники «волновой» теории фолликулогенеза считают, что несколько когорт, или «волн», антральных фолликулов вступают в фазу роста за один менструальный цикл или интеровуляторный интервал [5].
«Волновым» развитием фолликулов принято считать рекрутирование группы антральных фолликулов через равные промежутки времени в течение менструального цикла или интеровуляторного интервала. Фолликулы в каждой из волн имеют схожие, но не идентичные характеристики [5]. Как правило, один из рекрутируемых фолликулов становится доминантным, остальные же подвергаются атрезии. Новые волны манифестируют через равные промежутки времени, каждой из них предшествует небольшое увеличение концентрации ФСГ. В одном интеровуляторном интервале первая волна является ановуля- торной, последняя же всегда оканчивается овуляцией. В самом подробном из существующих на данный момент исследований яичники 63 нормально менструирующих женщин в возрасте от 19 до 43 лет (средний возраст 28 лет и 7 месяцев) изучены при помощи трансвагинального ультразвукового исследования [6].
Если исходить из предположения, что рекрутинг фолликулов из яичника происходит непрерывно в течение менструального цикла и не зависит от влияния центральных структур, то сам по себе яичник следует считать самодостаточной и саморегулирующейся структурой, в которой вследствие неясных пока механизмов осуществляются основные процессы роста, развития фолликулов и обеспечение полноценности ооцитов. То есть, проводя стимуляцию яичников в гонадотропин-зависимой фазе, мы имеем дело уже с вершиной айсберга, в которой в значительной степени предопределено число и качество имеющихся яйцеклеток.
Извлечение и дозревание незрелых фолликулов
Предпринимаются попытки извлечения и дозревания незрелых ооцитов [7]. Существует точка зрения, что первичные фолликулы могут быть более состоятельными в обеспечении репродукции, т.к. лишены дефектов, возможно, приобретаемых в процессе их развития и созревания. В клинической практике довольно успешно «дозревают» ооциты, находящиеся на стадии профазы первого мейоза — MI. Однако частота наступления беременности из «дозревших» и оплодотворенных ооцитов оказалась значительно ниже, чем в классической ситуации получения зрелых ооцитов [8]. Специалисты не без основания полагают, что причиной этого является не качество извлеченного незрелого ооцита, а условия культивирования in vitro, неспособные преодолеть асинхронное созревание ядра ооцита и интрацитоплазматических структур. Но эта проблема представляется решаемой путем модификации и совершенствования сред культивирования ооцита [8].
В этой связи в последнее время значительно вырос интерес к проблеме культивирования in vitro незрелых ооцитов, определения их способности к оплодотворению и выходу бластоцист. Определяются методики получения незрелых ооцитов, т.е. с минимальной стимуляцией или без таковой, и проводится оптимизация сред для дозревания [5].
Развитие направления сохранения репродуктивного материала онкологических больных способствовало получению незрелых ооцитов из ткани удаленного яичника [9]. Более того, предпринимаются попытки культивирования не только незрелых ооцитов на стадии профазы первого мейоза, но и на стадии герминального везикула [6].
В целом дозревание незрелых ооцитов может быть перспективным для клинической практики направлением.
Пренатальный фолликулогенез
Достижения фундаментальной эмбриологии и биологии развития позволяют сместить фокус исследований с последних 10 дней развития фолликула на процессы, происходящие до гонадотропин-зависи- мой фазы, и сам механизм селекции примордиальных фолликулов.
Сам по себе пренатальный фолликулогенез является загадкой. Так, к 26-й неделе развития плода в яичниках можно наблюдать от 6 до 7 млн примордиальных и антральных фолликулов. На момент рождения это число катастрофически снижается до 400 000 фолликулов. Далее до конца не изученные внутрио- вариальные механизмы активируют рост небольшого количества спящих первичных фолликулов, большая часть из которых уйдет впоследствии в атрезию; примерно к 50 годам у женщин остается около 1000 фолликулов [10].
Почему природа так жестко ограничила репродуктивный период женщины и можно ли это изменить? Не исключено, что репродуктивный запас вполне достаточен для поддержания человеческого вида, ведь природа предполагала, что женщина, достигнув половой зрелости, начнет рожать детей, что и было раньше, но не сейчас.
Можно ли пополнить запас яйцеклеток?
Пул примордиальных фолликулов считается единственным источником ооцитов для оплодотворения [11], хотя эта теория подвергалась сомнению в течение последнего десятилетия [12]. Группа Tilly J.L. была первой, сообщившей об обнаружении стволовых клеток в яичниках взрослых мышей [13]. Сообщалось об обнаружении клеток, активно экспрессирующих MVH (mouse vasa homologue) и BrdU (маркер пролиферации 5’-бромдезоксиуридин), в поверхностном эпителии яичников мыши (OSE). Позднее, с помощью проточной цитометрии этими же учеными в яичниках обнаружен VSELs (Very Small Embryonic-Like Stem Cells), фактор, относящийся к разряду плюрипотентных стволовых клеток и, предположительно, способный дать начало закладке OSC, специфичных предшественников ооцитов [13]. Позже группа Virant-Klun et al. [14] впервые сообщила о плюрипотентных стволовых клетках в организме человека. Эти стволовые клетки были очень малы по размеру (3—5 мкм), и VSELs, которые экспрессировали плюрипотентные маркеры на культуре, приводили к дифференцировке этих стволовых клеток в ооцитоподобные структуры размером >90 мкм с четко определенной прозрачной зоной. В то же время было показано, что ооцитоподобные структуры, полученные путем дифференцировки клеток, находящихся в ОСЕ, способны подвергаться оплодотворению и кортикальной реакции при инкубации со спермой. Было показано, что VSELs существуют в большом количестве в ткани яичников, собранной у женщин с пограничным раком и серозной карциномой яичников, что указывает на их потенциальную причастность к раку яичников [15]. Эти выводы были подтверждены другой группой ученых [16].
Многие ученые не поддерживают точку зрения о возможности пополнения пула фолликулов за счет стволовых клеток [16]. Так, недавние исследования Zhang et al. поддерживают традиционное мнение о том, что в послеродовой жизни у мышей и людей не происходит обновления фолликулов. Как только первичный пул фолликулов сформирован, у примордиальных фолликулов есть три варианта развития судьбы — поддерживать состояние покоя в качестве примордиальных фолликулов, быть активированными и вступить в фазу роста или подвергнуться атрезии [16]. Баланс между периодами покоя, активации и гибели примордиальных фолликулов считается решающим фактором в определении продолжительности репродуктивной жизни женщины.
Сигнальные пути и их значение во внутрияичниковом рекрутировании фолликулов и развитии ооцита
Для развития ооцита в фолликулярной структуре необходима непрерывная двусторонняя связь с клетками кумулюса, которые его окружают, а также с другими соматическими клетками, включенными в фолликул, такими как клетки теки и гранулезы. Эта связь обеспечивается преимущественно с помощью щелевых контактов (для молекул с малой молекулярной массой) и эндоцитоза, опосредованного рецепторами (для молекул с более крупной молекулярной массой). Указанное взаимодействие имеет важное значение для ядерного и цитоплазматического созревания.
PI3K сигнальный путь
Активация примордиальных фолликулов является первым этапом в развитии фолликулов и ключевым фактором, определяющим репродуктивную способность женщин. Существуют ограниченные сведения о сигнальных путях и молекулах, которые участвуют в этом процессе, и в основном данные получены в исследованиях на животных. Для большинства факторов регуляторная роль в рекрутировании примордиальных фолликулов была первоначально установлена с использованием нокаутных генов на грызунах. У людей процессы, аналогичные исследованиям на грызунах и демонстрирующие истощение резерва яичников за счет ускоренной активации примордиальных фолликулов, часто наблюдаются в случаях преждевременной недостаточности яичников. Текущая гипотеза об активации примордиальных фолликулов у мышей гласит, что белок-мишень рапамицина 1 млекопитающих (mTORC1) активируется в уплощенных клетках гранулезы примордиальных фолликулов, а затем Kit-лиганд, продуцируемый активированными клетками гранулезы, в свою очередь, активирует ооцит через фосфатидилинозитол- 3-киназу (PI3K) [17]. Однако до конца механизм активации примордиальных фолликулов остается неясным.
PI3K/AKT/mTOR (внутриклеточный сигнальный путь) — это один из универсальных сигнальных путей, характерных для большинства клеток человека. Он отвечает за рост, пролиферацию клеток, метаболизм, препятствует апоптозу, обладает тканеспецифической функцией. В яичниках млекопитающих путь PI3K/AKT/mTOR необходим для регуляции активации первичных фолликулов.
По своим структурным характеристикам и субстратной специфичности PI3K подразделяется на три класса (I, II и III) [18]. В свою очередь, PI3K класса I также делятся на два подсемейства: класс IA и класс IB. PI3K класса IA являются наиболее хорошо изученными и представляют собой липиды киназы, состоящей из регуляторной субъединицы p85 и каталитической субъединицы p110. Они фосфорилируют группу 3D на инозитоловом кольце фосфатидилинозитола- 3,4,5-трифосфата [19]. Каталитическая субъединица p110 может преобразовывать PIP2 в PIP3 [15].
PIP3 связывает как фосфатидилинозитол-зависи- мую киназу 1 (PDK1), так и протеин-серин-трео- ниновую киназу Akt на клеточной мембране, где PDK1 фосфорилирует и активирует Akt [20]. Akt впоследствии фосфорилирует ряд субстратов, включая ингибитор клеточного цикла p27 (также известный как p27KIP1), киназу гликогенсинтазы 3, комплекс туберозного склероза 2 (TSC2) и семейства факторов транскрипции forkhead box (FOXO) [21-24]. Таким образом, сигнальная сеть PI3K важна для пролиферации клеток, их развития и выживания [25]. Одним из немногих известных негативных регуляторов PI3K/ AKT/mTOR сигнального пути является фосфатаза с двойной субстратной специфичностью (PTEN), обнаруженная в клетках гранулезы первичных фолликулов человека на уровне экспрессии белка и генов. Как негативный регулятор функции PI3K, PTEN возвращает PIP3 обратно в PIP2 и тем самым подавляет передачу сигналов PI3K [26].
FOXO3, член семейства транскрипционных факторов Forkhead, служащий субстратом Akt, также вовлечен в регуляцию сигнальных путей PI3K/PTEN. В моделях на яичниках мышей показано, что FOXO3 импортируется в ядро во время формирования первичного фолликула, а затем экспортируется в цитоплазму при активации. В случае повышенной экспрессии FOXO3 у женщин формируется преждевременная недостаточность яичников. Необходимо отметить, что экспрессия FOXO3 в яичниках женщины в значительной степени отличается от описанной у мышей, у которых FOXO3 в основном экспрессируется в ядре всех примордиальных ооцитов, и его транслокация в цитоплазму совпадает с активацией и ростом фолликулов [27, 28]. В отличие от того, что наблюдается у мышей и других мышевидных грызунов, примордиальные фолликулы во взрослом яичнике млекопитающих, не являющихся грызунами, включая приматов, не являющихся людьми, не экспрессируют FOXO3 [29]. Исследования показали, что экспрессия FOXO3, в зависимости от возраста, варьировалась от очень низкой на стадии зародыша до более высокой в яичниках в пубертатном и старшем возрасте. В любом случае очевидное увеличение числа ооцитов, экспрессирующих FOXO3, в примордиальных фолликулах следует рассматривать осторожно, и в дальнейшем требуется провести углубленный анализ.
Предполагается, что PTEN и FOXO3, как часть пути PI3K/AKT, по-видимому, не играют существенной роли в формировании пула, активации и росте фолликулов в течение эмбрионального периода человека. Известно, что в младенческом и подростковом возрасте определяются примордиальные фолликулы, экспрессирующие FOXO3. Однако большинство фолликулов, которые начинают расти на этой стадии развития, будут подвергаться процессу атрезии через апоптоз [24], чему может способствовать экспрессия FOXO3, а также экспрессия PTEN в клетках гранулезы антральных фолликулов [25]. Было показано, что FOXO3 активирует апоптоз за счет усиления регуляции белков, содержащих только BH3, или за счет внешних апоптотических факторов, таких как FASL и TRIAL [30].
Таким образом, экспрессия ядерного FOXO3 в примордиальном фолликуле может указывать на неизбежность их апоптоза или, в качестве альтернативы, на предотвращение активации и дальнейшего развития, как это наблюдается у мышей. Результаты ограниченных исследований, основанных на наблюдательном описании и небольшом количестве образцов, не позволяют дифференцировать эти возможности. Однако низкая распространенность цитоплазматического FOXO3 может указывать на роль в предотвращении активации, а не в участии в апоптозе. Более того, яичник человека, по сравнению с млекопитающими, демонстрирует самый высокий показатель элиминации внутриутробных половых клеток путем апоптоза. При этом к половому созреванию остается примерно лишь 5% заложенного во время внутриутробной жизни [31]. Данный феномен позволяет предположить, что экспрессия ядерного FOXO3 сохраняет определенный пул фолликулов от активации и, следовательно, фертильность во взрослой репродуктивной жизни является ограниченной временным фактором. В то же время наличие в яичниках «спящих» примордиальных фолликулов открывает определенные горизонты их использования, если представленное предположение верно.
В яичниках ключевой киназой в сигнальном пути PI3K/AKT/mTOR является АКТ, которая экспрессируется как в ооцитах, так и в клетках гранулезы фолликулов человека. AKT имеет широкий спектр субстратов, играющих как прямую, так и косвенную роль в активации фолликулов.
Система IGF
В синергизме с гонадотропинами рассматривают систему IGF, которая может участвовать в процессах сохранения и выживания яйцеклеток. Система IGF, которая состоит из инсулина и инсулиноподобных факторов роста 1 (IGF-1) и 2 (IGF-2), участвует в росте, пролиферации и выживании клеток [31]. IGF, в частности IGF-1, могут синергически взаимодействовать с гонадотропинами для стимуляции фолликулярного стероидогенеза [32]. IGF-1 как самостоятельно, так и с ФСГ может увеличивать фосфорилирование AKT в клетках гранулезы [33].
Ras/ERK/MEK сигнальный путь
Ряд экспериментальных исследований показал, что параллельно с сигнальным путем PI3K/AKT/mTORC1 активируется Ras/ERK/MEK путь. Дефекты в пути Ras/RAF/митоген-активируемой протеинкиназы (MAPK) играют роль в росте и пролиферации клеток через активацию пути ERK. MAPK (митоген протеинкиназы) экспрессируется в нескольких тканях млекопитающих и играет важную роль в регуляции клеточной пролиферации и дифференцировки, реакции на стресс и иммунных реакций [34—37]. Активность MAPK3/1 изучалась у самок мышей в течение многих лет, и нарушение активности MAPK3/1 в клетках гранулезы яичников мыши показало, что эти киназы необходимы для возобновления процессов мейоза, овуляции и лютеинизации ооцитов, индуцированных ЛГ [36]. Исследования на яичниках крыс показали, что лечение PD98059 (inhibitor of the activation of mitogen-activated protein kinase) ингибитором MAPK значительно подавляет активацию первичных фолликулов [38]. Недавнее исследование продемонстрировало, что ингибирование передачи сигналов MAPK3/1 в яичниках мыши с помощью ингибитора MAPK3/1 U0126 (highly selective inhibitor of both MEK1 and MEK2) уменьшает количество растущих фолликулов, уровни фосфорилирования Tsc2 (Tuberous Sclerosis Complex 2), S6K1(Ribosomal protein) и rpS6 (Ribosomal Protein) и экспрессию KITL (KIT-ligand), что указывает на то, что передача сигналов MAPK3/1 участвует в активации первичных фолликулов посредством передачи сигналов mTORC1-KITL в клетках гранулезы. Кроме того, U0126 снижает уровни фосфорилирования Akt, что позволяет предположить, что сигнализация MAPK3/1 регулирует активацию первичных фолликулов через сигнал PI3K в ооцитах [39]. Следовательно, активность MAPK3/1 играет важную роль в активации первичных фолликулов через сигнальный путь mTORC1-KITL в клетках до гранулезы и через сигнализацию KIT-PI3K в ооцитах [31]. В свою очередь, пути AKT и MAPK3 участвуют в опосредовании эффектов гонадотропинов и IGF на пролиферацию фолликулярных клеток и развитие фолликулов [40], а IGF-1 может способствовать росту первичных фолликулов через сигнальный путь PI3K/ AKT [41].
Группа белков TGFfi
Семейство трансформирующего фактора роста бета (TGF-|3) представляет собой большую группу структурно родственных клеточных регуляторных белков, названо в честь своего первого члена, TGF-|31. TGF-|3 регулирует множество биологических процессов у млекопитающих, влияя на пролиферацию, рост, дифференцировку и апоптоз клеток [41, 42]. TGF-|3 связывается с рецепторами серин/треонин-протеинки- назы типов I и II на поверхности клетки с образованием комплекса, который активирует сигнальный путь Smad посредством фосфорилирования белков Smad. Фосфорилированные белки Smad затем связываются с общим Smad4 и перемещаются из цитоплазмы в ядро, где они регулируют транскрипцию гена-мишени [44-46].
Некоторые исследования показали, что IGF-1 пересекается с компонентами сигнального пути TGF-|3 на нескольких уровнях в клеточных линиях [47], хотя необходимы дальнейшие исследования для изучения взаимосвязи между этими двумя основными сигнальными путями в яичниках. Было выявлено, что TGF-|31 участвует в поддержании первичных фолликулов у мышей [48]. В культуре яичников мыши in vitro с TGF-|31 значительно ингибируется активация первичных фолликулов, TGF-|31 индуцирует апоптоз ооцитов и подавляет пролиферацию соматических клеток посредством регуляции сигнального пути TSC/mTORC1.
Таким образом, несмотря на скудные и далеко не однозначные сведения, полученные в основном на животных, определяются наиболее вероятные пути исследований. Это путь PI3K/AKT и связанные с ним части PTEN и FOXO3, при участии системы IGF, которая состоит из инсулина, IGF-1 и IGF-2. Объектом для изучения являются белки семейства TGF-|3, МАРК, которые, вероятно, взаимодействуют с основными сигнальными путями. Скорее всего, не только эти системы задействованы в обеспечении сложных процессов внутрияичникового фолликулогенеза, поэтому перспективным направлением исследований является поиск основных, наиболее значимых звеньев в этой цепочке.
Клинические работы, основанные на воздействии на внутрияичниковые сигнальные пути
Клинические работы, базирующиеся на указанной гипотезе, далеко неоднозначны и пока не поддержаны мировым сообществом. Так, в исследовании K. Kawamura у женщин с преждевременной недостаточностью яичников проводили активацию примордиальных фолликулов за счет стимулирования AKT путем ингибирования PTEN в кортикальных фрагментах яичников in vitro с последующим выполнением ретрансплантации овариальной ткани. Исследователи зафиксировали случаи живорождения после данной процедуры [49]. В свою очередь, было продемонстрировано, что использование ингибирования PTEN для инициации активации примордиальных фолликулов влияет на выживаемость фолликулов, в то время как на животных моделях, наоборот, наблюдалось прекращение репарации ДНК, приводящее к атрезии фолликулов. Таким образом, этот метод требует дальнейшего изучения для использования в клинической практике.
Также были проведены исследования на человеческих фолликулах, обработанных в условиях in vitro ингибитором mTORC1, которые продемонстрировали частичное снижение количества растущих фолликулов и последующее снижение экспрессии мРНК TSC1 (Tuberous sclerosis 1), что подтверждает роль этого каскада в активации первичных фолликулов.
Несмотря на экспериментальные доказательства того, что сигнальные пути PI3K/AKT/mTOR широко задействованы как в активации первичных фолликулов, так и в поддержании покоя, фундаментальное понимание этих процессов все еще очень ограничено, особенно у человека.
«Защита яичников» от повреждающего воздействия химиопрепаратов
Потеря или резкое снижение функции яичников, вызванное химиотерапией у женщин, больных раком, заставляет решать проблему защиты яичников, что тесно связано с исследованиями внутрияичникового фолликулогенеза и рекрутинга фолликулов. В исследовании K.N. Goldman провели ингибирование mTORC1 с помощью низкомолекулярных ингибиторов [50]. В качестве модели использовали яичники мышей, которые подвергли воздействию циклофосфамидом, и одновременно провели ингибирование комплекса mTOR-mTORC1 клинически одобренным препаратом эверолимус (RAD001) или mTORC1/2 — экспериментальным препаратом INK128 (potent and selective TORC1/2 inhibitor). Результаты показали, что ингибирование mTOR сохраняет резерв яичников, количество примордиальных фолликулов, уровни антимюллерова гормона в сыворотке и фертильность. Животные, получавшие химиотерапию, имели значительно меньше потомков по сравнению со здоровыми животными, тогда как совместное с химиопрепаратами назначение ингибиторов mTOR (the mechanistic target of rapamycin) сохраняло нормальную фертильность.
Также в контексте изучения механизмов формирования «ооцитарного» фактора бесплодия и разработки эффективных способов его преодоления чрезвычайно интересным представляется изучение динамики экспрессии генов и транскрипционной характеристики ооцитов на разных этапах фоллику- логенеза. Так, в недавнем исследовании показано, что, по аналогии с морфологической классификацией, при транскриптомном анализе обнаруживается 5 различных субпопуляций фолликулов [51]. Профили транскриптома ооцитов отражают физиологический статус созревания, а не индивидуальный генетический фон.
Заключение
Представленные исследования демонстрируют интерес ученых к изучению сложных вопросов вну- трияичникового фолликулогенеза, роли сигнальных путей в этом процессе, затрагивают вопросы молекулярно-генетического участия и транскриптомного анализа. Пока мало знаний для формирования целостного понимания происходящих процессов, но ясно одно: без накопления этих знаний и проведения фундаментальных исследований не произойдет дальнейшего развития репродуктивной медицины и решения вопросов патологии репродукции, тех ситуаций, которые сейчас мы не можем объяснить, а следовательно, эффективно лечить.



