Microbiota of the cervical canal and uterine cavity in hyperplastic diseases of the endometrium: comparative microbiological analysis
Sarkisyan R.M., Gavrilova T.Yu., Priputnevich T.V., Muravieva V.V., Denisov P.A., Goncharuk O.D., Adamyan L.V.
Objective: A comprehensive assessment of the microbiota composition in the cervical canal and endometrium in women with various forms of endometrial hyperplastic processes (EHP) – polyp/endometrial hyperplasia in combination with uterine myoma or adenomyosis – compared to patients without endometrial pathology, followed by the analysis of its potential pathogenetic role in hyperplastic changes in the endometrium.
Materials and methods: The study included 100 women of reproductive age, divided into 4 groups depending on the presence and nature of endometrial hyperplastic processes. Microbiological examination included polymerase chain reaction test (PCR test) of samples obtained from the cervical canal, as well as culture-based analysis of aspirates from the uterine cavity. To characterize microbial communities, an assessment of microbial saturation and biological diversity was carried out using alpha diversity indices (Shannon-Wiener diversity, Simpson diversity, Margalef's richness) and subsequent data statistical processing.
Results: We found that microbial richness and taxonomic diversity were significantly higher in the cervical canal compared to the endometrium in all study groups. The maximum frequency of endometrial sterility was observed in the control group (52%). However, this indicator was lower and amounted to 24% (p=0.042) in a combination of hyperplastic changes with adenomyosis. The leading representatives of normobiota were Lactobacillus jensenii, L. crispatus and L. gasseri. Opportunistic microorganisms, including Gardnerella vaginalis, Escherichia coli and Enterococcus faecalis, were more often detected in the endometrium of patients with EHP, which may indicate the presence of disorder in microbial homeostasis and the potential pathogenetic significance of these microorganisms.
Conclusion: The microbiological analysis revealed a decline in species diversity and an increase in the proportion of opportunistic microorganisms in the endometrium of patients with EHP. Such changes may indicate microbial transformation of the intrauterine environment and the supposed participation of microbiota in the pathogenesis of this pathology. The obtained results confirm the relevance of further research aimed at a detailed study of the interaction mechanisms between microbiota and endometrial tissue structures, as well as an assessment of its impact on the prevention and treatment of EHP.
Authors’ contributions: Adamyan L.V., Gavrilova T.Yu. – study concept and design; Sarkisyan R.M., Gavrilova T.Yu. – biological material collection and processing; Goncharuk O.D., Muravieva V.V. – laboratory processing; Sarkisyan R.M., Denisov P.A. – statistical data analysis, visualization; Sarkisyan R.M. – manuscript composition; Priputnevich T.V., Adamyan L.V. – manuscript editing.
Conflicts of interest: The authors declare no conflicts of interest.
Funding: The study had no sponsorship.
Ethical Approval: The study protocol was approved by the local ethics committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia.
Patient Consent for Publication: All patients signed an informed consent for data publication.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Sarkisyan R.M., Gavrilova T.Yu., Priputnevich T.V., Muravieva V.V., Denisov P.A., Goncharuk O.D., Adamyan L.V. Microbiota of the cervical canal and uterine cavity in hyperplastic diseases of the endometrium: comparative microbiological analysis.
Akusherstvo i Ginekologiya/Obstetreics and Gynecology. 2025; (9): 144-158 (in Russian)
https://dx.doi.org/10.18565/aig.2025.151
Keywords
In the past, it was thought that the microorganisms of the female genital tract inhabited only the vagina and vulva, and that the uterine cavity was sterile. Due to the presence of the cervix and the mucous plug of the cervix, the vagina and upper genital tract are completely isolated, and the sterility of the uterine cavity is maintained. However, there is growing evidence that the female reproductive tract is an open system. From the external to the internal genitalia a gradually changing microbiota continuum is observed. Bacterial abundance from the vagina to the ovary decreases, while bacterial diversity increases [1, 2]. In recent years, the “Human Microbiome” Project has shown that approximately 9% of the human microbiota resides in the female reproductive tract [3]. The endometrium appears to be an immunologically suitable niche for the microbiota, with its potential influence on the modulation of inflammatory and immune responses [4]. It was found that the uterine cavity contains 102–104 fewer bacteria than the vaginal microbiota [5]. Thus, the uterine cavity is characterized by low bacterial contamination.
Endometrial polyps (EP) are pathological changes in the endometrium that consist of endometrial glands and fibrous stroma containing blood vessels [6, 7]. The prevalence of EP ranges from 7.8% to 12%, with a higher incidence (32%) in women with infertility. EP can lead to abnormal uterine bleeding and female infertility [8]. In recent years, the microbiota of the uterine cavity has been actively studied as a potential factor in the pathogenesis of various gynecological diseases and the outcomes of reproductive technologies. According to Vanakova A.I. et al. (2023), the microbial composition of the endometrium differs from that of the vagina and cervical canal. Specifically, in the presence of endometrial polyps, an increase in the proportion of opportunistic anaerobic microorganisms is observed, which may indicate the role of dysbiotic disorders in the development of local chronic inflammation and restructuring of the endometrium [9]. Researches showed that among many endometrial diseases, chronic endometritis and endometrial polyps are more common in patients with infertility and become the main factors influencing the outcomes of assisted reproductive technology programs. [10, 11]. According to the study by Keburia L.K. et al. (2020), the predominance of Lactobacillus spp. in the endometrium was correlated with higher pregnancy rates in IVF, while the dominance of other microbial communities was associated with an increased incidence of implantation failure [12].
Endometrial hyperplasia (EH) is a hormone-dependent pathology that develops against the background of chronic exposure to estrogens with a relative or absolute deficiency of progesterone [13]. Lately, there has been a trend towards a “rejuvenation” of this disease: hyperplastic processes are increasingly being diagnosed in women of reproductive age, which is associated with an increase in the frequency of risk factors such as obesity, metabolic disorders and late childbearing [14]. These changes demonstrate the need for early diagnosis and an individualized approach to the treatment of young patients.
There are reports that the microbiome of patients with endometrial cancer or EH significantly differ from the microbiome of patients with benign diseases. Several microorganisms have been identified in higher numbers in samples from endometrial cancer: Firmicutes (Anaerostipes, ph2, Dialister, Peptoniphilus, 1-68, Ruminococcus and Anaerotruncus), Spirochaetes (Treponema), Actinobacteria (Atopobium), Bacteroidetes (Bacteroides and Porphyromonas). It is of high importance that the simultaneous presence of Atopobium vaginae and Porphyromonas spp. (99% match with P. somerae) in patients with endometrial cancer and EH had a sensitivity of 73–93% and a specificity of 67–90%, with sensitivity increasing to 100% at vaginal pH >4.5 [15].
A lot of attention is currently being paid to the study of the female reproductive tract microbiota as a potential reason for the pathogenesis of gynecological diseases. However, data on the microbiological characteristics of the cervical canal (CC) and uterine cavity in endometrial hyperplasia (EH), especially in combination with other structural changes, remains insufficient.
The goal of the study was to evaluate the microbial composition of the cervical cyst and endometrium in women with various forms of hyperplasia (polyps, hyperplasia in combination with myoma and/or adenomyosis) in comparison with healthy patients of reproductive age.
Materials and methods
In our work, we conducted an observational comparative study with a cross-sectional design (cross-sectional comparative observational study) aimed at a comparative analysis of the cervical canal microbiota and endometrium in women with various forms of endometrial hyperplasia and in patients without endometrial pathology. The research was carried out at the V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, in the Gynecological Department during the period of 2022–2025. Totally 100 women of reproductive age (18–45 years old) were included into the study. They were divided into 4 groups of 25 people each: group I – with endometrial polyps, group II – endometrial hyperplastic processes (polyp/hyperplasia) in combination with uterine fibroids, group III – endometrial hyperplastic processes (polyp/hyperplasia) in combination with adenomyosis, group IV – controls: patients without signs of endometrial hyperplastic processes, with a physiological menstrual cycle and normal ultrasound signs of the endometrium.
Inclusion criteria: presence of hyperplastic processes according to histological findings, absence of antibacterial therapy 30 days prior to sampling, consent to participate.
Exclusion criteria: malignant neoplasms, acute infections, severe somatic pathology.
All patients underwent a microbiological (culture) study of the uterine and cervix microbiota before surgery.
Microbiological study
To study the microbiota, the contents of the uterine cavity were collected using a bacteriological swab and placed in a tube containing AMIES transport medium (Copan, Italy). We used vacuum suction biopsy (pipelle biopsy) to collect biomaterial from the uterine cavity. In order to exclude contamination of the aspirate with the lower genital microflora, various loci had preliminary antiseptic preparation: mucus was removed from the cervix with a sterile swab and processed; after collecting the discharge from the cervical canal for examination, the cervix was processed twice with a bacteriological swab moistened with an antiseptic at 5-minute intervals. In order to minimize contamination with cervical microflora, aspiration of the endometrial contents was performed without preliminary dilation of the cervical canal using a 16 mm diameter catheter, through which a Pipelle probe was inserted behind the internal os into the uterine cavity. By creating negative pressure using a piston mechanism, a sample was obtained, which was then placed in a sterile container and delivered to the laboratory for microbiological analysis.
To isolate facultative anaerobes, a set of universal and selective nutrient media was used: Columbia agar, chocolate agar, mannitol-salt agar (Conda, Spain), Endo media and Sabouraud agar (State Research Center for Applied Biotechnology and Microbiology, Obolensk, Russia). Lactobacilli were cultured on Lactobacillus agar (State Research Center for Applied Biotechnology and Microbiology, Obolensk, Russia), while obligate anaerobes were cultured on reconstituted Schaedler agar (Conda, Spain) and Anaerobic Basal Agar (Oxoid, UK). To detect minimal quantities of microorganisms in endometrial biosamples, we used culture media (sugar and thioglycollate broths). Obligate anaerobes were grown in an anaerobic cabinet (Whitley DG 250 Anaerobic Workstation, UK) using an oxygen-free gas mixture (N2 – 80%; CO2 – 10%; H2 – 10%) for 48 hours. Microorganisms were identified by time-of-flight mass spectrometry (MALDI-TOF MS) using a MicroFlex mass spectrometer with MALDI BioTyper software (Bruker Daltonics, Germany), version 5.0.
Statistical analysis
Statistical processing of the obtained data was performed using descriptive and comparative statistics. For quantitative characteristics of microbial contamination, absolute and relative frequencies (%) of detected microorganisms were used, as well as the number of species and strains found in the cervical and endometrial samples.
The analysis of the biodiversity of microbial communities included the calculation of the following alpha diversity indices: the Shannon index, which was used to assess species diversity given the equitability of the species distribution; the Simpson index, to determine the dominant species in communities; and the Margalef species richness index, which made it possible to assess species richness taking into account the population size.
All obtained quantitative parameters were tested for compliance with normal distribution using the Kolmogorov–Smirnov test. In the case of a normal distribution, the mean (M) and standard deviation (SD) were calculated for each indicator; otherwise, the median (Me) and interquartile range (Q1; Q3) were identigied. Frequencies (%) were determined for qualitative and ordinal indicators. Statistical hypotheses for group comparisons were tested using the Mann–Whitney test or the Student's t-test, depending on the normality of the sample.
Pearson's χ² test helped to manage statistical processing of the differences between groups based on frequency characteristics. In cases with low frequencies (<5) in the contingency table, Fisher's exact test was used.
We also used the Spearman correlation analysis (ρ) to assess the relationships between microbiological parameters and clinical features (e.g., pathology type, diversity index).
We took p<0.05 as the critical level of statistical significance. When conducting multiple intergroup comparisons, the Bonferroni correction was applied to adjust the level of statistical significance. For the four comparison groups (endometrial polyps, EH combined with uterine myoma, EH with adenomyosis, and control group) the threshold p-value after correction was p<0.0125. If this criterion was met then the differences were considered statistically significant. Data processing was performed using such statistical programs as Microsoft Office Excel 2015, MedCalc v. 12.
Results
All patients included in the study represented women of reproductive age. Their average age was 35.0 (5.3) years. The average age was highest in the group of patients with hyperplastic endometrial hyperplasia (EH) combined with uterine fibroids: 39.2 (4.8) years. The minimum age was observed in the control group: 32.5 (4.2) years. Thus, patients with endometrial hyperplasia were statistically older than women without endometrial polyps (EP) (Table 1).
The most common complaints associated with endometrial polyps (groups I–III) included spotting before and/or after menstruation, as well as heavy and painful menstrual periods. The control group (group IV) was dominated by patients without significant complaints. A regular menstrual cycle was observed in 19/25 (76.0%) women in the control group and in 17/25 (68.0%) patients with endometrial polyps. At the same time, irregular menstruation was more often observed in women with EH combined with myoma (13/25, 52.0%) and adenomyosis (14/25, 56.0%), but the differences did not remain statistically significant after Bonferroni correction (p=0.03). Heavy menstruation occurred in 15/25 (60.0%) patients in the EH with uterine myoma group and in 17/25 (68.0%) in the group of EH with adenomyosis, which is statistically significantly more often than in the group with EP (7/25, 28.0%) and in the control group (6/25, 24.0%) (p=0.008). Painful menstruation was more common in the group with EH combined with adenomyosis: 18/25 (72.0%) compared with 10/25 (40.0%) in the group with EH and uterine fibroids, 9/25 (36%) in the group with EP, and 5/25 (20.0%) in the control group, but the differences did not reach statistical significance after adjustment for multiple comparisons (p=0.02). The incidence of obesity was significantly higher in the group of patients with EH combined with uterine fibroids: 10/25 (40.0%), compared with 3/25 (12.0%) in the control group, but statistical significance was not maintained after adjustment for multiple comparisons (p=0.04). The average BMI ranged from 25.2 (1.8) kg/m² in the control group to 27.6 (2.1) kg/m² in patients with EH combined with uterine fibroids, but the differences did not reach statistical significance (p>0.05). Complaints for prolonged blood discharge like “spotting” before and/or after menstruation were prevalent in groups with hyperplastic changes in the endometrium: 48% with EP, 60% with EH in combination with uterine fibroids and 56% with EH with adenomyosis, while in the control group – only 3 women (12%) (p=0.005).
According to ultrasound examination data, the endometrial thickness (M-echo) before surgery differed significantly between the study groups. In patients with EP this indicator was 7.2 (2.9) mm, in the group with EH combined with uterine fibroids – 6.9 (2.98) mm, with EH with adenomyosis – 6.81 (4.1) mm, while in the control group this indicator accounted for 7.82 (3.01) mm. A statistically significant decrease in the endometrial thickness was observed after surgical treatment: in the group with EP – up to 5.71 (1.49) mm, with EH with uterine fibroids – up to 5.3 (1.4) mm, with EH and adenomyosis – up to 6.06 (1.93) mm, and in the control group – up to 5.38 (1.18) mm (p<0.001). Before the procedure the endometrial echostructure in most patients with hyperplastic processes was heterogeneous, with the presence of hyperechoic inclusions. After treatment a significant proportion of patients experienced improvement of the echographic pattern (p<0.01).
As part of the microbiological study we made a comparison of the quantitative and qualitative composition of the cervix and endometrium microbiota in patients with EP, EH in combination with uterine myoma or adenomyosis, as well as in patients in the control group. It was found that the cervical canal had a higher microbial density compared to the endometrium in all study groups. The frequency of microflora growth absence in cervical samples ranged from 4% to 16%, with no statistically significant differences between the study groups (p>0.05) (Table 2).
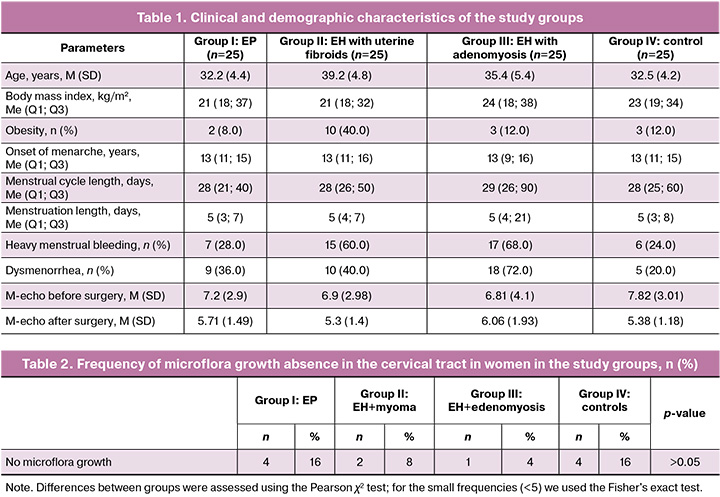
In the cervical canal, the microbiota was represented by four types of bacteria and one type of fungi. The Bacillota type was represented by eight bacteria genera: four facultative anaerobes (Staphylococcus, Streptococcus, Enterococcus, Gemella), three obligate anaerobes (Clostridium, Peptoniphilus, Veillonella), and one microaerophile (Lactobacillus). The Actinоmycetоta type included bacteria from three genera: Bifidоbacterium, Alloscardovia, and Gardnerella, primarily obligate anaerobes. The Pseudomonadоta type included four facultative anaerobes: Escherichia, Klebsiella, Proteus, and Haemophilus. The Bacterоidоta type was represented by Prevotella. The Ascomycota fungi type included Candida genus.
The endometrial microbiota, compared to the microbiota of the cervical canal, was characterized by lower taxonomic diversity and included 3 types of microorganisms – Bacillota, Pseudоmonadota and Actinоmycetоta and a smaller number of genera representing them (8 vs. 15 in the cervical canal). Bacillota was represented by 5 genera: 4 facultative anaerobic microorganisms (Staphylococcus, Streptococcus, Enterococcus, Мicrococcus) and one microaerophile (Lactobacillus). Pseudоmоnadоta included 2 types of microorganisms from facultative anaerobes: Escherichia and Klebsiella. Gardnerella was isolated from the Actinоmycetоta type. Analysis of the colon microbiota composition in the studied groups is shown in the heat map (Fig. 1).
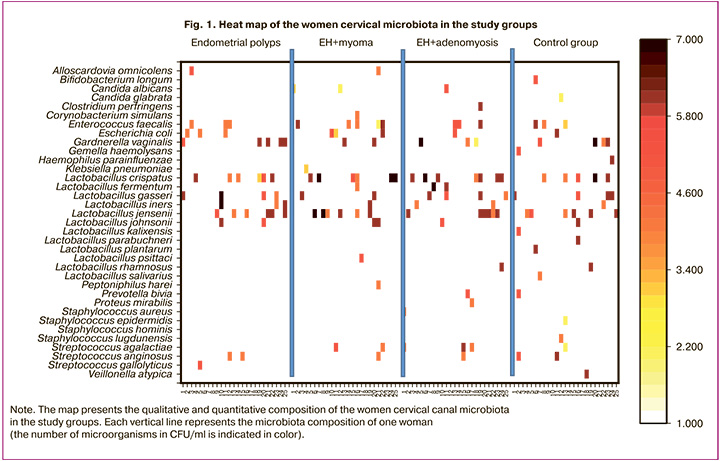
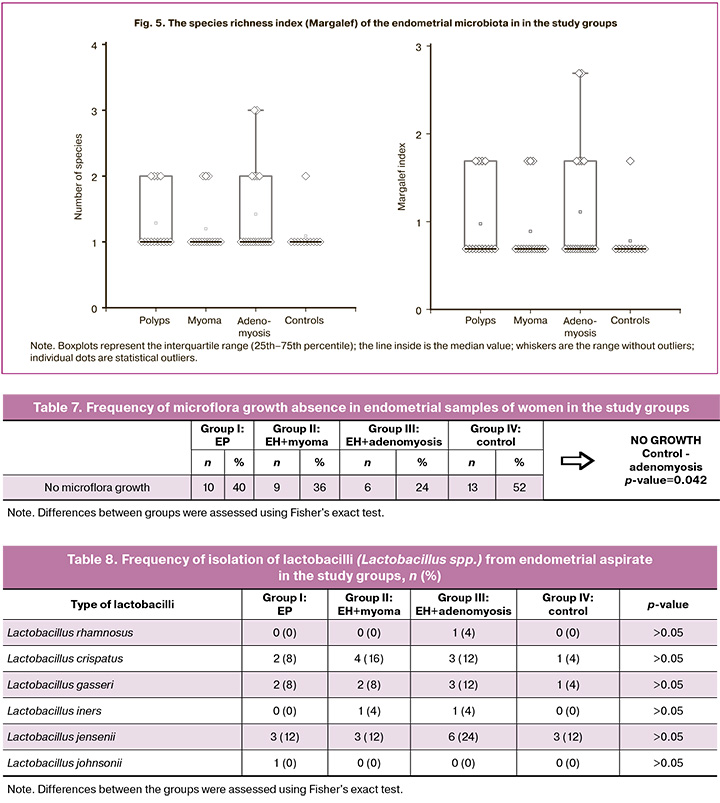
In our study, given the presence of samples with a relatively small number of microbial species, we used specially adapted biodiversity indicators (e.g., species richness or just their presence/absence; Margalef index) to assess biodiversity. The Shannon or Simpson indices should be used with caution or only when there is sufficient data to obtain reliable assessment. The highest species richness in the cervical microbiota was recorded in the control group: 43 strains of 25 species were isolated. In contrast, the group of women with EP demonstrated the lowest species richness (11 species) and the smallest number of isolated strains (37 strains). Comparison of cervical species richness microbiota (the Margalef index) showed that a statistically significant decrease in species richness was noted in group II (EH with uterine myoma) (p=0.01872) and in group III (EH with adenomyosis) (p=0.03582) compared to group IV (control) (Fig. 2).
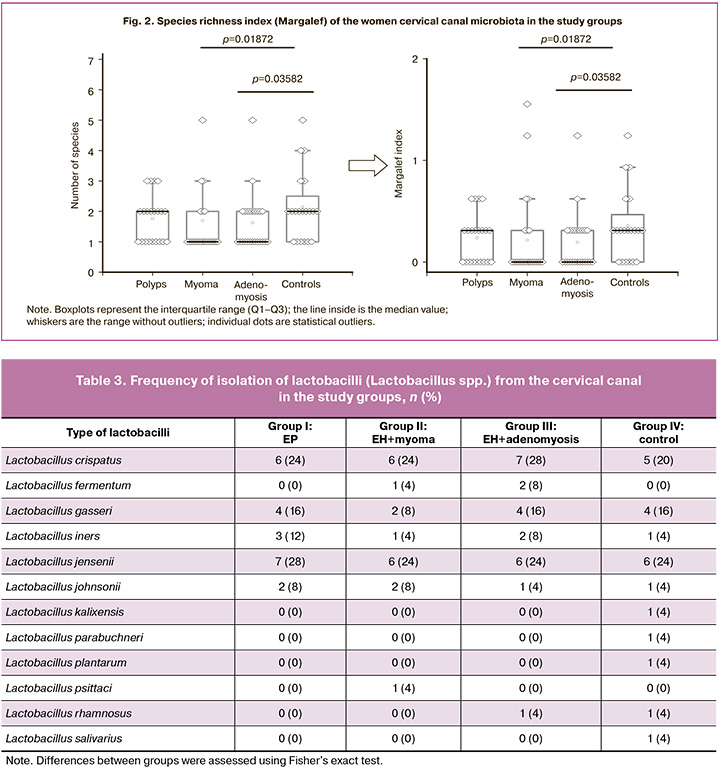
It is generally accepted that lactobacilli (Lactobacillus spp.), which detect the colonization resistance of the vaginal biotope, are associated with women's reproductive health. Accordingly, the presence of this group of microorganisms in the cervical canal also proves their protective function against colonization of the upper reproductive tract by opportunistic microorganisms. We conducted a detailed comparative assessment of the species composition of lactobacilli in the compared groups (Table 3, Fig. 2). The highest species diversity of lactobacilli was observed in the control group (10 species vs. 5–7 species in groups I–III) (Table 3). Three species dominated (like in other groups): Lactobacillus crispatus (L. crispatus) (20%), Lactobacillus gasseri (L. gasseri) (16%), Lactobacillus jensenii (L. jensenii) (24%). Other species were also present as associates in the control group, including those traditionally inhabiting the gastrointestinal tract (Lactobacillus kalixensis, Lactobacillus parabuchneri, Lactobacillus plantarum, Lactobacillus rhamnosus, and Lactobacillus salivarius), which may enhance the protective effect. The Lactobacillus iners (12% and 8%, respectively), often associated with bacterial vaginosis, was encountered more often in groups I (EP) and III (EH with adenomyosis), than in group IV (control) (4%), but without statistically significant difference.
The frequency of individual types of lactobacilli was also analyzed in relation to the total number of types of microorganisms isolated from patients in each group (Fig. 3).
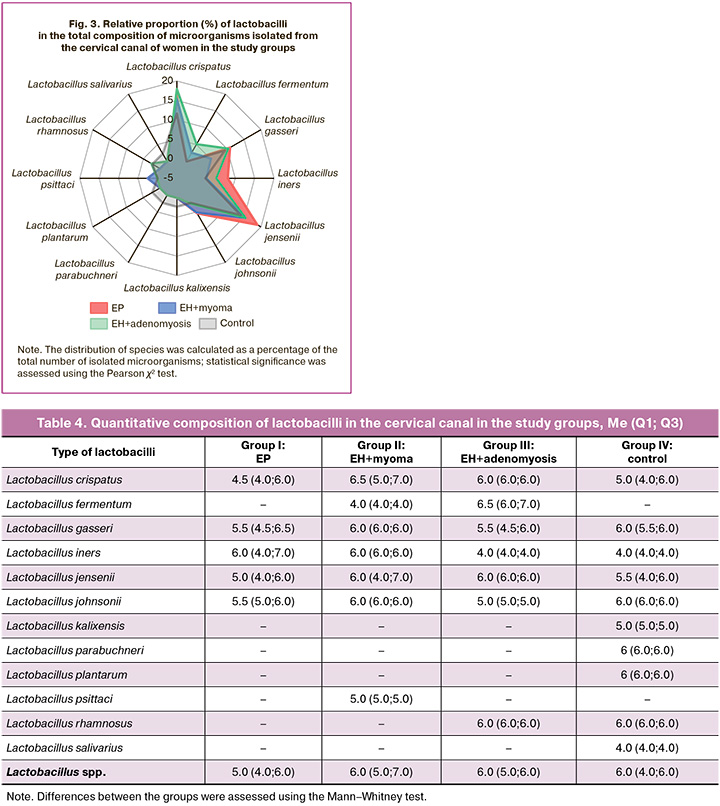
It was established that the most common species of lactobacilli accounted for 10–20% of the species structure of the cervical canal microbiota. A comparison of the relative species composition of the lactobacillary microflora of the central nervous system in the groups showed no significant difference in the prevalence of different lactobacilli species(p>0.05).
The analysis of quantitative indicators for all types of lactobacilli in the study groups showed that the level of lactobacilli colonization in the cervical canal in the examined women varied from 10 to 107 CFU/ml of secretion. Calculation of the median with the interquartile range for each type of lactobacilli showed that there was no statistically significant difference in the level of colonization of the cervical canal in the compared groups, including the most frequent species – L. crispatus, L. gasseri, L. jensenii и L. iners. Also, no difference was found in the quantitative parameters in all types of lactobacilli (Lactobacillus spp.): the median (Q1; Q3) in groups I–IV was 5.0 (4.0; 6.0); 6.0 (5.0; 7.0); 6.0 (5.0; 6.0) and 6.0 (4.0; 6.0), respectively (p>0.05) (Table 4).
Thus, no correlation was established between the species of lactobacilli isolated from the cervical canal and EH.
Opportunistic microorganisms, isolated from the cervical canal, are presented with the group of facultative anaerobes (Escherichia coli (E. coli), Proteus mirabilis (P. mirabilis), Klebsiella pneumoniaе (K. pneumoniaе), Enterococcus faecalis, (E. faecalis), Corynеbacterium simulans, Haemophilus parainfluenzae, Staphylococcus aureus, (S. aureus), Staphylococcus epidermidis, Staphylococcus hominis, Staphylococcus lugdunensis, Streptococcus agalactiae (S. agalactiae) Streptococcus anginosus (S. anginosus), Streptococcus gallolyticus; group of obligate anaerobes (Alloscardovia omnicolens, Clostridium perfringens, Peptoniphilus harei, Veillonella atypica) and a microaerophile – Gardnerella vaginalis (G. vaginalis) (Table 5). The most common in all groups (in groups I–IV, respectvely) were facultative anaerobes (E. faecalis – 12%, 20%, 12% and 12%; E. coli – 12%, 20%, 4% and 4%; Streptococcus anginosus – 8%, 4%, 4% and 8%; Streptococcus agalactiae – 0%, 8%, 12%, 8%) and G. vaginalis – 20%, 8%, 12% and 16%. However, no statistically significant difference was found in the frequency of isolation of various types of opportunistic microorganisms in the compared groups (p>0.05). Strict anaerobes were less common. At the same time, it should be noted that a wider range of microorganisms associated with vaginal infections was observed in the cervical canal of patients with myoma and adenomyosis: E. coli, P. mirabilis, K. pneumoniae, E. faecalis, S. aureus, S. agalactiae (causative agents of aerobic vaginitis), G. vaginalis (causative agent of bacterial vaginosis), Candida albicans (causative agent of candidal vulvovaginitis).
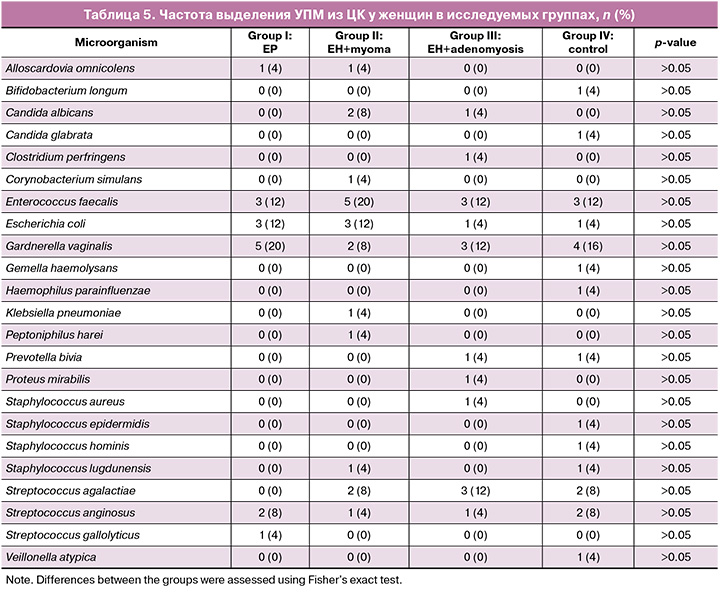
The level of colonization with opportunistic microorganisms ranged from 10 to 107 CFU/ml. A comparison of the median values for each type of opportunistic microorganisms found in different study groups revealed no statistically significant difference in colonization levels (p>0.05) (Table 6).
When studying the endometrial microbiota, the frequency of sterile cultures (no microbial growth) ranged from 24% to 52% (Table 7). More frequent endometrial sterility was recorded in the control group (52%), which is consistent with physiological nature of uterine cavity sterility. On the contrary, this indicator in the endometrium of patients with EH combined with adenomyosis was only 24%, which is significantly different from the control group (p=0.042), which demonstrates a probable microbial transformation in adenomyosis.

The analysis of the qualitative and quantitative composition of the endometrial microflora in the study patients is presented on the heat map (Fig. 4).
It is noteworthy that the endometrial microbiota is less diverse in terms of species composition and quantitative characteristics of isolated microorganisms compared to the cervical canal.
The highest species richness in the endometrial microbiota was detected in the patients with EH combined with adenomyosis: 28 strains of 12 species were isolated. The lowest species richness (9 species) and the smallest number of isolated strains (13 strains) were found in the control group, which may be due to the largest number of sterile samples. Comparison of the species richness of the endometrial microbiota across groups revealed no significant differences in such parameters as species richness and number of species. In most cases, the number of microorganisms was equal to one, which was reflected in the median across all groups. Overall, all groups exhibit similar levels of species richness, with values around 0.69 (Fig. 5). Higher values are less common and may indicate the presence of unique species or increased diversification in certain samples. Further research is needed to collect additional data for a more in-depth analysis and more informative results.
The assessment of the lactobacilla component in the endometrial microbiota revealed half as many lactobacilli species compared to the cervical canal (6 species versus 12). As in the cervical canal, three species dominated in the endometrium: L. crispatus, L. gasseri, and L. jensenii. When comparing the species composition of lactobacilli in the study groups (Table 8), we found that the number of lactobacilli species varied from 3 to 5. The highest number of lactobacilli was observed in group III (EH with adenomyosis). However, no statistically significant differences in the frequency of isolation of individual species were found (p>0.05). The L. iners was detected only in groups II (EH with uterine myoma) and III (EH with adenomyosis). Species rarely found in the reproductive tract (L. kalixensis, L. parabuchneri, L. plantarum, and L. salivarius), unlike in the cervical canal, were not detected in the endometrium.
Also, no statistically significant difference was found in the quantitative parameters in all types of lactobacilli (Lactobacillus spp.) in the study groups: Me (Q1; Q3) was 3.0 (2.0; 6.0); 2.0 (1.0; 6.0); 3.0 (2.0; 6.0) and 1.0 (1.0; 2.0) in groups I–IV, respectively, which is less than in the cervical canal (Table 9).

Thus, like during the examination of the cervical canal, no correlation was detected between the species of lactobacilli isolated from the endometrium and the EH.
The opportunistic endometrial microbiota differed from the cervical canal microbiota: almost two times fewer microorganism species were isolated (12 and 22, respectively); only facultative anaerobes (E. coli, K. pneumoniaе, E. faecalis, S. aureus, S. epidermidis, S. hominis, Staphylococcus auricularis (S. auricularis), S. agalactiae, S. anginosus, S. gallolyticus, Micrococcus luteus (M. luteus) and a microaerophile (G. vaginalis) were present (Table 10). Besides we identified the species that were not present in the cervical canal – S. auricularis и M. luteus. All this indicates the possibility of the formation of an independent microbiota in the uterine cavity. Among the opportunistic microorganisms found in the studied groups I–IV the most common were: S. epidermidis – 16%, 12%, 24% and 4%; E. faecalis – 4%, 8%, 4% and 4%; S. anginosus – 0%, 4%, 8% and 8%; Streptococcus agalactiae – 0%, 4%, 4%, 8%) and G. vaginalis – 12%, 0%, 8% and 8%. Overall, Streptococcus spp. were detected more frequently in groups II (EH with uterine myoma) and III (EH with adenomyosis). However, a statistically significant difference in the frequency of isolation of various types of opportunistic microorganisms in the study groups was found only for S. epidermidis, which was more often detected in group III (EH with adenomyosis) in comparison to the control group (p=0.042).
The results of assessment of endometrial colonization level by opportunistic microorganisms showed that in most cases the median level of endometrial contamination was significantly lower than in the cervical canal and was less than 3.5 lg CFU for most pathogens in all groups, which indicates a low level of colonization (Table 11). However, for some types of opportunistic microorganisms – E. faecalis (EH in combination with uterine myoma, EH in combination with adenomyosis – groups II and III, respectively), S. agalactiae (EH with adenomyosis – group III), G. vaginalis (EP – group I), the median was 4–6 lg CFU, which corresponds to a high level of colonization for the endometrium.
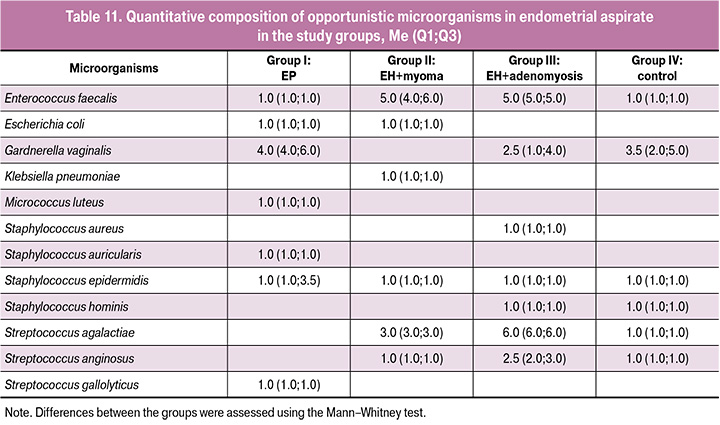
Thus, it was shown that, despite the absence of statistically significant differences in the frequency and level of endometrial colonization for most opportunistic microorganisms in the compared groups, microorganisms causing infectious and inflammatory diseases of the reproductive system were more frequently detected in the endometrium of patients with hyperplastic processes, as well as in the cervical canal. To clarify their role in the pathogenesis of EH, further research is needed on a larger patient population.
A correlation analysis using Spearman's rank correlation coefficient (ρ) revealed no statistically significant relationships between microbiological characteristics (species composition, alpha diversity indices, and bacterial counts) and clinical parameters (presence of complaints, body mass index, and endometrial thickness) in all study groups. Correlation coefficient did not exceed ρ=0.3; the statistical significance p-level exceeded 0.05 in all cases.
Discussion
Study by Al-Nasiry S. et al., based on molecular sequencing technology, showed that the female genital tract microbiota of a healthy woman is in dynamic balance. Thus, changes in the composition of the genital tract microbiota may be associated with various gynecological diseases, such as chronic endometritis, endometrial polyps, endometriosis, endometrial cancer, infertility, etc. [16]. By conducting a cluster analysis, Nijkang N.P. et al. found that depending on the localization, the number of microorganism species in the reproductive tract (vagina and uterine cavity) gradually increased. When classifying the diseases, the number of species of genital tract microorganisms in the group of healthy women was significantly higher than in the groups with chronic endometritis and EP. Also the number of species in patients with chronic endometritis was higher than in patients with EP [6]. This result indicates that the abundance of microorganisms gradually decreases from the lower to the upper genital tract, but the number of species gradually increases. Similar conclusions were reached in previous studies by Miles S.M. et al. [17]. The authors assessed the distribution of bacteria throughout the female reproductive tract, including the fallopian tubes and ovaries, and found that bacteria colonized the entire female reproductive tract, and the microbial community at each anatomical site highly correlated. The related research highlights the significance of studying the endometrial microbiota within the context of gynecological pathology and reproductive medicine. The importance of the uterine microbiota in the pathogenesis of EP was noted in the study of Vanakova A.I. et al. [9]. The authors suggest that dysbiotic changes contribute to the chronification of inflammation and structural reorganization of the endometrium, which is consistent with our results on the decrease in endometrial sterility in patients with hyperplastic processes. Fang R.L. et al. found that there were significant differences in the uterine microbiota between the chronic endometritis group patients and healthy women, and the patients in the control group had a more diverse intrauterine microbiota than the patients with chronic endometritis [18]. Similar results were obtained by Nijkang N.P. et al., indicating that the most common types of the genital tract microorganisms were detected within the normal population, which is necessary for maintaining a healthy microecology [6]. In turn, Ata B. et al. conducted several independent studies of the cervical microbiota, and most of them were conducted in conjunction with the vaginal microbiota. It was shown that Lactobacillus spp. are dominant in the cervical microbiota of healthy women, while other common bacteria included Prevotella spp., Streptococcus spp., and Fusobacterium spp., etc. [19]. Kimura F. et al. conducted further research and found that the proportion of Lactobacillus in the cervical microbiota is generally slightly lower than in the vaginal microbiota [20]. The literature data discusses the possible relationship between different lactobacilli species colonizing the vaginal biotope and the cervical canal with various pathological conditions. Our study of the lactobacilli species composition in the uterine cavity and endometrium microbiota revealed no significant correlation between lactobacilli composition and endometrial hyperplasia. At the same time, the L. iners species, which has less protective potential, was detected in the endometrium only in groups with EH, which requires further study. The study by Barinova V.V. et al., dedicated to the analysis of the endometrial microbiota in patients with IVF failures, confirms the existence of a low-biomass endometrial microbial ecosystem and its possible impact on reproductive outcomes [21]. At the same time, the results of the study by Keburia L.K. et al. emphasize the need to assess the microbiological status of the endometrium when preparing patients for assisted reproductive technology programs. Dysbiotic changes detected in the endometrium may explain cases of repeated embryo implantation failures, even in the absence of overt clinical uterine pathology [12]. Our study complements these data by revealing a link between decreased endometrial sterility and the presence of hyperplastic processes, which may indicate the pathogenetic involvement of the microbiota in the development of this pathology.
Thus, the obtained results confirm the need for further research aimed at the interaction of microbiota with endometrial tissues and the possibilities of its modulation for the prevention and treatment of hyperplastic diseases.
Conclusion
The study identified significant features in the composition of the cervical and endometrium microbiota in patients with EH. It was found that the cervical canal was colonized by greater microbial richness and biodiversity compared to the endometrium in all studied groups. The highest sterility of the endometrium was detected in the control group, which confirms the idea of physiological sterility of the uterine cavity. At the same time, a statistically significant decrease in endometrial sterility is observed in patients with EH in combination with adenomyosis, which confirms the presence of microbial restructuring of the intrauterine environment during hyperplastic changes in the endometrium.
The cervical microbiota demonstrates higher species and strain diversity, with the majority of the microbial community consisting of members of the genus Lactobacillus, primarily L. jensenii, L. crispatus, and L. gasseri. Opportunistic microorganisms such as Gardnerella vaginalis, Escherichia coli, Enterococcus faecalis, and rare species including Staphylococcus auricularis, Micrococcus luteus, and Streptococcus anginosus were detected predominantly in the endometrium of patients with EH. Analysis of species richness (Margalef index) showed that the most balanced microbial ecosystem was observed in the cervical samples of the control group.
The observed association between endometrial hyperplastic processes, accompanied by decreased sterility and increased microbial load, may indicate a potential pathogenetic role of the microbiota. These data support the need for further in-depth research aimed at exploring the mechanisms of microbiota interaction with female reproductive system tissues and its impact on the development of gynecological pathologies.
References
- Kitaya K., Matsubayashi H., Yamaguchi K., Nishiyama R., Takaya Y., Ishikawa T. et al. Chronic endometritis: potential cause of infertility and obstetric and neonatal complications. Am. J. Reprod. Immunol. 2015; 75(1): 13-22. https://dx.doi.org/10.1111/aji.12438
- Кулаков В.И., Адамян Л.В., ред. Лапароскопия и гистероскопия в гинекологии и акушерстве. М.: ПАНТОРИ; 2012. 124 с. [Kulakov V.I., Adamyan L.V., eds. Laparoscopy and Hysteroscopy in Gynecology and Obstetrics. Moscow: PANTORI; 2012. 124 p. (in Russian)].
- Margulies S.L., Flores V., Parkash V., Pal L. Chronic endometritis: a prevalent yet poorly understood entity. Int. J. Gynecol. Obstet. 2022; 158: 194-200. https://dx.doi.org/10.1002/ijgo.13962
- Molina N.M., Sola-Leyva A., Saez-Lara M.J., Plaza-Diaz J., Tubić-Pavlović A., Romero B. et al. New opportunities for endometrial health by modifying uterine microbial composition: present or future? Biomolecules. 2020; 10(4): 593. https://dx.doi.org/10.3390/biom10040593
- American Association of Gynecologic Laparoscopists. AAGL practice report: practice guidelines for the diagnosis and management of endometrial polyps. J. Minim. Invasive Gynecol. 2012; 19(1): 3-10. https://dx.doi.org/10.1016/j.jmig.2011.09.003
- Nijkang N.P., Anderson L., Markham R., Manconi F. Endometrial polyps: pathogenesis, sequelae and treatment. SAGE Open Med. 2019: 7, 2050312119848247. https://dx.doi.org/10.1177/2050312119848247
- Lee Y., Kim K.A., Song M.J., Park Y.S., Lee J., Choi J.W. et al. Multiparametric magnetic resonance imaging of endometrial polypoid lesions. Abdom. Radiol. 2020; 45(11): 3869-81. https://dx.doi.org/10.1007/s00261-020-02567-7
- Munro M.G. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil. Steril. 2019; 111(4): 629-40. https://dx.doi.org/10.1016/j.fertnstert.2019.02.008
- Ванакова А.И., Долгушина Н.В., Припутневич Т.В. Роль микробиоты полости матки в генезе полипов эндометрия. Акушерство и гинекология. 2023; 11: 43-7. [Vanakova A.I., Dolgushina N.V., Priputnevich T.V. The role of the uterine microbiota in the genesis of endometrial polyps. Obstetrics and Gynecology. 2023; (11): 43-7 (in Russian)]. https://dx.doi.org/10.18565/aig.2023.201
- Peric A., Weiss J., Vulliemoz N., Baud D., Stojanov M. Bacterial colonization of the female upper genital tract. Int. J. Mol. Sci. 2019; 20(14): 3405. https://dx.doi.org/10.3390/ijms20143405
- Ворошилина Е.С., Зорников ДЛ, Копосова О.В., Исламиди Д.К., Игнатова К.Ю., Абакумова Е.И., Курбатова Н.В., Плотко Э.Э. Возможности оценки микробиоты полости матки с использованием ПЦР в реальном времени. Вестник РГМУ. 2020; 1: 12-7. [Voroshilina E.S., Zornikov D.L., Koposova O.V., Islamidi D.K., Ignatova K.Yu., Abakumova E.I., Kurbatova N.V., Plotko E.E. The use of real-time PCR for evaluation of endometrial microbiota. Bulletin of RSMU. 2020; 1: 12-7 (in Russian)]. https://dx.doi.org/10.24075/vrgmu.2020.012
- Кебурия Л.К., Смольникова В.Ю., Припутневич Т.В., Муравьева В.В., Калинина Е.А. Микробиота эндометрия и репродуктивный исход в программах вспомогательных репродуктивных технологий. Акушерство и гинекология. 2020; 4: 166-72. [Keburia L.K., Smolnikova V.Yu., Priputnevich T.V., Muravyeva V.V., Kalinina E.A. Endometrial microbiota and reproductive outcome in assisted reproductive technology programs. Obstetrics and Gynecology. 2020; (4): 166-72 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.4.166-172
- Petersdorf K., Groettrup-Wolfers E., Overton P.M., Seitz C., Schulze-Rath R. Endometrial hyperplasia in pre-menopausal women: a systematic review of incidence, prevalence, and risk factors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022; 271: 158-71. https://dx.doi.org/10.1016/j.ejogrb.2022.02.015
- Beavis A.L., Blechter B., Najjar O., Fader A.N., Katebi Kashi P., Rositch A.F. Identifying women 45 years and younger at elevated risk for endometrial hyperplasia or cancer. Gynecol. Oncol. 2023; 174: 98-105. https://dx.doi.org/10.1016/j.ygyno.2023.04.019
- Walter-Antonio M.R., Chen J., Multinu F., Hokenstad A., Distad T.J., Cheek E.H. et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016; 8(1): 122. https://dx.doi.org/10.1186/s13073-016-0368-y
- Al-Nasiry S., Ambrosino E., Schlaepfer M., Morré S.A., Wieten L., Voncken J.W. et al. The interplay between reproductive tract microbiota and immunological system in human reproduction. Front. Immunol. 2020; 11: 378. https://dx.doi.org/10.3389/fimmu.2020.00378
- Miles S.M., Hardy B.L., Merrell D.S. Investigation of the microbiota of the reproductive tract in women undergoing a total hysterectomy and bilateral salpingo-oophorectomy. Fertil Steril. 2017; 107(3): 813-20.e1. https://dx.doi.org/10.1016/j.fertnstert.2016.11.028
- Fang R.L., Chen L.X., Shu W.S., Yao S.Z., Wang S.W., Chen Y.Q. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am. J. Transl. Res. 2016; 8(3): 1581-92.
- Ata B., Yildiz S., Turkgeldi E., Pérez Brocal V., Dinleyici E.C., Moya A. et al. The endobiota study: comparison of vaginal, cervical, and gut microbiota between women with stage III/IV endometriosis and healthy controls. Sci. Rep. 2019; 9(1): 2204. https://dx.doi.org/10.1038/s41598-019-39700-6
- Kimura F., Takebayashi A., Ishida M., Nakamura A., Kitazawa J., Morimune A. et al. Review: chronic endometritis and its effect on reproduction. J. Obstet. Gynaecol. Res. 2019; 45(5): 951-60. https://dx.doi.org/10.1111/jog.13937
- Баринова В.В., Кузнецова Н.Б., Буштырева И.О., Оксенюк О.С., Дудурич В.В., Шаталов А.Е. Микробиом эндометрия при многократных неудачах вспомогательных репродуктивных технологий и у здоровых женщин: где норма и где патология? Акушерство и гинекология. 2021; 6: 105-14. [Barinova V.V., Kuznetsova N.B., Bushtyreva I.O., Oksenyuk O.S., Dudurich V.V., Shatalov A.E. Endometrial microbiome in women with and without a history of repeated failures of assisted reproductive technology: what are norm and pathology? Obstetrics and Gynecology. 2021; (6): 105-14 (in Russian)]. https://dx.doi.org/10.18565/aig.2021.6.105-114
Received 11.06.2025
Accepted 11.09.2025
About the Authors
Rita M. Sarkisyan, PhD student, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, ritamisakovna@mail.ru, https://orcid.org/0000-0002-4097-5537Tatyana Yu. Gavrilova, Dr. Med. Sci., obstetrician-gynecologist at the Gynecological Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, t_gavrilova@oparina4.ru, https://orcid.org/0000-0001-7424-4292
Tatyana V. Priputnevich, Corresponding Member of the Russian Academy of Sciences, Dr. Med. Sci., Director of the Institute of Microbiology, Antimicrobial Therapy and Epidemiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia,
Moscow, Ac. Oparin str., 4, priput1@gmail.com, https://orcid.org/0000-0002-4126-9730
Vera V. Muravieva, PhD, Senior Researcher at the Laboratory of Molecular Microbiology, Department of Molecular Microbiology and Bioinformatics, Institute of Microbiology, Antimicrobial Therapy and Epidemiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, v_muravieva@oparina4.ru, https://orcid.org/0000-0003-0383-0731
Pavel A. Denisov, Researcher at the Laboratory of Bioinformatic Analysis, Institute of Microbiology, Antimicrobial Therapy and Epidemiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4,
denisov@neuro.nnov.ru, https://orcid.org/0000-0003-1813-6718
Olga D. Goncharuk, Head of the Laboratory of Medical Microbiology, Institute of Microbiology, Antimicrobial Therapy and Epidemiology, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4, o_goncharuk@oparina4.ru, https://orcid.org/0000-0001-5876-8424
Leila V. Adamyan, Academician of the Russian Academy of Sciences, Dr. Med. Sci., Professor, Deputy Director for Research, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparin str., 4; Chief Specialist in Gynecology of
the Ministry of Health of Russia; Head of the Department of Reproductive Medicine and Surgery of the Faculty of Postgraduate Education, Russian University of Medicine, Ministry of Health of Russia, adamyanleila@gmail.com, https://orcid.org/0000-0002-3253-4512
Corresponding author: Rita M. Sarkisyan, ritamisakovna@mail.ru



