Analysis of genetic risk factors for endometrial hyperplasia in overweight and obese women
Churnosov V.I., Ponomarenko I.V., Ponomarenko M.S., Churnosov M.I.
Objective: To study the association between single nucleotide variants (SNVs) related to body sex hormone levels and the occurrence of endometrial hyperplasia (EH) in overweight and obese women.
Materials and methods: This study included 727 women with a body mass index (BMI) ≥25 kg/m², comprising 324 patients with simple EH (glandular/glandular-cystic forms) and 403 controls. Molecular genetic analysis was conducted on four SNVs associated with sex hormone levels based on data from previous genome-wide association studies (GWAS): NC_000012.12 (ANO2):g.5902324C>A (rs117585797), NC_000016.10 (CHD9):g.52913718A>C (rs117145500), NC_000011.10 (FSHB):g.30193714T>A (rs11031002), and NC_000011.9 (SLC22A24):g.62915346C>A (rs112295236). Associations between SNVs and EH were analyzed using logistic regression.
Results: An association was found between SNV rs11031002 T>A in FSHB and EH across the three genetic models (p=0.001 and pperm=0.001): OR=0.45, 95% CI 0.31–0.66 (allelic model); OR=0.44, 95% CI 0.30–0.64 (additive model); OR=0.45, 95% CI 0.30–0.66 (dominant model). The minor allele A of SNV rs11031002 T>A in FSHB demonstrated a protective effect against disease development (OR<1). Additionally, SNV rs11031002 T>A in FSHB and five polymorphic loci (rs11031005, rs11031006, rs11031010, rs74485684, rs10835638) in linkage disequilibrium with it exhibit significant functionality, influencing the interaction of the FSHB gene promoter with 38 transcription factors and affecting the transcription level of the ARL14EP gene in subcutaneous adipose tissue.
Conclusion: SNV rs11031002 T>A in FSHB is associated with EH risk in overweight and obese women.
Authors' contributions: Churnosov V.I. – literature search and analysis, data summarization, drafting of the manuscript; Ponomarenko I.V. – study conception and design, drafting of the manuscript; Ponomarenko M.S. – statistical analysis; Churnosov M.I. – critical revision, final editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: The study was supported by a grant from the Russian Science Foundation No. 25-25-00034,
https://rscf.ru/project/25-25-00034/.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Belgorod State National Research University.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Churnosov V.I., Ponomarenko I.V., Ponomarenko M.S., Churnosov M.I. Analysis of
genetic risk factors for endometrial hyperplasia in overweight and obese women.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (5): 58-65 (in Russian)
https://dx.doi.org/10.18565/aig.2025.39
Keywords
Endometrial hyperplasia (EH) is a condition characterized by excessive proliferation of endometrial glands, resulting from disruption of the normal gland-to-stroma ratio within the uterine mucosa [1]. The incidence rate of EH in the general female population aged 18 to 90 years is reported to be 132 cases per 100,000 women-years. This figure includes simple EH at a rate of 58 per 100,000 women-years, complex EH at 63 per 100,000 women-years, and atypical EH at 17 per 100,000 women-years [2]. EH is age-dependent; it is occasionally recorded in women under 30 years of age (6 per 100,000 women-years), while its incidence peaks in the age groups of 50–54 years (simple EH – 142, complex EH – 212 per 100,000 women-years) and 60–64 years (atypical EH – 54 per 100,000 women-years) [2]. The most common and pronounced symptom of EH is abnormal uterine bleeding, which significantly reduces the quality of life for affected women [3–5]. Additionally, EH is associated with an increased risk of endometrial cancer, with rates reaching 29% across different forms of EH [6].
The impact of obesity and overweight as risk factors for EH is well established [6–10]. Women with a body mass index (BMI) greater than 40 kg/m² exhibit significantly higher risks of developing EH, with a 13-fold increase in atypical EH and a 23-fold increase in EH without atypia [9]. In patients experiencing abnormal uterine bleeding and having a BMI greater than 30 kg/m², complex EH or endometrial cancer is observed four times more frequently than in women with a normal BMI [11]. Obesity and overweight are believed to contribute to hyperestrogenism due to increased extragonadal synthesis of estrogens from androstenedione, a decrease in sex hormone-binding globulin levels, and the development of anovulation, leading to more pronounced proliferation of endometrial cells [6–10, 12, 13].
Several genetic and epidemiological studies have highlighted the significant role of hereditary factors, including single nucleotide variants (SNVs) in specific candidate genes related to growth factors, tumor necrosis, cytochromes, chemokines, interleukins, apoptosis, estrogen receptors, and menarche, in the development of EH [14–21]. However, the number of such studies is limited, and the findings are often fragmentary, failing to account for the combined effects of risk factors, such as obesity and SNVs in candidate genes. This underscores the relevance of this study.
This study aimed to investigate the association between SNVs related to sex hormone levels and EH occurrence in overweight and obese women.
Materials and methods
The study involved 727 women with BMI ≥25 kg/m², including 324 patients with simple EH and 403 controls. These women were examined at the Perinatal Center of the Belgorod Regional Clinical Hospital of St. Joasaph. between 2008 and 2013. To diagnose EH, gynecological department doctors performed hysteroscopy with targeted therapeutic and diagnostic curettage of the uterine cavity. Histological examination of the biological material obtained during curettage was conducted at the pathomorphological department of the Belgorod Regional Pathological Anatomy Bureau. The presence of simple EH without atypia (glandular or glandular-cystic form) served as the basis for inclusion in the patient group. In contrast, the absence of clinical or ultrasound signs of benign pelvic organ diseases was the criterion for inclusion in the control group, which was established during a population medical examination. All women included in this study were unrelated, Russian, born in the Central Black Earth Region of Russia [19, 21], and provided written consent to participate. The study was conducted under the supervision of the Ethics Committee of Belgorod State University.
A molecular genetic study of four SNVs: NC_000012.12 (ANO2):g.5902324C>A (rs117585797), NC_000016.10 (CHD9):g.52913718A>C (rs117145500), NC_000011.10 (FSHB):g.30193714T>A (rs11031002), NC_000011.9 (SLC22A24):g.62915346C>A (rs112295236) was performed. The linking of individual SNVs to specific genes was carried out in accordance with the RefSeq database data presented in the Haploreg database (access date: 15.02.2024)), associated with the level of sex hormones (progesterone, estradiol, testosterone, free androgen index, luteinizing hormone (LH), follicle-stimulating hormone (FSH) in the body, according to the data of previously performed GWAS [22–24]. DNA from the biobank of the Department of Medical and Biological Disciplines was used for the genotyping. Genotyping was performed by allele-specific polymerase chain reaction in real time using TаqМаn probes (TestGen LLC kits, Russia) on CFX96 detection amplifiers (Bio-Rad, USA). DNA samples were taken at a concentration of 10–20 ng/μl (A260/A280 was 1.7–2.0) [25].
The quality of the genotyping DNA samples was assessed by analyzing the expected versus observed distribution of genotypes in patients with EH and the control group for all four SNVs considered. Associations between SNVs and EH were studied using logistic regression (via the gPLINK program [26]) across four genetic models (additive, dominant, recessive, and allelic), while accounting for covariates such as age and BMI and adjusting for multiple comparisons through an adaptive permutation test with pperm calculation [27]. A pperm value of less than 0.05 was accepted as statistically significant [28]. The direction of association was evaluated based on the odds ratio (OR) and 95% confidence interval (CI) [29]. For the rs11031002 T>A FSHB locus, which was associated with EH (and strongly linked to SNVs with r²=0.64–0.79 and D'=0.95–0.99, including rs11031005, rs11031006, rs11031010, rs74485684, and rs10835638), the functional significance was investigated through in silico analysis [21] using data from the HaploReg (access date: 15.02.2024) [30] and GTE xportal (access date: 18.02.2024) [31] databases.
Results and discussion
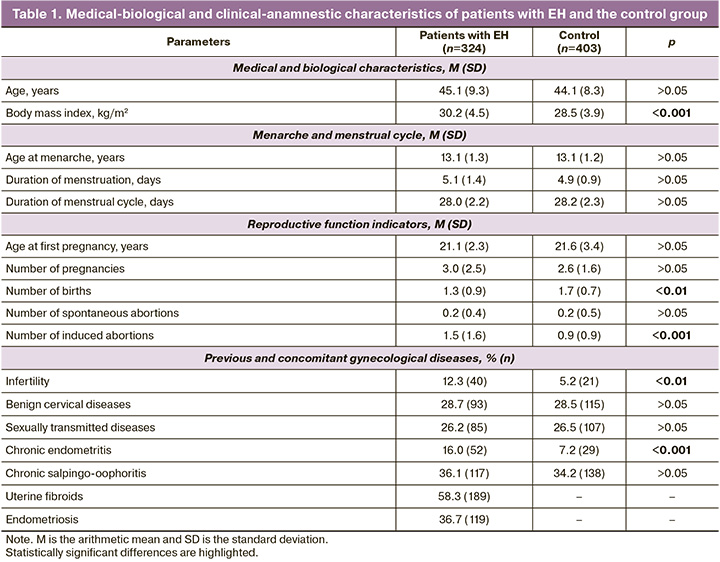
Analysis of the medical and biological characteristics of patients with EH and a control group with a BMI of ≥25 kg/m² showed that the mean age of women in the control group (44.1 years) was similar to that of the patients with EH (45.1 years) (p > 0.05, Mann–Whitney test). However, the BMI in the control group (28.5 kg/m²) was lower than that of the patients with EH (30.2 kg/m²) (p<0.001, Mann–Whitney test).
Patients with EH had fewer pregnancies that resulted in childbirth (1.30 times less frequently, p<0.01) and more pregnancies that ended in artificial abortions (1.73 times more frequently, p<0.001). Their medical histories also showed a significantly higher incidence of infertility (2.37 times more, p<0.01) and chronic endometritis (2.23 times more, p<0.001). Additionally, over half of the patients with EH had uterine myoma (58.3%) and more than one-third had endometriosis (36.7%) (Table 1).
Among patients with EH and in the control group with BMI ≥25 kg/m2, the distribution of genotypes for all the loci considered was in full compliance with the Hardy–Weinberg law: pHWE=0.06–1.00 (EH), pHWE=0.15–1.00 (control) (Table 2). Associations of SNVs rs11031002 T>A FSHB with the occurrence of EH in women with BMI≥25 kg/m2 were found, confirmed by permutation testing: OR=0.45, 95% CI 0.31–0.66, p=0.001, pperm=0.001 (allelic model); OR=0.44, 95% CI 0.30–0.64, p=0.001, pperm =0.001 (additive); OR=0.45, 95% CI 0.30–0.66, p=0.001, pperm=0.001 (dominant) (Table 2). These data indicate that the alternative (minor) allele A of rs11031002 FSHB serves as a protective factor for EH formation in women with obesity or increased BMI (OR<1).
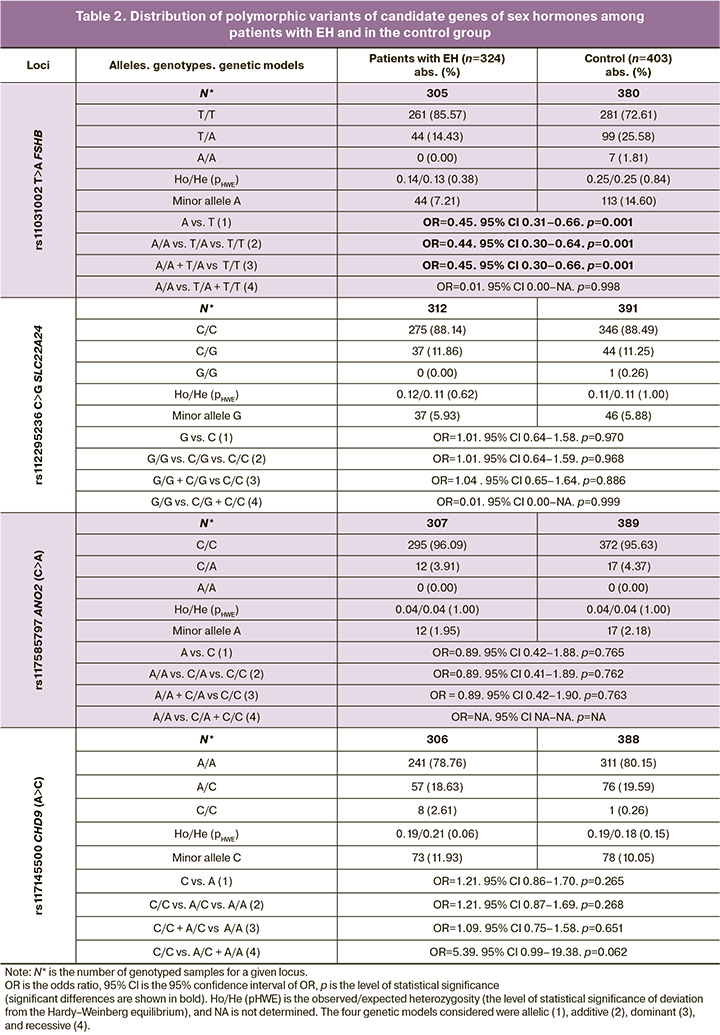
Using the materials of the HaploReg database (accessed on 15.02.2024), it was revealed that SNVs rs11031002 T>A FSHB is localized at a distance of 37 thousand nucleotide pairs from the 5' region of the FSHB gene (near the putative promoter region of this gene [32]) and determines the interaction of this DNA region with four transcription factors (TF): HDAC2, Pou2f2, Pou6f, and Zfp105. Interestingly, the A allele of these SNVs, which is associated with a low risk of EH formation in women with obesity or increased BMI, is associated with an increased affinity of this DNA region located near the putative promoter region of the FSHB gene for TFs HDAC2, Pou2f2, and Zfp105 (resulting in a more pronounced interaction of DNA with these TFs) and a decreased affinity of DNA for the action of TF Pou6f1 (resulting in a less pronounced interaction of DNA with these TFs). It is important to emphasize that in ovaries, the DNA region in which rs11031002 is located acts as an enhancer. Another important point should be noted: in strong linkage disequilibrium (at r2=0.64–0.79 and D'=0.95–0.99) with SNVs rs11031002 T>A FSHB there are SNVs (rs11031005, rs11031006, rs11031010, rs74485684, rs10835638), which affect the interaction of the DNA region located near the putative promoter region of the FSHB gene with 34 other different TFs: FXR, ERalpha-a, Foxa, Otx2, Zfp281, Foxo, Foxl1, Foxj1, Foxj2, Foxk1, Foxp3, Foxf2, Foxd1, TCF4, HNF4, HNF1, Hdx, CEBPA, Lhx3, NR4A, Dbx1, LRH1, SF1, Pax7, RORalpha1, Mef2, RXRA, Nkx6-1, Sox, HDAC2, Zfp187, MZF1, Spz1, p300.
According to the experimental data presented in the bioinformatics database GTEx portal (accessed on 18.02.2024), SNV rs11031002 T>A in the FSHB gene is associated with the expression levels of the ARL14EP gene in subcutaneous adipose tissue. Specifically, the A allele of this SNV, which serves as a marker of low risk for endometrial hyperplasia (EH), is correlated with increased transcription of the ARL14EP gene (NES indicator=0.25; p=2.9×10-6). Importantly, the transcriptional activity of the ARL14EP gene in subcutaneous adipose tissue is also influenced by five SNVs in FSHB, which are strongly linked to rs11031002 (with r²=0.64–0.79 and D'=0.95–0.99): rs11031005, rs11031006, rs11031010, rs74485684, and rs10835638. Based on these data, it can be concluded that the SNV rs11031002 T>A in FSHB, along with the five SNVs in linkage disequilibrium, demonstrated significant functionality. These variations affected the interaction of the DNA region near the putative promoter of the FSHB gene with 38 transcription factors and influenced the transcription levels of the ARL14EP gene in subcutaneous adipose tissue. This could possibly serve as a biological mechanism that “explains” the association of this SNV with the development of EH in women with a BMI ≥25 kg/m².
Another potential mechanism may be related to the medical and biological basis of the involvement of SNV rs11031002 T>A in the formation of EH. Previous GWAS data indicated that the minor allelic variant A of rs11031002, which has protective implications against the development of EH (as per our findings), is associated with high levels of LH [22] and low concentrations of FSH [24]. This hormonal imbalance may contribute significantly to the development of EH.
The literature clearly indicates the primary role of hormonal factors in EH development [6–8, 10]. An increase in FSH levels, an elevated FSH/LH ratio, a decrease in LH, and an imbalance in the estrogen-progesterone system (such as absolute hyperestrogenism or normal estrogen levels coupled with a deficiency of progesterone) predispose individuals to developing EH [6–8, 10]. Hormonal imbalances involving FSH, LH, and the FSH/LH ratio can lead to anovulatory cycles in women [33, 34]. Chronic anovulation, particularly prevalent during perimenopause, is recognized as a significant hormone-mediated risk factor for EH [6, 10]. During anovulatory cycles, estrogen levels, which stimulate endometrial proliferation, dominate without the counteracting effects of progesterone (which has an anti-proliferative effect on endometrial cells) produced by the corpus luteum after ovulation. This imbalance in the estrogen-progesterone system results in continued endometrial proliferation, thereby increasing the risk of EH [10]. In a study by Hambridge H.L. et al. (involving 250 healthy premenopausal women), it was shown that women with one anovulatory cycle had lower peak concentrations of LH and lower levels of sex hormones (progesterone and estradiol) by 38%, 22%, and 25%, respectively, than women with two ovulatory cycles [34]. The most pronounced reductions in progesterone (> fourfold) and estradiol (-60%) were observed in women with two anovulatory cycles compared to those with two ovulatory cycles [34]. Moreover, in a study conducted by Burger H.G. et al., anovulatory cycles in women over 45 years of age were typically characterized by increased levels of FSH and reduced levels of inhibin B [33]. The authors attributed this to an age-related decline in the number of primordial ovarian follicles (down to 100), which was reflected in a decrease in small antral follicles (the site of inhibin B production). This leads to a reduction in inhibin B (which normally represses FSH synthesis), resulting in elevated FSH levels that maintain circulating estradiol levels [33]. Additionally, elevated FSH levels combined with significantly reduced progesterone levels can result in abnormal endometrial growth [35], thereby serving as a risk factor for EH [6, 8]. It is crucial to emphasize that several SNVs strongly linked to the EH-associated locus rs11031002 T>A in the FSHB gene (with linkage parameters r²≥0.64–0.79 and D'=0.95–0.99) also exhibited significant associations with LH and FSH levels (rs11031006 [36–38], rs10835638 [35, 39–42], rs11031010 [43], rs12294104 [44]). Consequently, these associations may contribute to the LH/FSH-mediated phenotypic effects of SNV rs11031002 T>A in EH.
Conclusion
This study identified a link between SNV rs11031002 T>A in the FSHB gene and EH in women with BMI ≥25 kg/m² (for the minor allele A, OR<1). Furthermore, this SNV and five closely associated polymorphic loci demonstrated significant functional activity, influencing the interaction between the DNA region near the proposed promoter of the FSHB gene and 38 transcription factors as well as the transcription levels of the ARL14EP gene in subcutaneous adipose tissue.
References
- Cree I.A., White V.A., Indave B.I., Lokuhetty D. Revising the WHO classification: female genital tract tumours. Histopathology. 2020; 76(1): 151-6. https://dx.doi.org/10.1111/his.13977
- Reed S.D., Newton K.M., Clinton W.L., Epplein M., Garcia R., Allison K. et al. Incidence of endometrial hyperplasia. Am. J. Obstet. Gynecol. 2009; 200(6): 678.e1-678.e6786. https://dx.doi.org/10.1016/j.ajog.2009.02.032
- Протасова А.Э., Адамян Л.В., Собивчак М.С., Цыпурдеева А.А. Эндометриальная гиперплазия: современные концепции этиопатогенеза. Проблемы репродукции. 2023; 29(4): 75-80. [Protasova A.E., Adamyan L.V., Sobivchak M.S., Tsypurdeeva A.A. Endometrial hyperplasia: modern concepts of etiopathogenesis. Russian Journal of Human Reproduction. 2023; 29(4): 75 80. (in Russian)]. https://dx.doi.org/10.17116/repro20232904175
- Думановская М.Р., Чернуха Г.Е., Табеева Г.И., Асатурова А.В. Гиперплазия эндометрия: поиск оптимальных решений и стратегий. Акушерство и гинекология. 2021; 4: 23-31. [Dumanovskaya M.R., Chernukha G.E., Tabeeva G.I., Asaturova A.V. Endometrial hyperplasia: search for optimal solutions and strategies. Obstetrics and Gynecology. 2021; (4): 23-31. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.4.23-31
- Подзолкова Н.М., Коренная В.В. Современные представления об этиологии, патогенезе и принципах лечения гиперплазии эндометрия. Акушерство и гинекология. 2021; 8: 192-9. [Podzolkova N.M., Korennaya V.V. Modern ideas about the etiology, pathogenesis and principles of treatment of endometrial hyperplasia. Obstetrics and Gynecology. 2021; (8): 192-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2021.8.192-199
- Sanderson P.A., Critchley H.O., Williams A.R., Arends M.J., Saunders P.T. New concepts for an old problem: the diagnosis of endometrial hyperplasia. Hum. Reprod. Update. 2017; 23(2): 232-54. https://dx.doi.org/10.1093/humupd/dmw042
- Chandra V., Kim J.J., Benbrook D.M., Dwivedi A., Rai R. Therapeutic options for management of endometrial hyperplasia. J. Gynecol. Oncol. 2016; 27(1): e8. https://dx.doi.org/10.3802/jgo.2016.27.e8
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Гиперпластические процессы эндометрия: этиопатогенез, факторы риска, полиморфизм генов-кандидатов. Акушерство и гинекология. 2019; 1: 13-8. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Endometrial hyperplastic processes: etiopathogenesis, risk factors, polymorphism of candidate genes. Obstetrics and Gynecology. 2019; (1): 13-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.1.13-18
- Габидуллина Р.И., Смирнова Г.А., Нухбала Ф.Р., Валеева Е.В., Орлова Ю.И., Шакиров А.А. Гиперпластические процессы эндометрия: современная тактика ведения пациенток. Гинекология. 2019; 21(6): 53-8. [Gabidullina R.I., Smirnova G.A., Nuhbala F.R., Valeeva E.V., Orlova Y.I., Shakirov A.A. Hyperplastic processes of the endometrium: modern tactics of patient management. Gynecology. 2019; 21(6): 53-8. (in Russian)]. https://dx.doi.org/10.26442/20795696.2019.6.190472
- Nees L.K., Heublein S., Steinmacher S., Juhasz-Böss I., Brucker S., Tempfer C.B. et al. Endometrial hyperplasia as a risk factor of endometrial cancer. Arch. Gynecol. Obstet. 2022; 306(2): 407-21. https://dx.doi.org/10.1007/s00404-021-06380-5
- Wise M.R., Gill P., Lensen S., Thompson J.M., Farquhar C.M. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am. J. Obstet. Gynecol. 2016; 215(5): 598.e1-598.e8. https://dx.doi.org/10.1016/j.ajog.2016.06.006
- Alsudairi H.N., Alrasheed A.T., Dvornyk V. Estrogens and uterine fibroids: an integrated view. Research Results in Biomedicine. 2021; 7(2): 156-63. https://dx.doi.org/10.18413/2658-6533-2021-7-2-0-6
- Ponomarenko M.S., Reshetnikov E.A., Churnosova M.M., Reshetnikova Y.N., Churnosov V.I., Ponomarenko I.V. Comorbidity and syntropy of benign proliferative diseases of the female reproductive system: non-genetic, genetic, and epigenetic factors (review). Research Results in Biomedicine. 2023; 9(4): 544-56. https://dx.doi.org/10.18413/2658- 6533-2023-9-4-0-9
- Demakova N.A., Altuchova O.B., Orlova V.S., Pachomov S.P., Krikun E.N. Associations of cytokines genetic polymorphisms with development of endometrial hyperplasia. Research Journal of Pharmaceutical, Biological and Chemical. 2014; 5(5): 1041-5.
- van der Putten L.J.M., van Hoof R., Tops B.B.J., Snijders M.P.L.M., van den Berg-van Erp S.H., van der Wurff A.A.M. et al. Molecular profiles of benign and (pre)malignant endometrial lesions. Carcinogenesis. 2017; 38(3): 329-35. https://dx.doi.org/10.1093/carcin/bgx008
- Altuchova O.B., Demakova N.A., Koneva O.A., Pachomov S.P., Orlova V.S., Golovchenko O.V. Genetic factors of uterine hyperplastic processes. Research Journal of Pharmaceutical, Biological and Chemical. 2014; 6(5): 1397-400.
- Демакова Н.А. Молекулярно-генетические характеристики пациенток с гиперплазией и полипами эндометрия. Научный результат. Медицина и фармация. 2018; 4(2): 26-39. [Demakova N.A. Molecular and genetic characteristics of patients with hyperplasia and endometric polyps. Research Result. Medicine and Pharmacy. 2018; 4(2): 26-39 (in Russian)]. https://dx.doi.org/10.18413/2313-8955-2018-4-2-0-4
- Ivanova T.I., Krikunova L.I., Ryabchenko N.I., Mkrtchyan L.S., Khorokhorina V.A., Salnikova L.E. Association of the apolipoprotein E 2 allele with concurrent occurrence of endometrial hyperplasia and endometrial carcinoma. Oxid. Med. Cell. Longev. 2015; 2015: 593658. https://dx.doi.org/10.1155/2015/593658
- Пономаренко И.В., Полоников А.В., Чурносов М.И. Ассоциация полиморфизма rs4986938 гена ESR2 с развитием гиперплазии эндометрия. Акушерство и гинекология. 2019; 4: 66-72. [Ponomarenko I.V., Polonikov A.V., Churnosov M.I. Association of ESR2 rs4986938 polymorphism with the development of endometrial hyperplasia. Obstetrics and Gynecology. 2019; (4): 66-72. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.4.66-72
- Фаткуллин И.Ф., Габидуллина Р.И., Смирнова Г.А., Нухбала Ф.Р., Валеева Е.В., Орлова Ю.И., Шакиров А.А. Ассоциация полиморфизма RS2414098 гена CYP19A1 с риском развития гиперплазии эндометрия. Акушерство и гинекология. 2020; 2: 125-30. [Fatkullin I.F., Gabidullina R.I., Smirnova G.A., Nukhbala F.R., Valeeva E.V., Orlova Yu.I., Shakirov A.A. The association between rs2414098 polymorphism of the CYP19A1 gene and the risk of developing endometrial hyperplasia. Obstetrics and Gynecology. 2020; (2): 125-30. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.2.125-130
- Ponomarenko I., Reshetnikov E., Polonikov A., Sorokina I., Yermachenko A., Dvornyk V. et al. Candidate genes for age at menarche are associated with endometrial hyperplasia. Gene. 2020; 757: 144933. https://dx.doi.org/10.1016/j.gene.2020.144933
- Ruth K.S., Campbell P.J., Chew S., Lim E.M., Hadlow N., Stuckey B.G. et al. Genome-wide association study with 1000 genomes imputation identifies signals for nine sex hormone-related phenotypes. Eur. J. Hum. Genet. 2016; 24: 284-90. https://dx.doi.org/10.1038/ejhg.2015.102
- Ruth K.S., Day F.R., Tyrrell J., Thompson D.J., Wood A.R., Mahajan A. et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat. Med. 2020; 26(2): 252-8. https://dx.doi.org/10.1038/s41591-020-0751-5
- Gudjonsson A., Gudmundsdottir V., Axelsson G.T., Gudmundsson E.F., Jonsson B.G., Launer L.J. et al. A genome-wide association study of serum proteins reveals shared loci with common diseases. Nat. Commun. 2022; 13(1): 480. https://dx.doi.org/10.1038/s41467-021-27850-z
- Пономарева Т.А. Генетические варианты глобулина, связывающего половые гормоны, и гормональный профиль больных генитальным эндометриозом. Научные результаты биомедицинских исследований. 2025; 11(1): 75-90. [Ponomareva T.A. Genetic variants of sex hormone-binding globulin and hormonal profile in patients with genital endometriosis. Research Results in Biomedicine. 2025; 11(1): 75-90. (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2025-11-1-0-4
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A., Bender D. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007; 81(3): 559-75. https://dx.doi.org/10.1086/519795
- Che R., Jack J.R., Motsinger-Reif A.A., Brown C.C. An adaptive permutation approach for genome-wide association study: evaluation and recommendations for use. BioData Min. 2014; 7: 9. https://dx.doi.org/10.1186/1756-0381-7-9
- Пасенов К.Н. Особенности ассоциаций SHBG-связанных генов с раком молочной железы у женщин в зависимости от наличия наследственной отягощенности и мутаций в генах BRCA1/CHEK2. Научные результаты биомедицинских исследований. 2024; 10(1): 69-88. [Pasenov K.N. Features of associations of SHBG-related genes with breast cancer in women, depending on the presence of hereditary burden and mutations in the BRCA1/CHEK2 genes. Research Results in Biomedicine. 2024; 10(1): 69-88. (in Russian)]. https://dx.doi.org/10.18413/2658-6533-2024-10-1-0-4
- Yarosh S.L., Kokhtenko E.V., Churnosov M.I., Solodilova M.A., Polonikov A.V. Joint effect of glutathione S-transferase genotypes and cigarette smoking on idiopathic male infertility. Andrologia. 2015; 47(9): 980-6. https://dx.doi.org/10.1111/and.12367
- Ward L.D., Kellis M. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016; 44(D1): D877-81. https://dx.doi.org/10.1093/nar/gkv1340
- GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020; 369(6509): 1318-30. https://dx.doi.org/10.1126/science.aaz1776
- McGrath I.M., Mortlock S., Montgomery G.W. Genetic regulation of physiological reproductive lifespan and female fertility. Int. J. Mol. Sci. 2021; 22(5): 2556. https://dx.doi.org/10.3390/ijms22052556
- Burger H.G., Hale G.E., Dennerstein L., Robertson D.M. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause. 2008; 15(4 Pt 1): 603-12. https://dx.doi.org/10.1097/gme.0b013e318174ea4d
- Hambridge H.L., Mumford S.L., Mattison D.R., Ye A., Pollack A.Z., Bloom M.S. et al. The influence of sporadic anovulation on hormone levels in ovulatory cycles. Hum. Reprod. 2013; 28(6): 1687-94. https://dx.doi.org/10.1093/humrep/det090
- Ruth K.S., Beaumont R.N., Tyrrell J., Jones S.E., Tuke M.A., Yaghootkar H. et al. Genetic evidence that lower circulating FSH levels lengthen menstrual cycle, increase age at menopause and impact female reproductive health. Hum. Reprod. 2016; 31(2): 473-81. https://doi.org/10.1093/humrep/dev318
- Hayes M.G., Urbanek M., Ehrmann D.A., Armstrong L.L., Lee J.Y., Sisk R. et al.; Reproductive Medicine Network. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat. Commun. 2015; 6: 7502. https://dx.doi.org/10.1038/ncomms8502
- Mbarek H., van de Weijer M.P., van der Zee M.D., Ip H.F., Beck J.J., Abdellaoui A. et al. Biological insights into multiple birth: genetic findings from UK Biobank. Eur. J. Hum. Genet. 2019; 27(6): 970-9. https://dx.doi.org/10.1038/s41431-019-0355-z
- Pietzner M., Wheeler E., Carrasco-Zanini J., Cortes A., Koprulu M., Wörheide M.A. et al. Mapping the proteo-genomic convergence of human diseases. Science. 2021; 374(6569): eabj1541. https://dx.doi.org/10.1126/science.abj1541
- Bianco B., Loureiro F.A., Trevisan C.M., Peluso C., Christofolini D.M., Montagna E. et al. Effects of FSHR and FSHB variants on hormonal profile and reproductive outcomes of infertile women with endometriosis. Front. Endocrinol. (Lausanne). 2021; 12: 616. https://dx.doi.org/10.3389/fendo.2021.760616
- Rull K., Grigorova M., Ehrenberg A., Vaas P., Sekavin A., Nõmmemees D. et al. FSHB -211 G>T is a major genetic modulator of reproductive physiology and health in childbearing age women. Hum. Reprod. 2018; 33(5): 954-66. https://dx.doi.org/10.1093/humrep/dey057
- Trevisan C.M., de Oliveira R., Christofolini D.M., Barbosa C.P., Bianco B. Effects of a polymorphism in the promoter region of the follicle-stimulating hormone subunit beta (FSHB) gene on female reproductive outcomes. Genet. Test. Mol. Biomarkers. 2019; 23(1): 39-44. https://dx.doi.org/10.1089/gtmb.2018.0182
- Schubert M., Pérez Lanuza L., Wöste M., Dugas M., Carmona F.D., Palomino-Morales R.J. et al. A GWAS in idiopathic/unexplained infertile men detects a genomic region determining follicle-stimulating hormone levels. J. Clin. Endocrinol. Metab. 2022; 107(8): 2350-61. https://dx.doi.org/10.1210/clinem/dgac165
- Tian Y., Zhao H., Chen H., Peng Y., Cui L., Du Y. et al. Variants in FSHB are associated with polycystic ovary syndrome and luteinizing hormone level in han chinese women. J. Clin. Endocrinol. Metab. 2016; 101(5): 2178-84. https://dx.doi.org/10.1210/jc.2015-3776
- Saxena R., Bjonnes A.C., Georgopoulos N.A., Koika V., Panidis D., Welt C.K. Gene variants associated with age at menopause are also associated with polycystic ovary syndrome, gonadotrophins and ovarian volume. Hum. Reprod. 2015; 30(7): 1697-703. https://dx.doi.org/10.1093/humrep/dev110
Received 19.02.2025
Accepted 30.04.2025
About the Authors
Vladimir I. Churnosov, PhD student at the Department of Biomedical Disciplines, Belgorod State National Research University, 308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, vladimirchurnosov@rambler.ru, https://orcid.org/0009-0007-8519-4594Irina V. Ponomarenko, Dr. Med. Sci., Professor at the Department of Medical and Biological Disciplines, Belgorod State National Research University, 308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, ponomarenko_i@bsuedu.ru, https://orcid.org/0000-0002-5652-0166
Marina S. Ponomarenko, PhD student at the Department of Biomedical Disciplines, Belgorod State National Research University, 308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, ponomarenkomc@yandex.ru, https://orcid.org/0009-0009-0312-0829
Mikhail I. Churnosov, Dr. Med. Sci., Head of the Department of Medical and Biological Disciplines, Belgorod State National Research University, 308015, Russia, Belgorod, Pobedy str., 85, +7(4722)30-13-83, churnosov@bsuedu.ru, https://orcid.org/0000-0003-1254-6134
Corresponding author: Irina V. Ponomarenko, ponomarenko_i@bsuedu.ru



