Endometrial hyperplasia in women with comorbid obesity working in contact with organic solvents
Objective: To determine the effect of organic solvents on the clinical, morphological, and molecular characteristics of endometrial hyperplasia in relation to obesity. Materials and methods: This single-center cross-sectional study included 140 women with histologically confirmed endometrial hyperplasia and occupational exposure to organic solvents; of these, 70 women were obese. The comparison group comprised140 women with endometrial hyperplasia without occupational health risks; of these, 68 were obese. The participants were 57 (45; 64) and 56 (46; 63) years of age and had been menopausal for more than 2 years. The concentrations of organic solvents exceeded the maximum permissible exposure limit by a factor of 1.1 to 5, and the duration of exposure was 22 (18; 28) years. All patients underwent pelvic ultrasound, hysteroscopy with biopsy, and histological and immunohistochemical (Ki-67, estrogen, and progesterone receptor) examinations. Protein concentrations in the blood were determined using a solid-phase enzyme immunoassay. Results: Organic solvent exposure was associated with a higher incidence of atypical endometrial hyperplasia, mainly in the obese subgroup – 24/70 (34.3%). In the non-obese subgroup, it was 13/70 (18.6%) in the comparison group (7/68 (10.3%) vs. 5/72 (6.9%), p=0.003). There was simultaneous increase in Ki-67 expression (for atypical endometrial hyperplasia 55.7% (53.5; 60,1)% и 42,0 (40,4; 45.6)% in obese and non-obese subgroups), increased frequency of abnormal uterine bleeding – 20/70 (28.5%) and 11/70(15.7%), M-echo thickness – 12 (11; 13) and 9.0 (9; 9.5) mm), blood concentrations of interleukins 1β and 6 (higher in obesity), transforming growth factor β1, platelet-derived growth factor-AB, fibroblast growth factor-2 (independent of obesity), p<0.01. The organic solvent exposure group showed correlations between atypical endometrial hyperplasia and age, maximum single concentrations of toluene, waist circumference, Ki-67, and platelet-derived growth factor-AB. Conclusion: This study identified the clinical and pathogenetic features of endometrial hyperplasia in women exposed to organic solvents associated with comorbid obesity. Authors' contributions: Marinkin I.O. – conception and design of the study, reviewing, editing, and final approval for submission; Shpagina L.A. – conception and design of the study, manuscript editing, material collection and analysis, final approval for submission; Lisova E.S. – conception and design of the study, material collection and analysis, statistical analysis, manuscript drafting; Kotova O.S., Kuznetsova G.V., Loktin E.M., Karmanovskaya S.A. – material collection and analysis, statistical analysis, manuscript drafting. Conflicts of interest: The authors have no conflicts of interest to declare. Funding: There was no funding for this study. Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Novosibirsk State Medical University, Ministry of Health of the Russian. Patient Consent for Publication: All patients provided informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Marinkin I.O., Shpagina L.A., Lisova E.S., Kotova O.S., Kuznetsova G.V., Loktin E.M., Karmanovskaya S.A. Endometrial hyperplasia in women with comorbid obesity working in contact with organic solvents. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (3): 57-64 (in Russian) https://dx.doi.org/10.18565/aig.2022.289Marinkin I.O., Shpagina L.A., Lisova E.S., Kotova O.S., Kuznetsova G.V., Loktin E.M., Karmanovskaya S.A.
Keywords
Occupational health hazards to working women are primarily related to the high sensitivity of the reproductive system to toxic chemicals. Exposure to reprotoxins is associated with a wide range of endocrine disorders and cytotoxic effects [1, 2]. It is particularly important that many chemical compounds are risk factors for the development of neoplasms and precancerous conditions [3]. In particular, organic solvents (hydrocarbons and their derivatives) are carcinogenic to humans, with a level of evidence ranging from IIb (xylene) to I (trichloroethylene), according to the IARC classification [4–6]. These are among the most common industrial toxicants.
Endometrial hyperplasia is a common disease prone to malignant transformation [7, 8]. However, cancer risk is characterized by significant heterogeneity. For example, atypical hyperplasia has a 20–50% chance of malignant transformation [9]. Simple hyperplasia transforms into malignancy in less than 5% of the cases [10]. Recently, there has been a growing interest in research on the biomechanisms of the disease and factors influencing its clinical manifestations, and scientific search for biomarkers of unfavorable course of the disease to identify risk groups. The specific characteristics of endometrial hyperplasia in women exposed to organic solvents have not been sufficiently studied. At the same time, it is necessary to consider obesity as a proven etiological factor in the development of endometrial hyperplasia [7], as well as the cumulative effect of organic solvents on adipose tissue, which significantly affects toxicity [11].
This study aimed to determine the effects of organic solvents on the clinical, morphological, and molecular characteristics of endometrial hyperplasia in relation to obesity.
Materials and methods
This was a single-center, single-stage, observational, comparative study. The study group included 140 women with endometrial hyperplasia and workplace exposure to organic solvents; of these, 70 were obese and 70 had a normal body weight. The comparison group was comprised of women with endometrial hyperplasia and no chemical exposure (n=140, obese subgroup of 68, and non-obese subgroup of 72).
Patients were included in the study based on the following criteria: written informed consent to participate in the study, age 50–65 years, menopause for 2 years or more, diagnosis of endometrial hyperplasia, occupational contact with organic solvents for at least 5 years in the study; in the control group, no occupational exposure to chemical agents (including concentrations below the maximum allowable) during the entire period of work activity.
Women were not included in the study if they did not give informed consent to participate in the study, if they were unable or unwilling to understand and comply with the requirements of the study protocol, if they had contraindications to the diagnostic measures provided for in the study protocol, if they had taken estrogen-progestin grugs during the month prior to enrolment, or if their body mass index was less than 18; those with malignant neoplasms of any location, inflammatory diseases of the pelvic organs, chronic inflammatory diseases of other locations, polycystic ovary syndrome, severe diabetes mellitus in the stage of decompensation, cirrhosis of the liver, or hepatitis with liver failure; and in the presence of anamnestic data on the absence of childbirth, early age of onset of menstruation, family history of ovarian, colon, or uterine cancer.
Endometrial hyperplasia was diagnosed based on histological examination of the endometrium [12, 13].
Obesity was diagnosed according to the WHO Health Organization criteria of body mass index > 30 kg/m2. Abdominal obesity was defined according to the 2005 IDF criteria, including waist circumference > 80 cm [14] and waist-to-hip ratio > 0.85 [15].
All patients underwent history taking, anamnesis, physical examination, bimanual gynecological examination, two ultrasound examinations of the pelvic organs, hysteroscopy with biopsy, or fractional diagnostic curettage with histological examination, according to standard indications [12].
Histological samples were prepared according to standard procedures and examined by light microscopy with hematoxylin and eosin (H&E) staining and immunohistochemistry. The expression of progesterone and estrogen receptors in epithelial and stromal cells and the expression of the protein proliferation marker Ki-67 were determined.
The serum concentrations of C-reactive protein, interleukin 1β and 6, transforming growth factor β1 (TGFβ1), fibroblast growth factor-2 (FGF-2), platelet-derived growth factor (PDGF AB), leptin, adiponectin, and ferritin were determined using enzyme immunoassay. Blood fibrinogen concentration was measured using the Clauss method.
The occupation of the patients exposed to the chemical factor was painters, and the place of work was a mechanical engineering enterprise (aircraft production, OKVED code 2022 30.30). The levels of organic solvents in the working area air were measured by the photometric method during routine monitoring carried out by experts of the Russian Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing in Novosibirsk Region. The concentrations of organic solvents in the air of the work area were 1.1 to 5 times higher than the maximum allowable concentrations.
Statistical analysis
Standard methods were used for descriptive statistics. Quantitative variables showing normal distribution were expressed as mean (M) and standard deviation (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) was reported. The distribution of continuous variables was tested for normality using the Kolmogorov–Smirnov test. Categorical variables were reported as frequencies and percentages. The Kruskal–Wallis test was used to compare numerical data between three or more groups, and categorical variables were compared using the χ2 test. Post-hoc pairwise comparisons were performed to identify significantly different groups. Correlation analysis was conducted by calculating the Spearman's rank correlation coefficients. Variable "atypical endometrial hyperplasia" was included in the analysis as a dichotomous variable. Differences between the groups were considered statistically significant at p<0.01, adjusted for Bonferroni correction (four compared groups, six pairs of comparisons). The significance threshold for correlation analysis was defined as 0.05. Statistical analysis was performed using the SPSS 28 software.
The study was performed in accordance with the ethical principles established by the Declaration of Helsinki of the World Medical Association as well as with the observance of ethical norms and rules stipulated by the Bulletin of the Higher Attestation Commission of the Ministry of Education of Russia No. 3 of 2002. "On Procedures for Biomedical Research in Human Subjects.” The study was reviewed and approved by the Research Ethics Committee of Novosibirsk State Medical University of the Ministry of Health of Russia.
Results
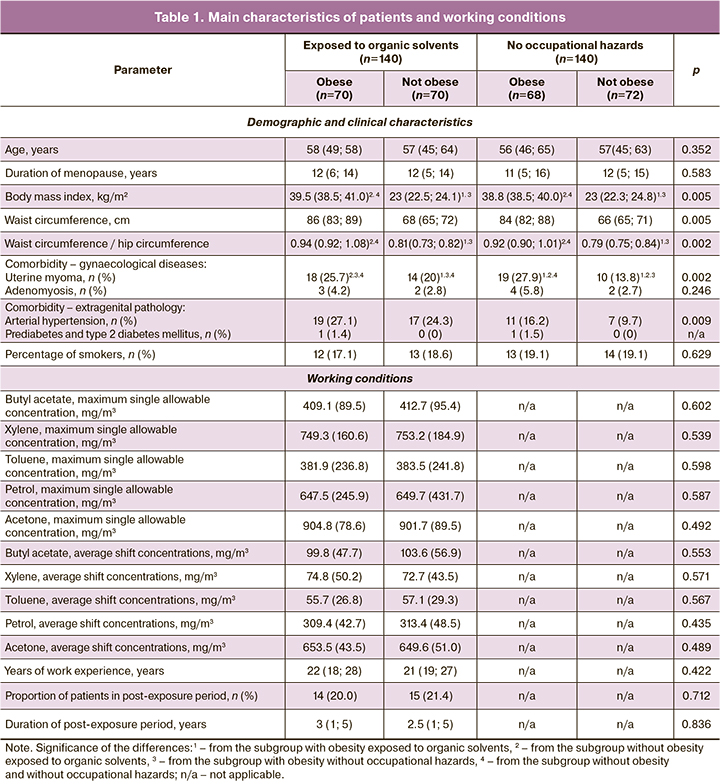
The main characteristics of the patients and hygienic characteristics of their working conditions are presented in Table 1.
In women with chemical exposure, endometrial hyperplasia was characterized by frequent atypical forms, highest in the obese subgroup, 24/70 (34.3%) cases, compared with 13/70 (18.6%) in the non-obese subgroup and 7/68 (10.3%) and 5/72 (6.9%) in the non-occupational risk group in obese and normal-weight women, respectively (p=0.0035). Differences were statistically significant between obese and non-obese workers, obese workers and controls, obese workers, and non-obese controls. The rate of atypical endometrial hyperplasia did not differ between the obese and non-obese subgroups in the control group.
An increase in the incidence of abnormal uterine bleeding (AMB) in patients with endometrial hyperplasia exposed to organic solvents was noted in the clinical and anamnestic findings, predominantly in obese patients (Table 2). In the same subgroup, an increasing trend was observed in the number of patients with severe AMB accompanied by hemodynamic disorders, decreased hematocrit levels, and the need for transfusions of blood components. In addition, the rates of anemia and lower serum ferritin levels were higher in the worker group. These changes were more pronounced in the obese group.
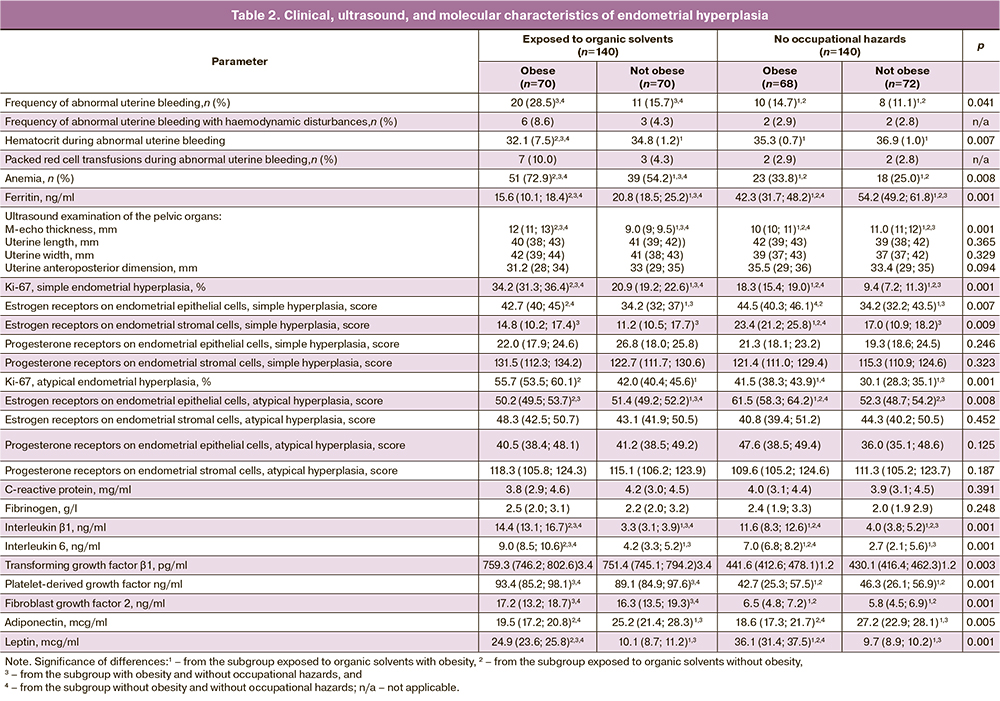
Ultrasonography of the pelvic organs showed increased M-echo thickness in the subgroup of women exposed to organic solvents and the obese subgroup.
The study participants with comorbid obesity exposed to organic solvents had the highest levels of Ki-67 protein, a proliferation marker expressed by endometrial cells. This pattern was observed in both simple and atypical endometrial hyperplasia. When comparing the subgroups, Ki-67 expression was higher in the organic solvent-exposed versus unexposed subgroups and in the obese versus normal weight subgroups.
In simple endometrial hyperplasia, the expression of estrogen receptors in the epithelium was greater in obese subjects and was independent of chemical exposure. In the comparison group, estrogen receptor expression in the obese subgroup was also greater in the stromal cells. Among patients with atypical endometrial hyperplasia, the expression of estrogen receptors was higher in the obese subgroup and the highest in the absence of chemical factor effects.
Blood concentrations of the standard acute phase indicators C-reactive protein and fibrinogen did not differ between the study groups. Levels of proinflammatory cytokines, interleukin 1β and 6, were higher in obese patients after exposure to organic solvents. Systemic circulating levels of profibrotic growth factors, TGFβ, FGF-2, and PDGF-AB, were increased in women exposed to organic solvents and were independent of obesity.
The decrease in serum adiponectin concentrations in the obese subgroups was independent of toxicant exposure. Blood leptin levels in obese workers exposed to organic solvents were elevated but lower than those in the control group.
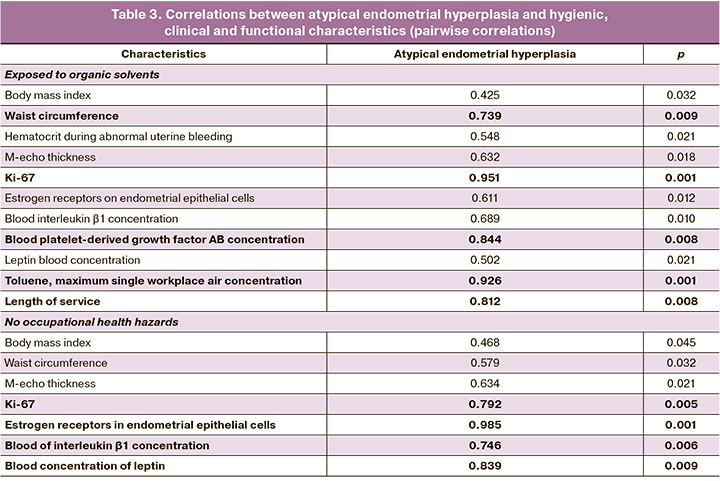
Correlation analysis showed a strong correlation between the atypical variant of endometrial hyperplasia and waist circumference, Ki-67 expression level, blood concentration of PDGF-AB, maximum single allowable concentration of toluene in the air of the working area, and work experience. We also observed statistically significant moderate-strength correlations with hematocrit during AMI, M-echo thickness, level of estrogen receptor expression in endometrial cells, and blood concentrations of interleukin-1β and leptin; weak correlation was observed with body mass index.
The control group showed strong correlations between atypical endometrial hyperplasia and Ki-67 expression, estrogen receptor expression in endometrial epithelial cells, concentrations of interleukin-1β and leptin in the systemic circulation, medium strength correlations with waist circumference and M-echo thickness, and weak correlations with body mass index (Table 3).
Discussion
Obesity is a known risk factor for endometrial hyperplasia [7]. This study found that exposure to organic solvents increased the frequency of atypical forms and contributed to the adverse course of the disease, with a high incidence and severity of AMC. Increased Ki-67 expression reflects the greater proliferative activity of endometrial cells under chemical exposure [16], especially with concurrent obesity, which is an unfavorable sign of possible malignant transformation.
The role of hyperestrogenism and changes in endometrial cell expression of estrogen receptors in the pathogenesis of endometrial hyperplasia is well established [7]. In the study participants, obesity was significantly associated with endometrial estrogen receptor levels, which is consistent with the literature [17]. However, inflammatory processes and growth factors, especially profibrotic factors, are associated with cell proliferation, development of neoplasms, and precancerous lesions [18–20]. This study identified an increase in serum concentrations of interleukin 1β [21] and 6 [22] in relation to exposure to both chemical factors and obesity, and an increase in the profibrotic factors TGF-β1 [18,19], FGF-2 [23], and PDGF [24] in women exposed to occupational hazards. According to correlation analysis, atypical variants of endometrial hyperplasia in women exposed to organic solvents were associated with the profibrotic cytokine platelet growth factor. In patients not exposed to chemical factors, the expression levels of estrogen receptors, serum concentrations of leptin (obesity and inflammation), and the proinflammatory cytokine interleukin 1β were related. Therefore, it can be assumed that organic solvents influence the clinical and pathogenetic features of endometrial hyperplasia by inducing an inflammatory response and fibrosis, whereas obesity influences by inducing inflammation. The increased Ki-67 expression in women exposed to organic solvents, the relationship between the toluene concentration in the air of the work area, and atypical endometrial hyperplasia does not rule out a direct effect of the toxicant. The synergistic effect of chemical factors and obesity on proliferation, inflammation, and fibrosis markers observed in the study groups may result in a severe course of the disease.
Conclusion
In women exposed to organic solvents, endometrial hyperplasia is characterized by a higher incidence of atypical forms, abnormal uterine bleeding, increased M-echo thickness, increased Ki-67 expression in the endometrium, and increased blood concentrations of interleukins 1β and 6, transforming growth factor β1, platelet-derived growth factor, and fibroblast growth factor 2. Comorbid obesity exacerbates the effects of organic solutes on the phenotype of endometrial hyperplasia.
References
- Green M.P., Harvey A.J., Finger B.J., Tarulli G.A. Endocrine disrupting chemicals: Impacts on human fertility and fecundity during the peri-conception period. Environ. Res. 2021; 194: 110694. https://dx.doi.org/10.1016/j.envres.2020.110694.
- Spinder N., Prins J.R., Bergman J.E.H., Smidt N., Kromhout H., Boezen H.M. et al. Congenital anomalies in the offspring of occupationally exposed mothers: a systematic review and meta-analysis of studies using expert assessment for occupational exposures. Hum. Reprod. 2019; 34(5): 903-19.https://dx.doi.org/10.1093/humrep/dez033.
- Yu Q., Zhang L., Hou K., Li J., Liu S., Huang K. et al. Relationship between air pollutant exposure and gynecologic cancer risk. Int. J. Environ. Res. Public Health. 2021; 18(10): 5353. https://dx.doi.org/10.3390/ijerph18105353.
- Karami S., Bassig B., Stewart P.A., Lee K.M.., Rothman N., Moore L.E. et al. Occupational trichloroethylene exposure and risk of lymphatic and haematopoietic cancers: a meta-analysis. Occup. Environ. Med. 2013; 70(8): 591-9. https://dx.doi.org/10.1136/oemed-2012-101212.
- Onyije F.M., Hosseini B., Togawa K., Schüz J., Olsson A. Cancer incidence and mortality among petroleum industry workers and residents living in oil producing communities: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2021; 18: 4343. https://dx.doi.org/10.3390/ijerph18084343.
- International Agency for the Research on Cancer. List of classifications. Available at: https://monographs.iarc.who.int/list-of-classifications
- Подзолкова Н.М., Коренная В.В. Современные представления об этиологии, патогенезе и принципах лечения гиперплазии эндометрия. Акушерство и гинекология. 2021; 8: 192-9. [Podzolkova N.M., Korennaya V.V. Modern ideas about the etiology, pathogenesis and principles of treatment of endometrial hyperplasia. Obstetrics and Gynecology. 2021; (8): 192-9. (in Russian)].https://dx.doi.org/10.18565/aig.2021.8.192-199.
- Клинышкова Т.В., Турчанинов Д.В., Фролова Н.Б. Клинико-эпидемиологические аспекты рака тела матки с позиции профилактики рецидивирования гиперплазии эндометрия. Акушерство и гинекология. 2020; 1: 135-40. [Klinyshkova T.V., Turchaninov D.V., Frolova N.B. Clinical and epidemiological aspects of corpus uteri cancer in the context of prevention of recurrent endometrial hyperplasia. Obstetrics and Gynecology. 2020; (1): 135-140. (in Russian)]. https://dx.doi.org/10.18565/aig.2020.1.135-140.
- Salman M.C., Usubutun A., Boynukalin K., Yuce K. Comparison of WHO and endometrial intraepithelial neoplasia classifications in predicting the presence of coexistent malignancy in endometrial hyperplasia. J. Gynecol. Oncol. 2010; 21(2): 97-101. https://dx.doi.org/10.3802/jgo.2010.21.2.97.
- Vilos G.A., Oraif A., Vilos A.G., Ettler H., Edris F., Abu-Rafea B. Long-term clinical outcomes following resectoscopic endometrial ablation of non-atypical endometrial hyperplasia in women with abnormal uterine bleeding. J. Minim. Invasive Gynecol. 2015; 22(1): 66-77. https://dx.doi.org/10.1016/j.jmig.2014.07.009.
- Измеров Н.Ф., ред. Профессиональная патология. Национальное руководство. М.: ГЭОТАР-Медиа; 2011. 784с. [Izmerov N.F., ed. Occupational medicine. National guidelines. Мoscow: GEOTAR-Media; 2011. 784p.(in Russian)].
- Адамян Л.В., Андреева Е.Н., Артымук Н.В., Башмакова Н.В., Беженарь В.Ф., Белокриницкая Т.Е. и др. Гиперплазия эндометрия. Федеральные клинические рекомендации. Доступно по: https://cr.minzdrav.gov.ru/schema/646_1 [Adamyan L.V., Andreeva E.N., Artymuk N.V., Bashmakova N.V.,Bezhenar V.F., Belokrinickaya T.E. et al. Endometrial hyperplasia. Federal clinical guidelines. Available at: https://cr.minzdrav.gov.ru/schema/646_1 (in Russian)].
- Raffone A., Travaglino A., Saccone G., Insabato L., Mollo A., De Placido G., Zullo F. Endometrial hyperplasia and progression to cancer: which classification system stratifies the risk better? A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2019; 299(5): 1233-42. https://dx.doi.org/10.1007/s00404-019-05103-1.
- Alberti K.G.M.M., Zimmet P., Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006; 23(5): 469-80. https://dx.doi.org/10.1111/j.1464-5491.2006.01858.x.
- Bojanic D., Ljubojevic M., Krivokapic D., Gontarev S. Waist circumference, waist-to-hip ratio, and waist-to-height ratio reference percentiles for abdominal obesity among Macedonian adolescents. Nutr. Hosp. 2020; 37(4): 786-93. https://dx.doi.org/10.20960/nh.03006.
- Liang Q., Ma D., Gao R.F., Yu K.D. Effect of Ki-67 Expression levels and histological grade on breast cancer early relapse in patients with different immunohistochemical-based subtypes. Sci. Rep. 2020; 10(1): 7648.https://dx.doi.org/10.1038/s41598-020-64523-1.
- Hetemaki N., Savolainen-Peltonen H., Tikkanen M.J., Wang F., Paatela H., Hamalainen E. et al. Estrogen metabolism in abdominal subcutaneous and visceral adipose tissue in postmenopausal women. J. Clin. Endocrinol. Metab. 2017; 102(12): 4588-95. https://dx.doi.org/10.1210/jc.2017-01474.
- Caja L., Dituri F., Mancarella S., Caballero-Diaz D., Moustakas A., Giannelli G., Fabregat I. TGF-β and the tissue microenvironment: relevance in fibrosis and cancer. Int. J. Mol. Sci. 2018; 19(5): 1294. https://dx.doi.org/10.3390/ijms19051294.
- Chung J.Y., Chan M.K., Li J.S., Chan A.S., Tang P.C., Leung K.T. et al. TGF-β signaling: from tissue fibrosis to tumor microenvironment. Int. J. Mol. Sci. 2021; 22(14): 7575. https://dx.doi.org/10.3390/ijms22147575.
- Bent R., Moll L., Grabbe S., Bros M. Interleukin-1 Beta-A friend or foe in malignancies? Int. J. Mol. Sci. 2018; 19(8): 2155. https://dx.doi.org/10.3390/ijms19082155.
- Mantovani A., Dinarello C.A., Molgora M., Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity. 2019; 50(4): 778-95. https://dx.doi.org/10.1016/j.immuni.2019.03.012.
- Unver N., McAllister F. IL-6 family cytokines: key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev. 2018; 41: 10-7. https://dx.doi.org/10.1016/j.cytogfr.2018.04.004.
- Hillege M.M.G., Galli Caro R.A., Offringa C., de Wit G.M.J., Jaspers R.T., Hoogaars W.M.H. TGF-β regulates collagen type i expression in myoblasts and myotubes via transient Ctgf and Fgf-2 expression. Cells. 2020; 9(2): 375.https://dx.doi.org/10.3390/cells9020375.
- Paolini C., Agarbati S., Benfaremo D., Mozzicafreddo M., Svegliati S., Moroncini G. PDGF/PDGFR: a possible molecular target in scleroderma fibrosis. Int. J. Mol. Sci. 2022; 23(7): 3904. https://dx.doi.org/10.3390/ijms23073904.
Received 03.12.2022
Accepted 22.02.2023
About the Authors
Igor O. Marinkin, Dr. Med. Sci., Professor, Merited Doctor of the Russian Federation, Rector, Head of the Department of Obstetrics and Gynecology, Novosibirsk State Medical University, Ministry of Health of Russia, rector@ngmu.ru, https://orcid.org/0000-0002-9409-4823, 630091, Russia, Novosibirsk, Krasnyi Ave., 52.Lyubov A. Shpagina, Dr. Med. Sci., Professor, Merited Doctor of the Russian Federation, Head of the Internal Medicine and Rehabilitation Department, Novosibirsk State Medical University, Ministry of Health of Russia, lashpagina@gmail.com, https://orcid.org/0000-0003-0871-7551, 630051, Russia, Novosibirsk, Polzunova str., 21.
Evgeniya S. Lisova, PhD Student at the Internal Medicine and Rehabilitation Department, Novosibirsk State Medical University, Ministry of Health of Russia,
mkb-2@yandex.ru, https://orcid.org/0000-0002-3469-8756, 630051, Russia, Novosibirsk, Polzunova str., 21.
Olga S. Kotova, Dr. Med. Sci., Associate Professor at the Internal Medicine and Rehabilitation Department, Novosibirsk State Medical University, Ministry of Health of Russia, ok526@yandex.ru, https://orcid.org/0000-0003-0724-1539, 630051, Russia, Novosibirsk, Polzunova str., 21.
Galina V. Kuznetsova, PhD, Associate Professor at the Internal Medicine and Rehabilitation Department, Novosibirsk State Medical University, Ministry of Health of Russia, mkb-2@yandex.ru, https://orcid.org/0000-0001-7428-9159, 630051, Russia, Novosibirsk, Polzunova str., 21.
Evgenij M. Loktin, Dr. Med. Sci., Associate Professor at the Internal Medicine and Rehabilitation Department, Novosibirsk State Medical University,
Ministry of Health of Russia, mkb-2@yandex.ru, https://orcid.org/0000-0002-7370-6958, 630051, Russia, Novosibirsk, Polzunova str., 21.
Svetlana A. Karmanovskaya, Dr. Med. Sci., Associate Professor at the Internal Medicine and Rehabilitation Department, Novosibirsk State Medical University,
Ministry of Health of Russia, mkb-2@yandex.ru, https://orcid.org/0000-0003-3446-8018, 630051, Russia, Novosibirsk, Polzunova str., 21.



