Stillbirths in the Russian Federation in 2020 (COVID-19 pandemic year)
Objective: To compare maternal and placental conditions that caused stillbirths in the Russian Federation in the years 2020 (COVID-19 pandemic year) and 2019.Shchegolev A.I., Tumanova U.N., Chausov A.A., Shuvalova M.P.
Materials and methods: The study analyzed statistical forms А-05 of the Federal State Statistics Service (Rosstat) for the years 2019 and 2020 based on medical records of perinatal deaths related to stillbirths. Maternal and placental conditions that caused stillbirths were divided into 4 groups: I, maternal conditions unrelated to the index pregnancy; II, maternal complications of the index pregnancy; III, placental, umbilical cord and sheath complications; and IV, other complications of labor and other maternal conditions. Stillbirth rates were calculated as the ratio of the number of stillbirths to the total number of newborns born alive and dead, multiplied by 1000.
Results: According to Rosstat data, in 2020 the absolute number of stillbirths and the stillbirth rate increased by 1.1% and 4.2%, respectively, compared to 2019. At the same time, the stillbirth rate as a result of respiratory disorders and a group of endocrine, metabolic, and other disorders specific to the perinatal period increased by 4.7% and 4.3%, respectively. The stillbirth rate from congenital anomalies, on the contrary, decreased by 15.4%. Among the conditions that contributed to stillbirth in 2020, dominated placental, umbilical cord, and fetal membrane lesions, which were noted in 45.7% of the observations. The stillbirth rate due to placental abnormalities increased by 5.6% compared to 2019. In 2020 compared to 2019, there was an increase in the proportion of parasitic diseases (by 29.5%) and the group of so-called other maternal respiratory and circulatory diseases (by 25.9%), as well as the number of multiple pregnancies (by 17.2%). There were significant differences between the rates of conditions that caused stillbirths in different Federal Districts of the Russian Federation.
Conclusion: According to Rosstat data, in 2020 (the year of the COVID-19 pandemic) the absolute number of stillbirths and the stillbirth rate increased by 1.1% and 4.2%, respectively, compared to 2019. Analysis of the incidence of conditions that caused stillbirths showed an increase in the proportion and rate of stillbirths for maternal conditions unrelated to the index pregnancy and placental disorders.
Keywords
The emergence of a new coronavirus strain, SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) caused a the COVID-19 pandemic, also known as the new coronavirus pandemic [1]. The risk of severe illness with COVID-19 increases with age, with older adults at highest risk [2].
At the same time, pregnant women also have a higher risk of developing severe COVID-19 [3] with adverse maternal and fetal outcomes [4, 5]. According to the literature, pregnant women with COVID-19 have a higher incidence of intrauterine distress and fetal growth restriction, premature rupture of membranes, preterm delivery, stillbirth, and neonatal death [6, 7]. Previously, based on the analysis of Rosstat data, we showed a decrease in early neonatal mortality in 2020 (the year of the COVID-19 pandemic) [8] with a simultaneous increase in the total number of stillbirths by 1.12% and the stillbirth rate by 4.2%, compared to the data for 2019 for the whole Russian Federation [9]. At the same time, there was an increase in the number of stillbirths as a result of respiratory disorders and with an unspecified cause of death.
In this regard, given the specifics of the formulation of the initial cause of perinatal death, a separate analysis of the conditions responsible for stillbirth during the COVID-19 pandemic is essential to understand the role of SARS-CoV-2 infection and the course of COVID-19 in pregnant women.
The present study aimed to compare maternal and placental conditions that caused stillbirths in the Russian Federation in the years 2020 and 2019.
Materials and methods
The study analyzed statistical forms А-05 of the Federal State Statistics Service (Rosstat) for the years 2019 and 2020 based on medical certificates of perinatal death related to stillbirths.
Rosstat Forms A-05 tables show the main diseases (the original causes of death) in rows and maternal conditions that caused (contributed to) the fetal deaths in columns. The latter are divided into 4 groups: I – maternal conditions unrelated to the index pregnancy, II – maternal complications of the index pregnancy, III – placental, umbilical cord and sheath complications, IV – other complications of labor, and other maternal conditions, and were the subject of this study.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics v26. The stillbirth rate was calculated as the ratio of the number of stillbirths to the total number of neonates born alive and dead, multiplied by 1000. Data were compared by Chi-square test (χ2) and relative risk (RR) with a 95% confidence interval (95% CI).
Results and discussion
Since 2012 Russian legislation introduced new definitions of live birth and stillbirths as birth weight 500 g or more with a gestational age of 22 weeks or more. The introduction of these criteria, expanding the boundaries for categorizing newborns, naturally led to an increase in the number of stillbirths, and then, beginning in 2014, to a steady decline in their number (Table 1). However, in 2020, according to Rosstat, an increase, compared with the previous year 2019, in the number of stillbirths by 1.1% and the stillbirth rate by 4.2% was registered. That is, against the background of a 6-year trend of declining stillbirth rates, in 2020, there was an increase in the absolute number of stillbirths and the stillbirth rate.
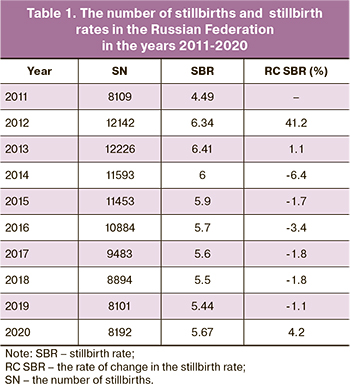
As we have already indicated, according to Rosstat data, in 2019, there were 8101 stillbirths in the Russian Federation. At the same time, the leading cause of death was respiratory disorders, which were registered in 86.4% of stillbirths. As in previous years [10], antenatal (intrauterine) fetal hypoxia traditionally dominated among the diseases of this group, accounting for 78.2% of all stillbirths; intrapartum hypoxia (during delivery) was the second (6.7%). The stillbirth rates were 4.256‰ and 0.365‰ for antenatal and intrapartum hypoxia, respectively. Congenital anomalies ranked second among the causes of stillbirth (6.7%) and diseases related to endocrine, metabolic, and other disorders specific to the perinatal period ranked third (2.6%). Their stillbirth rates were 0.363‰ and 0.139‰ respectively.
In 2020 (the year of the COVID-19 pandemic), 8192 stillbirths were registered in the Russian Federation, which exceeded the number of stillbirths in 2019 by 1.1%. Moreover, the stillbirth rate in 2020 (5.67‰) exceeded the corresponding figure in 2019 (5.44‰) by 4.23%. Among the major disease groups, three leading causes of stillbirth in 2020 were respiratory disorders, congenital anomalies, and a group of endocrine, metabolic, and other disorders specific to the perinatal period. However, in 2020, compared with 2019, stillbirth rates due to respiratory disorders and the group of endocrine, metabolic, and other disorders specific to the perinatal period increased by 4.7% (4.919‰ versus 4.699‰) and 4.3% (0.145‰ versus 0.139‰), respectively. In contrast, the stillbirth rate from congenital anomalies decreased by 15.4% (0.307‰ vs. 0.363‰).
At the same time, in 2020, the number of cases of antenatal hypoxia as the initial cause of death increased by 4.1%, accounting for 80.5% of all stillbirths, while the number of cases of intrapartum hypoxia as the main disease decreased by 24.8%, accounting for 5.0%. As a result, stillbirth rates due to antenatal hypoxia increased by 7.3% in 2020 compared to 2019, and due to intrapartum hypoxia decreased by 22.5%.
The analysis of the number of stillbirths and stillbirth rates has established their differences in various federal districts of the Russian Federation. In 2020, compared to 2019, the absolute number of stillbirths increased in three federal districts: Southern (by 10.4%), Ural (by 6.7%) and Siberian (by 4.8%). However, the stillbirth rate increased in 6 federal districts: Central (by 0.4%), Northwestern (by 0.8%), Southern (by 13.4%), Volga (by 4.0%), Siberian (by 9.4%) and Far Eastern (by 0.6%).
At the same time, the distribution of major disease rates did not change. Respiratory disorders, congenital anomalies, and a group of endocrine, metabolic, and other disorders specific to the perinatal period were the most frequently registered as the initial cause of death. There were two differences: first, in the Ural Federal District, the endocrine, metabolic, and other disorders specific to the perinatal period ranked second, and congenital anomalies ranked third; second, in the Northwestern Federal District in 2019 and in the Far Eastern Federal District in 2020, the group of the hemorrhagic and hematological disorders diseases ranked third [9].
A comparative analysis of stillbirth rates depending on the cause of death revealed an increase in their values in 2020 compared to 2019 for respiratory disorders in all federal districts (except the North Caucasian) and multidirectional changes (increase or decrease) for other groups of major diseases.
Unfortunately, according to the medical certification rules, which include cases and stillbirths, some pathological conditions and processes cannot be defined as a major disease (initial death). It is recommended to indicate such diseases and affection in two separate columns: "the main maternal disease or pathological condition that had an adverse effect on the fetus or child" and "other maternal diseases or pathological conditions that had an adverse effect on the fetus or child". The first of these diseases is included in the Rosstat statistical form A-05 and forms the vertical part of the tables reflecting maternal conditions that caused fetal affection and death, divided into 4 groups, as we pointed out in the "Materials and Methods" section.
Information on the frequency of conditions that contributed to (caused) stillbirth in the Russian Federation federal districts in 2019 and 2020 is presented in Table 2. Analysis of Table 2 shows that placental, umbilical cord, and fetal membrane affections (group III) were the most frequent (45.1%) in the Russian Federation as a whole in 2019 (Table 2). Among the latter, so-called other (i.e., other than placenta previa and placental abruption) and unspecified placental abnormalities were the most frequent, accounting for 53.5% of Group III observations and 24.1% of all stillbirth cases. In turn, placental abruption was observed in 26.7% and 12.0%, respectively.
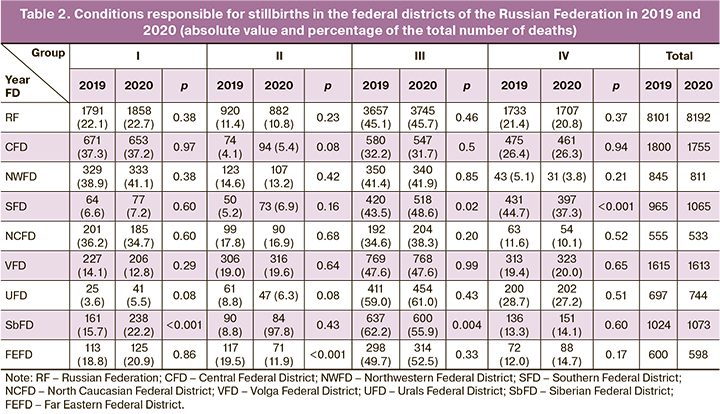
Group I and Group IV diseases were reported about 2 times less frequently as conditions contributing to stillbirth (22.1% and 21.4%, respectively). At the same time, the so-called "Other diseases not related to pregnancy, including maternal nutritional disorders, trauma, and surgery" dominated in Group I, accounting for 86.3% of the diseases of this group and 19.1% of all stillbirths. Group IV was dominated by observations with an unspecified maternal cause, accounting for 87.5% and 18.7% of the conditions in this group and all stillbirths, respectively.
Among the conditions that contributed to stillbirth in 2020, placental, umbilical cord, and fetal membrane lesions also predominated, accounting for 45.7% of cases. In turn, the stillbirth rate due to placental pathology increased by 5.6%, compared to 2019 (2.592‰ vs. 2.456‰). The largest increase was for placenta previa (28.3%, p=0.105).
Along with placental abnormalities, there was a slight increase (2.6%) in the proportion of group I (maternal conditions not related to the index pregnancy) and a decrease in the proportions of group II (maternal complications of the index pregnancy) and group IV (other complications of labor and other maternal conditions) by 5.2% and 2.6%, respectively. However, in group I, which combines diseases unrelated to the index pregnancy, the proportion of parasitic diseases increased (by 29.5%, p=0.037) and so-called other respiratory and circulatory diseases (by 25.9%, p=0.567). And the number of multiple pregnancies increased (by 17.2%, p=0.021) in group II.
The analysis of the conditions contributing to stillbirths reveals their significant differences between the federal districts. The predominance of placental abnormalities in stillbirths in the Russian Federation as a whole is characteristic of most federal districts. At the same time, the highest proportion of placental pathology among all stillbirths was observed in 2019 in the Siberian Federal District (62.2%) and in 2020 in the Ural Federal District (61.0%). The lowest proportion of this pathology, which accounted for almost one-third of all stillbirths, were observed in the Central Federal District in 2019 and 2020. At the same time, the relative proportion of placental pathology as a cause of stillbirths increased in 2020 compared to 2019 both in the Russian Federation as a whole (by 1.3%) and in 5 federal districts (from 1.2% in the Northwestern Federal District to 11.8% in the Southern Federal District). A decrease in the proportion of registered placental abnormalities was observed in 3 districts: from 0.01% in the Volga Federal District to 10.1% in the Siberian Federal District.
The proportion of maternal diseases and conditions that were not related to index pregnancy but contributing to stillbirth (group I) in both studied periods had the highest values in the Northwestern Federal District (38.9% in 2019 and the 41.1% in 2020) and lowest in the Urals Federal District (3.6% and 5.5% in 2019 and 2020, respectively). In 2020 compared to 2019, the relative share of such maternal diseases increased in 5 federal districts (from 5.5% in the Northwestern Federal District to 53.7% in the Urals Federal District) and decreased in 3 districts (from 0.2% in the Central Federal District to 9.1% in the Volga Federal District). As a result, in the Russian Federation as a whole, the proportion of group I conditions among all stillbirths increased by 2.6%.
The proportion of group IV conditions, which included other complications of labor and other maternal conditions, had the highest values in the Southern Federal District (44.7% in 2019 and 37.3% in 2020) and the lowest in the Northwestern Federal District (5.1% and 3.8% in 2019 and 2020). At the same time, the relative share of group IV conditions in 2020 compared to 2019 increased in 3 federal districts (from 3.3% in the Volga Federal District to 22.6% in the Far Eastern Federal District) and decreased in 5 federal districts (from 0.5% in the Central Federal District to 24.9% in the Northwestern Federal District). Accordingly, in the Russian Federation as a whole, the proportion of such conditions among all stillbirths decreased by 2.6%.
In 2020 as compared with 2019 in the Russian Federation there was also a 5.2% decrease in group II conditions, including maternal complications and diseases that developed during the index pregnancy. At the same time, the proportion of these conditions was highest in the Volga Federal District (18.9% and 19.6% in 2019 and 2020, respectively) and lowest in the Central Federal District (4.1% and 5.3% in 2019 and 2020, respectively). However, in 2020 compared with 2019, the incidence of maternal complications treated as a condition that contributed to stillbirth increased in 3 federal districts (from 3.4% in the Volga Federal District to 32.3% in the Southern Federal District) and decreased respectively in 5 districts (from 5.3% in the North Caucasian Federal District to 39.1% in the Far Eastern Federal District).
Our calculations are the most conclusive parameters for estimating changes in stillbirth rates for the studied four groups of conditions contributing to stillbirth in the Russian Federation and federal districts (Table 3).
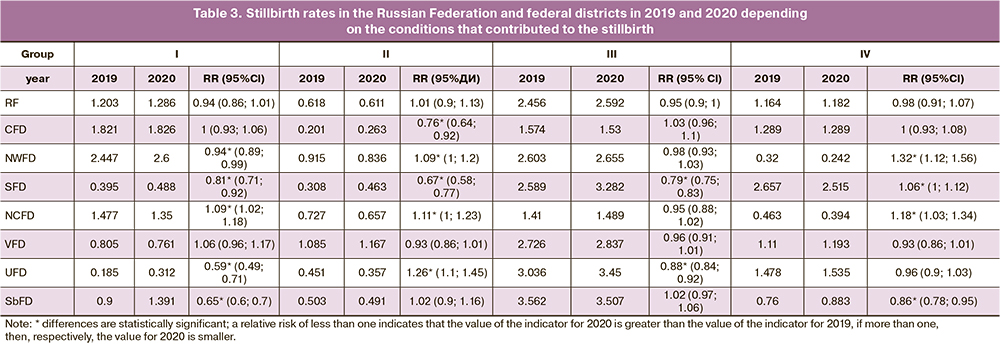
During the studied period, the lowest stillbirth rates were observed in the North Caucasian Federal District (4.077‰ and 3.889‰ in 2019 and 2020, respectively). The highest stillbirth rates were registered in 2019 in the Far Eastern Federal District (6.545‰) and in 2020 in the Southern Federal District (6.748‰). It is noteworthy that in 2020, compared with 2019, an increase in stillbirth rate was established in 7 federal districts; the greatest increase (by 13.4%) was observed in the Southern Federal District. Only in the North Caucasian Federal District there was a decrease in the stillbirth rate by 4.6%.
Among the conditions responsible for stillbirths, the greatest contribution was established for group III, including placental pathology. Stillbirth rates for this group in 2019 and 2020 were highest in the Siberian Federal District (3.562‰ and 3.507‰) and lowest in the North Caucasian Federal District (1.410‰ and 1.489‰). At the same time, 6 federal districts had increased stillbirth rates in 2020 compared to 2019 from 2.0% in the Northwestern Federal District to 26.8% in the Southern Federal District. In the Central and Siberian federal districts, the stillbirth rates in the placental pathology group decreased by 2.8% and 1.5%, respectively.
Stillbirth rates for maternal diseases and conditions not related to the index pregnancy but contributing to stillbirth (group I) in 2019 and 2020 had the highest values in the Northwestern Federal District: 2.447 and 2.600‰, respectively. Lowest values were observed in the Ural Federal District: 0.185‰ and 0.312‰, respectively. Six federal districts registered an increase in stillbirth rates in 2020; the greatest increase was observed in the Urals (by 68.7%) and Siberian (by 54.5%) federal districts. In the North Caucasian and Volga federal districts, there was a decrease in stillbirth rates compared to 2019, by 8.6% and 5.5%, respectively.
For group II, which includes maternal complications in the index pregnancy, the highest stillbirth rates in 2019 were observed in the Far Eastern Federal District (1.277‰), and in 2020 - in the Volga Federal District (1.167‰). The lowest values were observed in the Central Federal District: 0.201‰ and 0.263‰ in 2019 and 2020, respectively. At the same time, in 2020 compared to 2019, stillbirth rates for group II conditions increased only in 3 federal districts: the Southern (by 50.0%), Central (by 30.9%), and Volga (by 7.6%). Consequently, five federal districts demonstrated a decrease in the stillbirth rate, most pronounced in the Far Eastern (by 38.7%) and Ural (by 20.8%) federal districts.
The highest stillbirth rates for group IV conditions during the studied period were in the Southern Federal District (2.657‰ and 2.515‰ in 2019 and 2020, respectively), and the lowest in the Northwestern Federal District (0.320‰ and 0.242‰, respectively). Along with this, an increase in the stillbirth rate for group IV conditions in half of the federal districts was established in 2020 compared to 2019; the most pronounced increase (by 23.5%) was registered in the Far Eastern Federal District. In the other four federal districts, a decrease in the stillbirth rate was observed, the most pronounced (by 24.3%) in the Northwestern Federal District.
Thus, a comparative analysis of Rosstat data on stillbirths for 2020 and 2019 in the Russian Federation revealed an increase in the total number of stillbirths and the stillbirth rate in the COVID-19 pandemic year, as well as changes in the frequency of stillbirth-causing conditions compared to the previous year 2019.
It is reasonable to assume that these changes are to some extent due to the COVID-19 pandemic, which, according to the literature, is associated with changes in both the number and structure of perinatal complications and losses.
Changes in the incidence and pattern of perinatal complications were due both to SARS-Cov-2 infection of pregnant women and fetuses and to the introduction of restrictive measures during the pandemic, including lockdown. Indeed, the development of the COVID-19 pandemic, accompanied by quarantine in selected cities, provinces, and entire countries, significantly altered the daily lives of all residents, including pregnant women. For example, there have been significant disruptions in health services, including disruptions in delivery [11], as well as limited or even unavailable obstetric and perinatal care [12]. According to Khoury et al. [13], 47.9% of pregnant women experienced difficulty accessing prenatal classes, 73.2% switched to telehealth care, and 23% cancelled all prenatal appointments altogether. Thus, pregnant women faced stress and anxiety about the health and well-being of their unborn child [14]. Increased levels of depression (25–31%), anxiety (34–42%), and psychological distress (70%) were noted in pregnant women and mothers of newborns [15, 16].
Such conditions are undoubtedly accompanied by the development of prenatal distress with excessive production of stress hormones, particularly cortisol. Although cortisol is necessary for fetal growth, excessive exposure to it is associated with lower fetal and newborn weight [17]. Importantly, excessive stress during pregnancy also leads to dysregulation of immune responses, leading to impaired fetal development [18].
Pregnant women have been shown to be more susceptible to SARS-CoV-2 infection [19], have more severe forms of the disease [20] and are at increased risk of pregnancy complications [21]. Moreover, COVID-19, even in its mild form, is accompanied by endothelial damage with cytokine release, development of an inflammatory response and tissue damage [22], manifesting as acute respiratory distress syndrome, disseminated intravascular coagulation and cardiovascular complications [23].
According to Ayala-Ramrez et al. [24], the course of COVID-19 in pregnant women shows typical symptoms and proceeds with varying degrees of severity: a mild course was observed in 81-86% of cases, a severe course 9.3–14%, and a critical course in 5%. The most common symptoms were fever (65%), cough (60%), and shortness of breath (24%) [25]. Approximately 5% of pregnant women were hospitalized in the intensive care unit and 35.87% of patients were intubated [24]. According to Lambelet et al. [26] the number of hospitalizations in the ICU was 6–8%, and the mortality rate among pregnant women reached 2.7%.
It should be noted that the presence of COVID-19 in pregnant women has been associated with a high risk of several adverse outcomes, such as fetal distress, fetal growth restriction, premature rupture of membranes, preterm delivery [27], spontaneous abortion, and stillbirth [28]. According to Han et al. [25], the proportion of preterm births was 25%.
A multicenter retrospective cohort study by Hernandez-Pacheco et al. [29] identified gestational hypertensive disorders in 37.5% of hospitalized (between October 2020 and December 2021) pregnant women with SARS-CoV-2 infection. Based on a meta-analysis that included 42 studies from 48548 pregnant women, Wei et al. [30] found that COVID-19 was associated with pre-eclampsia [odds ratio (OR) 1.33], preterm delivery (OR 1.82), and stillbirth (OR 2.11). Severe COVID-19 was associated with preeclampsia (OR 4.16), gestational diabetes, and preterm birth, in contrast to mild COVID-19 [30]. In another meta-analysis involving 28 studies of 790954 pregnant women, SARS-CoV-2 infection during pregnancy was accompanied by a statistically significant increased risk of eclampsia (OR 1.97), severe preeclampsia (OR 1.76) and HELLP syndrome (OR 2.10) [31].
The development of gestational hypertensive disorders and preeclampsia is based on vascular endothelial damage due to impaired functioning of the renin-angiotensin system during pregnancy [32, 33]. In this regard, pregnant women constitute a special potentially vulnerable group because pregnancy is characterized by changes in the expression of angiotensin-converting enzyme-2 receptors (ACE-2, angiotensin converting enzyme 2) in placental structures that provide invasion and intracellular replication of SARS-CoV-2, as well as in serum ACE-2 and angiotensin (1-7) levels [20, 32, 33].
Regarding preeclampsia, it should be noted that pregnant women with COVID-19 may develop the so-called preeclampsia-like syndrome, i.e., clinical symptoms of preeclampsia that disappear before labor after the elimination of respiratory distress [34]. One of the causes of arterial hypertension in pregnant women with COVID-19 is the compensatory increase in the level of vascular endothelial growth factor (VEGF) expression in syncytiotrophoblasts and vascular endotheliocytes of placental villi in parturient women with COVID-19 in response to hypoxia [35]. Similar changes in placental villi and the mechanism of hypertension development are characteristic of preeclampsia, where the expression of VEGF in villi depends on the severity of the disease [36].
A characteristic feature of the pathogenesis of COVID-19 in pregnant women is considered direct and mediated placental damage. To date, convincing data have been obtained on the detection of SARS-CoV-2 in placental structures, mainly in the syncytiotrophoblast [37–39], and endotheliocytes of the fetal placental vascular compartment [40], including in observations with vertical (transplacental) transmission of infection.
Placental lesions have been found to manifest as inflammatory reactions and disorders of the maternal and fetal blood circulation compartments [41, 42]. Inflammatory changes in the placenta of pregnant women with COVID-19 are determined in the intervillous space in the form of histiocytic intervillositis and in the villi of the chorion (villositis) with the presence of macrophages (CD68) and T lymphocytes (CD3) [37, 38, 40]. Higher levels of interleukin-10 were observed in the cord blood of pregnant women with COVID-19 compared to those of the control group (p=0.04), and tumor necrosis factor α levels were positively correlated with preterm birth [43].
More frequent preterm birth in pregnant women with COVID-19 was also combined with a higher frequency of detection of placental vascular lesions [43]. The latter are mainly represented by disorders of the maternal blood supply (maternal vascular insufficiency) of the placenta: decidual arteriopathy, accelerated maturation and distal villous hypoplasia, perivisceral fibrin deposits, edema, and villous infarction [42, 44]. The presence of one or more types of such lesions was observed in the maternal circulatory compartment in 77% of placentas of SARS-CoV-2-infected women infected with SARS-CoV-2 in labor [45]. The severity of the lesions increased with increasing severity of COVID-19 in pregnant patients [46].
These vascular abnormalities indicate the development of preplacental hypoxia and are a morphological substrate of placental insufficiency. The presence of preplacental hypoxia in women in labor with COVID-19 is also confirmed by our findings of an increased number of syncytial nodules and bridges [35], the severity of which is considered a morphological indicator of the development of preplacental hypoxia [47] and a criterion of reduction in the degree of uteroplacental blood flow [48]. In addition, using morphometric study of placentas of women in labor with COVID-19, we found a decrease in the degree of villi vascularization [49], that is, impaired fetal compartment blood flow, indicating the development of placental hypoxia and fetal intrauterine hypoxia [50].
According to the literature [51], placental lesions, including the umbilical cord, are naturally the leading factor in the development of perinatal death. However, according to the rules for selecting the initial cause of death and issuing a medical certificate of perinatal death, such lesions cannot appear as the main disease in perinatal death. They should be listed as the condition that caused fetal death or neonatal death. Therefore, the most frequent initial cause of stillbirth is hypoxia antenatal (intrauterine) and intrapartum (during delivery), and the condition that causes fetal death is placental pathology. Thus, in the Russian Federation as a whole in 2012, hypoxia was recorded as a cause of stillbirth in 81.8% of cases, and placental abnormalities as a cause of stillbirth in 44.5% of cases [52]. In cases of early neonatal death, respiratory disorders were the most frequently reported underlying disease, accounting for 37.3% of cases in 2010. 37.3% of the cases, while placental abnormalities were observed in 17.2% of the cases [53]. In 2016, placental lesions caused early neonatal death in 22.2% of cases [54].
Our analysis of the causes of stillbirth during the COVID-19 pandemic showed a 1.3% increase in the proportion of placental abnormalities as the condition responsible for stillbirth (group III) in 2020 compared to 2019. At the same time, the stillbirth rate of group III increased by 5.6%, which, in our opinion, is due to the direct and indirect effect of SARS-CoV-2 on placental development. There was also a 31.2% increase in the stillbirth rate for respiratory and circulatory diseases not related to index pregnancy. The treatment of non-obstetric diseases that women had prior to the onset of pregnancy should be performed by physicians of appropriate specialties.
Conclusion
According to Rosstat data, in 2020 (the year of the COVID-19 pandemic), the absolute number of stillbirths and the stillbirth rate increased by 1.1% and 4.2%, respectively, compared to 2019. Analysis of the incidence of conditions that caused stillbirths showed an increase in the proportion and rate of stillbirths for maternal conditions unrelated to the index pregnancy and placental disorders. The highest increase was noted in cases of placenta previa. An important point is the presence of marked differences in different federal districts. To elucidate the role of SARS-CoV-2 infection in the development of conditions resulting in stillbirth, clinical and morphological comparisons should be made.
References
- Coronavirus Disease (COVID-19) Pandemic. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Li J., Huang D.Q., Zou B., Yang H., Hui W.Z., Rui F. et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021; 93(3): 1449-58.https://dx.doi.org/10.1002/jmv.26424.
- Torres-Torres J., Martinez-Portilla R.J., Espino Y.S.S., Estrada-Gutierrez G., Solis-Paredes J.M., Villafan-Bernal J.R. et al. Comorbidity, poverty and social vulnerability as risk factors for mortality in pregnant women with confirmed SARS-CoV-2 infection: analysis of 13 062 positive pregnancies including 176 maternal deaths in Mexico. Ultrasound Obstet. Gynecol. 2022; 59(1): 76-82. https://dx.doi.org/10.1002/uog.24797.
- Huntley B.J.F., Mulder I.A., Di Mascio D., Vintzileos W.S., Vintzileos A.M., Berghella V. et al. Adverse pregnancy outcomes among individuals with and without severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a systematic review and meta-analysis. Obstet. Gynecol. 2021; 137(4): 585-96. https://dx.doi.org/10.1097/AOG.0000000000004320.
- Wei S.Q., Bilodeau-Bertrand M., Liu S., Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021; 193(16): E540-8. https://dx.doi.org/10.1503/cmaj.202604.
- Papapanou M., Papaioannou M., Petta A., Routsi E., Farmaki M., Vlahos N. et al. Maternal and neonatal characteristics and outcomes of COVID-19 in pregnancy: an overview of systematic reviews. Int. J. Environ. Res. Public Health. 2021; 18(2): 596. https://dx.doi.org/10.3390/ijerph18020596.
- Yan H., Ding Y., Guo W. Mental health of pregnant and postpartum women during the coronavirus disease 2019 pandemic: a systematic review and meta-analysis. Front. Psychol. 2020; 11: 617001. https://dx.doi.org/10.3389/fpsyg.2020.617001.
- Tumanova U.N., Shchegolev A.I., Chausov A.A., Shuvalova M.P. Analysis of causes of early neonatal mortality during covid-19 pandemic in 2020 in Russia. Bulletin of RSMU. 2021; 5: 71-7. https://dx.doi.org/10.24075/brsmu.2021.045.
- Щеголев А.И., Туманова У.Н., Чаусов А.А., Шувалова М.П. Сравнительный анализ причин мертворождения в Российской Федерации в 2019 и 2020 годах. Акушерство и гинекология. 2022; 2: 80-90. https://dx.doi.org/10.18565/aig.2022.2.80-90. [Shchegolev A.I., Tumanova U.N., Chausov A.A., Shuvalova M.P. Comparative analysis of stillbirth causes and rates in the Russian Federation in 2019 and 2020. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2022; 2: 80-90. (in Russian)]https://dx.doi.org/10.18565/aig.2022.2.80-90.
- Щеголев А.И., Туманова У.Н., Шувалова М.П., Фролова О.Г. Гипоксия как причина мертворождаемости в Российской Федерации. Здоровье, демография, экология финно-угорских народов. 2014; 3: 96-8. [Shchegolev A.I., Tumanova U.N., Shuvalova M.P., Frolova O.G. Hypoxia as a cause of stillbirth in the Russian Federation. Zdorov'e, demografija, jekologija finno-ugorskih narodov/Health, Demography, and Ecology of Finno-Ugric Peoples. 2014; 3: 96-8. (in Russian)].
- Salari N., Hosseinian-Far A., Jalali R., Vaisi-Raygani A., Rasoulpoor S., Mohammadi M. et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global. Health. 2020; 16(1): 57. https://dx.doi.org/10.1186/s12992-020-00589-w.
- Meaney S., Leitao S., Olander E.K., Pope J., Matvienko-Sikar K. The impact of COVID-19 on pregnant womens' experiences and perceptions of antenatal maternity care, social support, and stress-reduction strategies. Women Birth. 2022; 35(3): 307-16. https://dx.doi.org/10.1016/j.wombi.2021.04.013.
- Khoury J.E., Atkinson L., Bennett T., Jack S.M., Gonzalez A. Prenatal distress, access to services, and birth outcomes during the COVID-19 pandemic: Findings from a longitudinal study. Early Hum. Dev. 2022; 170: 105606.https://dx.doi.org/10.1016/j.earlhumdev.2022.105606.
- Khoury J.E., Atkinson L., Bennett T., Jack S.M., Gonzalez A. COVID-19 and mental health during pregnancy: The importance of cognitive appraisal and social support. J. Affect. Disord. 2021; 282: 1161-9. https://dx.doi.org/10.1016/j.jad.2021.01.027.
- Fan S., Guan J., Cao L., Wang M., Zhao H., Chen L. et al. Psychological effects caused by COVID-19 pandemic on pregnant women: a systematic review with meta-analysis. Asian J. Psychiatr. 2021; 56: 102533. https://dx.doi.org/10.1016/j.ajp.2020.102533.
- Pariente G., Wissotzky Broder O., Sheiner E., Lanxner Battat T., Mazor E., Yaniv Salem S. et al. Risk for probable post-partum depression among women during the COVID-19 pandemic. Arch. Womens Ment. Health. 2020; 23(6): 767-73. https://dx.doi.org/10.1007/s00737-020-01075-3.
- Bolten M.I., Wurmser H., Buske-Kirschbaum A., Papoušek M., Pirke K.M., Hellhammer D. Cortisol levels in pregnancy as a psychobiological predictor for birth weight. Arch. Womens Ment. Health. 2011; 14(1): 33-41.https://dx.doi.org/10.1007/s00737-010-0183-1.
- Coussons-Read M.E. Effects of prenatal stress on pregnancy and human development: mechanisms and pathways. Obstet. Med. 2013; 6(2): 52-7.https://dx.doi.org/10.1177/1753495X12473751.
- Wastnedge E., Reynolds R., van Boeckel S., Stock S., Denison F., Maybin J. et al. Pregnancy and COVID-19. Physiol. Rev. 2021; 101(1): 303-12.https://dx.doi.org/10.1152/physrev.00024.2020.
- Liu H., Wang L.L., Zhao S.J., Kwak-Kim J., Mor G., Liao A.H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J. Reprod. Immunol. 2020; 139: 103122. https://dx.doi.org/10.1016/j.jri.2020.103122.
- Di Mascio D., Khalil A., Saccone G., Rizzo G., Buca D., Liberati M. et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2020; 2(2): 100107. https://dx.doi.org/10.1016/j.ajogmf.2020.100107.
- Evans P.C., Rainger G.E., Mason J.C., Guzik T.J., Osto E., Stamataki Z. et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020; 116(14): 2177-84.https://dx.doi.org/10.1093/cvr/cvaa230.
- Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020; 17(5): 259-60. https://dx.doi.org/10.1038/s41569-020-0360-5.
- Ayala-Ramírez P., González M., Escudero C., Quintero-Arciniegas L., Giachini F.R., Alves de Freitas R. et al. Severe acute respiratory syndrome coronavirus 2 infection in pregnancy. A non-systematic review of clinical presentation, potential effects of physiological adaptations in pregnancy, and placental vascular alterations. Front. Physiol. 2022; 13: 785274.https://dx.doi.org/10.3389/fphys.2022.785274.
- Han Y., Ma H., Suo M., Han F., Wang F., Ji J. et al. Clinical manifestation, outcomes in pregnant women with COVID-19 and the possibility of vertical transmission: a systematic review of the current data. J. Perinat. Med. 2020; 48(9): 912-24. https://dx.doi.org/10.1515/jpm-2020-0431.
- Lambelet V., Vouga M., Pomar L., Favre G., Gerbier E., Panchaud A. et al. SARS-CoV-2 in the context of past coronaviruses epidemics: Consideration for prenatal care. Prenat. Diagn. 2020; 40(13): 1641-54. https://dx.doi.org/10.1002/pd.5759.
- Mullins E., Evans D., Viner R.M., O'Brien P., Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet. Gynecol. 2020; 55(5): 586-92. https://dx.doi.org/10.1002/uog.22014.
- Della Gatta A.N., Rizzo R., Pilu G., Simonazzi G. Coronavirus disease 2019 during pregnancy: a systematic review of reported cases. Am. J. Obstet. Gynecol. 2020; 223(1): 36-41. https://dx.doi.org/10.1016/j.ajog.2020.04.013.
- Hernandez-Pacheco J.A., Torres-Torres J., Martinez-Portilla R.J., Solis-Paredes J.M., Estrada-Gutierrez G., Mateu-Rogell P. et al. sFlt-1 is an independent predictor of adverse maternal outcomes in women with SARS-CoV-2 infection and hypertensive disorders of pregnancy. Front. Med. (Lausanne). 2022; 9: 894633. https://dx.doi.org/10.3389/fmed.2022.894633.
- Wei S.Q., Bilodeau-Bertrand M., Liu S., Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021; 193(16): E540-8. https://dx.doi.org/10.1503/cmaj.202604.
- Papageorghiou A.T., Deruelle P., Gunier R.B., Rauch S., García-May P.K., Mhatre M. et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am. J. Obstet. Gynecol. 2021; 225(3): e289. https://dx.doi.org/10.1016/J.AJOG.2021.05.014.
- Anton L., Merrill D.C., Neves L.A., Stovall K., Gallagher P.E., Diz D.I. et al. Activation of local chorionic villi angiotensin II levels but not angiotensin (1-7) in preeclampsia. Hypertension. 2008; 51(4): 1066-72.https://dx.doi.org/10.1161/HYPERTENSIONAHA.107.103861.
- Gilbert J.S., LaMarca B.B., Granger J.P. ACE2 and ANG-(1-7) in the gravid uterus: the new players on the block. Am. J. Physiol. 2008; 294(3): 915-6. https://dx.doi.org/10.1152/ajpregu.00018.2008.
- Mendoza M., Garcia-Ruiz I., Maiz N., Rodo C., Garcia-Manau P., Serrano B. et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020; 127(11): 1374-80.https://dx.doi.org/10.1111/1471-0528.16339.
- Shchegolev A.I., Kulikova G.V., Lyapin V.M., Shmakov R.G., Sukhikh G.T. The number of syncytial knots and VEGF expression in placental villi in parturient woman with COVID-19 depends on the disease severity. Bull. Exp. Biol. Med. 2021; 171(3): 399-403. https://dx.doi.org/10.1007/s10517-021-05236-x.
- Dubova E.A., Pavlov K.A., Lyapin V.M., Shchyogolev A.I., Sukhikh G.T. Vascular endothelial growth factor and its receptors in the placental villi of pregnant patients with pre-eclampsia. Bull. Exp. Biol. Med. 2013; 154(6): 792-5.https://dx.doi.org/10.1007/s10517-013-2058-8.
- Sisman J., Jaleel M.A., Moreno W., Rajaram V., Collins R.R.J., Savani R.C. et al. Intrauterine transmission of SARS-COV-2 infection in a preterm infant. Pediatr. Infect. Dis. J. 2020; 39(9): e265-7. https://dx.doi.org/10.1097/INF.0000000000002815.
- Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., do Cao J. et al. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020; 11(1): 3572. https://dx.doi.org/10.1038/s41467-020-17436-6.
- Sukhikh G., Petrova U., Prikhodko A., Starodubtseva N., Chingin K., Chen H., Bugrova A., Kononikhin A., Bourmenskaya O., Brzhozovskiy A.., Polushkina E., Kulikova G., Shchegolev A., Trofimov D., Frankevich V., Nikolaev E., Shmakov R.G. Vertical transmission OF SARS-COV-2 in second trimester associated with severe neonatal pathology. Viruses. 2021; 13(3): 447.https://dx.doi.org/10.3390/v13030447.
- Hsu A.L., Guan M., Johannesen E., Stephens A.J., Khaleel N., Kagan N. et al. Placental SARS-CoV-2 in a pregnant woman with mild COVID-19 disease. J. Med. Virol. 2021; 93(2): 1038-44. https://dx.doi.org/10.1002/jmv.26386.
- Oltean I., Tran J., Lawrence S., Ruschkowski B.A., Zeng N., Bardwell C. et al. Impact of SARS-CoV-2 on the clinical outcomes and placental pathology of pregnant women and their infants: a systematic review. Heliyon. 2021; 7(3): e06393. https://dx.doi.org/10.1016/j.heliyon.2021.e06393.
- Щеголев А.И., Туманова У.Н., Серов В.Н. Поражения плаценты у беременных с SARS-CoV-2-инфекцией. Акушерство и гинекология. 2020: 12: 44-52. https://dx.doi.org/10.18565/aig.2020.12.44-52. [Shchegolev A.I., Tumanova U.N., Serov V.N. Placental lesions in pregnant women with SARS-COV-2 infection. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2020; 12: 44-52. (in Russian)] https://dx.doi.org/10.18565/aig.2020.12.44-52.
- Boelig R.C., Aghai Z.H., Chaudhury S., Kazan A.S., Chan J.S.Y., Bergmann-Leitner E. Impact of COVID-19 disease and COVID-19 vaccination on maternal or fetal inflammatory response, placental pathology, and perinatal outcomes. Am. J. Obstet. Gynecol. 2022: S0002-9378(22)00414-8.https://dx.doi.org/10.1016/j.ajog.2022.05.049.
- Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020; 154(1): 23-32. https://dx.doi.org/10.1093/ajcp/aqaa089.
- Meyer J., Roman A., Limaye M., Grossman T., Flaifel A., Vaz M. et al. Association of SARS-CoV-2 placental histopathology findings with maternal fetal comorbidities and severity of COVID-19 hypoxia. J. Matern. Fetal Neonatal Med. 2021 Sep. 20: 1-7.https://dx.doi.org/10.1080/14767058.2021.1977791.
- Edlow A.G., Li J.Z., Collier A.R.Y., Atyeo C., James K.E., Boatin A.A. et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open. 2020; 3(12): e2030455.https://dx.doi.org/10.1001/jamanetworkopen.2020.30455.
- Щеголев А.И., Ляпин В.М., Туманова У.Н., Воднева Д.Н., Шмаков Р.Г. Гистологические изменения плаценты и васкуляризация ее ворсин при ранней и поздней преэклампсии. Архив патологии. 2016; 78(1): 13-8. https://dx.doi.org/10.17116/patol201678113-18. [Shchegolev A.I., Lyapin V.M., Tumanova U.N., Vodneva D.N., Shmakov R.G. Histological changes in the placenta and vascularization of its villi in early- and late-onset preeclampsia. Arhiv patologii/Pathology Archive. 2016; 78(1): 13-8.(in Russian)] https://dx.doi.org/10.17116/patol201678113-18.
- Щеголев А.И., Туманова У.Н., Ляпин В.М., Серов В.Н. Синцитиотрофобласт ворсин плаценты в норме и при преэклампсии. Акушерство и гинекология. 2020; 6: 21-8. https://dx.doi.org/10.18565/aig.2020.6.21-28. [Shchegolev A.I., Tumanova U.N., Lyapin V.M., Serov V.N. The syncytiotrophoblast of the placental villi in health and in preeclampsia. Akusherstvo i ginekologiya/Obstetrics and Gynecology. 2020; 6: 21-8. (in Russian)]https://dx.doi.org/10.18565/aig.2020.6.21-28.
- Shchegolev A.I., Kulikova G.V., Tumanova U.N., Shmakov R.G., Sukhikh G.T. Morphometric parameters of placental villi in parturient women with COVID-19. Bull. Exp. Biol. Med. 2021; 172(1): 85-9.https://dx.doi.org/10.1007/s10517-021-05337-7.
- Shchyogolev A.I., Dubova E.A., Pavlov K.A., Lyapin V.M., Kulikova G.V., Shmakov R.G. Morphometric characteristics of terminal villi of the placenta in pre-eclampsia. Bull. Exp. Biol. Med. 2012; 154(1): 92-5.https://dx.doi.org/10.1007/s10517-012-1883-5.
- Туманова У.Н., Щеголев А.И. Поражения плаценты в генезе мертворождения (обзор литературы). Международный журнал прикладных и фундаментальных исследований. 2017; 3(ч. 1): 77-81. [Tumanova U.N., Shchegolev A.I. Placental lesions as the cause of stillbirth (review). Mezhdunarodnyj zhurnal prikladnyh i fundamental'nyh issledovanij/ International Journal of Applied and Basic Research. 2017; 3 (Part 1): 77-81. (in Russian)].
- Щеголев А.И., Туманова У.Н., Шувалова М.П., Фролова О.Г. Сравнительный анализ мертворождаемости в Российской Федерации в 2010 и 2012 годах. Российский вестник перинатологии и педиатрии. 2015; 3: 58-62. [Shchegolev A.I., Tumanova U.N., Shuvalova M.P., Frolova O.G. Comparative analysis of stillbirth rates in the Russian Federation in 2010 and 2012. Rossijskij vestnik perinatologii i pediatrii/ Russian Bulletin of Perinatology and Pediatrics. 2015; 3: 58-62. (in Russian)].
- Щеголев А.И., Павлов К.А., Дубова Е.А., Фролова О.Г. Ранняя неонатальная смертность в Российской Федерации в 2010г. Архив патологии. 2013; 75(4): 15-9. [Shchegolev A.I., Pavlov K.A., Dubova E.A., Frolova O.G. Early neonatal mortality in the Russian Federation in 2010. Arhiv patologii/Pathology Archive. 2013;(75)4: 15-19. (in Russian)].
- Щеголев А.И., Туманова У.Н., Шувалова М.П. Роль хориоамнионита в генезе мертворождения. Международный журнал прикладных и фундаментальных исследований. 2017; 2(ч. 2): 205-9. [Shchegolev A.I., Tumanova U.N., Shuvalova M.P. The role of chorioamnionitis at the cause of stillbirth. Mezhdunarodnyj zhurnal prikladnyh i fundamental'nyh issledovanij/ International Journal of Applied and Basic Research. 2017; 2 (Part 2): 205-9. (in Russian)].
Received 21.08.2022
Accepted 10.10.2022
About the Authors
Alexander I. Shchegolev, Dr. Med. Sci., Professor, Head of the 2nd Department of Anatomic Pathology, Academician V.I. Kulakov National Medical Research Centerfor Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, ashegolev@oparina4.ru, https://orcid.org/0000-0002-2111-1530,
4, Oparina str., Moscow, 117997, Russia.
Uliana N. Tumanova, Dr. Med. Sci., Leading Researcher at the 2nd Department of Anatomic Pathology, Academician V.I. Kulakov National Medical Research Center
for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, u.n.tumanova@gmail.com, https://orcid.org/0000-0002-0924-6555,
4, Oparina str., Moscow, 117997, Russia.
Andrey A. Chausov, Head of the Information and Analytical Center of the Department of regional cooperation and integration, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, a_chausov@oparina4.ru, https://orcid.org/0000-0002-3094-7209,
4, Oparina str., Moscow, 117997, Russia.
Marina P. Shuvalova, Ph.D., Associate Professor, Deputy director – Head of the Department of regional cooperation and integration, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, m_shuvalova@oparina4.ru, https://orcid.org/0000-0002-6361-9383,
4, Oparina str., Moscow, 117997, Russia.
Authors’ contributions: Shchegolev A.I. – conception and design of the study, Rosstat data analysis and summary, review of the relevant literature, manuscript editing; Tumanova U.N. – Rosstat data analysis, search and review of the relevant literature, manuscript drafting; Chausov A.A. – Rosstat data analysis, statistical analysis; Shuvalova M.P. – Rosstat data analysis, manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Shchegolev A.I., Tumanova U.N., Chausov A.A., Shuvalova M.P.
Stillbirths in the Russian Federation in 2020 (COVID-19 pandemic year).
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 11: 131-140 (in Russian)
https://dx.doi.org/10.18565/aig.2022.11.131-140



