Matrix metalloproteinase-9 as a potential marker of preterm birth
Skorobogatova O.V., Belousova V.S., Ignatko I.V., Zarova E.A., Bogomazova I.M., Pesegova S.V., Kardanova M.A., Kuzmina T.E.
Relevance: Preterm birth (PB) is a significant issue worldwide as it is the leading cause of perinatal morbidity and mortality. International statistics indicate that 5–18% of pregnancies result in PB.
Objective: This study aimed to investigate changes in blood plasma and cervicovaginal secretion concentrations of matrix metalloproteinase-9 (MMP-9) in pregnant women with threatened PB, as well as the dynamics of MMP-9 concentrations in response to tocolytic therapy with hexoprenaline.
Materials and methods: We examined 45 pregnant women with threatened PB. The control group consisted of 40 women with healthy pregnancies. The inclusion criterion was a singleton spontaneous pregnancy at 24–31 weeks and 6 days of gestation, with signs of threatened PB. Exclusion criteria were multiple pregnancies, acute phase or exacerbation of chronic diseases, history of cervical pathology, cancer and autoimmune diseases, preeclampsia and its complications, chromosomal abnormalities and congenital fetal malformations, pregnancies resulting from assisted reproductive technologies, and premature rupture of membranes.
Results: Blood plasma MMP-9 concentrations in women with threatened PB and those with normal pregnancies were virtually identical, measuring 116 and 120 ng/ml, respectively. In patients with threatened PB, the mean cervical secretion concentration of MMP-9 was 6.48 pc/g. After tocolytic therapy with hexoprenaline, the concentration decreased to 1.5 pc/g, which was comparable to the cervical secretion concentration of MMP-9 in pregnant women in the control group (1.58 pc/g).
Conclusion: Our study demonstrated that blood plasma MMP-9 cannot serve as a marker of PB because we did not observe any changes in its concentration during threatened PB. However, MMP-9 in cervicovaginal fluid may be considered a marker of PB, as its concentration increases in threatened PB and decreases in response to tocolytic therapy with hexoprenaline, reaching levels typical of a normal pregnancy.
Authors' contributions: Skorobogatova O.V., Belousova V.S., Ignatko I.V. – conception and design of the study; Skorobogatova O.V., Pesegova S.V., Zarova E.V., Bogomazova I.M. – material collection and processing; Zarova E.A., Kuzmina T.E. – statistical analysis; Skorobogatova O.V., Belousova V.S. – drafting of the manuscript; Belousova V.S., Kardanova M.A. – editing of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was conducted within the framework of the scientific and qualifying work of O.V. Skorobogatova. The study was reviewed and approved by the Research Ethics Committee of the I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University) (Ref. No: 22-22 of November 3, 2022) and the Research Ethics Committee of the S.S. Yudin Clinical Hospital (Ref. No: 9 of December 13, 2022).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Skorobogatova O.V., Belousova V.S., Ignatko I.V., Zarova E.A., Bogomazova I.M., Pesegova S.V.,
Kardanova M.A., Kuzmina T.E. Matrix metalloproteinase-9 as a potential marker of preterm birth.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (7): 74-80 (in Russian)
https://dx.doi.org/10.18565/aig.2024.66
Keywords
Preterm birth (PB) is a pressing issue in the modern world. Preterm birth is defined as birth occurring between 220 and 366 weeks of pregnancy. It is the leading cause of perinatal morbidity and mortality. According to international health statistics, 5–18% of pregnancies end in PB [1]. Nursing premature babies remains a serious medical and social problem that requires the use of modern, expensive technologies.
Currently, it is recommended to measure cervical length using ultrasound cervicometry and to perform a test for phosphorylated protein-1 in cervical secretions (Actim Partus test) to predict PB, including in high-risk patients [2]. However, the predictive value of cervicometry does not exceed 35–40%, and 46–58% for the Actim Partus test [3]. Several other tests are used to detect PB markers. One of them is a combined test for the determination of interleukin-6 and insulin-like growth factor-1 binding protein, both total and native (PremaQuick Test). The sensitivity and specificity of this test are 87.1% and 92.4%, respectively [4]. Another test is available for the determination of placental alpha microglobulin-1 (PartoSure Test), with sensitivity and specificity of 84% and 95%, respectively [5].
However, the incidence of PB does not show a decreasing trend; therefore, the search for a highly sensitive and specific marker of threatening PBs remains highly relevant.
During childbirth, both at term and preterm, the cervix undergoes remodeling due to the disorganization and breakdown of collagen fibers. The cervix is mainly composed of connective tissue with collagen as its main component. It is the "rigid structure" of collagen that allows the cervix to perform the important function of retaining the fertilized egg in the uterine cavity during pregnancy. As the cervix ripens, collagen and elastic fibers degrade, fluid flow increases, and a local inflammatory response develops. Cervical remodeling occurs under the influence of matrix metalloproteinases (MMPs) [6].
MMPs are a family of zinc- and calcium-dependent endopeptidases that can degrade components of the extracellular matrix of connective tissues. Fibroblasts, macrophages, and neutrophils are sources of MMPs. Currently, 28 MMPs are known, and their role is being studied in various physiological and pathological processes, including trophoblast invasion, spiral artery remodeling, cervical ripening, and rupture of membranes [7].
MMP-9, also known as gelatinase, is a type-IV collagenase. It is produced by various cells including epithelial cells, fibroblasts, keratinocytes, osteoblasts, dendritic cells, macrophages, granulocytes, and T cells. MMP-9 is involved in inflammation, tissue remodeling, repair, and mobilization of matrix-related growth factors and affects the development of PB. Increased concentrations of MMP-9 cause rupture of membranes, myometrial contraction, and cervical remodeling. According to the literature, the concentration of MMP-9 in maternal blood plasma remains constant during uncomplicated pregnancy, and during cervicovaginal secretion, MMP-9 is present at very low concentrations and increases with the onset of cervical remodeling [8].
This study aimed to investigate the concentrations of MMP-9 in the blood plasma and cervicovaginal secretion of pregnant women with threatened PB to evaluate MMP-9 as a potential marker of PB.
Materials and methods
This prospective study included 85 women with a singleton pregnancy. The study group consisted of 45 pregnant women with signs of threatened PB at gestational ages 240–316 weeks. The control group comprised 40 women with physiological pregnancies at the same gestational age.
All pregnant women were observed in the Department of Pregnancy Pathology at the Perinatal Center at the S.S. Yudin City Clinical Hospital from 2022 to 2024.
The inclusion criterion was a singleton spontaneous pregnancy at 240–316 weeks with signs of threatened PB, including cervical length shortening to less than 25 mm and lower abdominal pain.
The exclusion criteria were multiple pregnancies, acute phase or exacerbation of chronic diseases, history of cervical pathology, cancer and autoimmune diseases, preeclampsia and its complications, chromosomal abnormalities and congenital malformations of the fetus, pregnancy resulting from assisted reproductive technologies, and premature rupture of membranes. The gestational age was determined by the first day of the last menstruation as well as by ultrasound screening in the first and second trimesters of pregnancy.
On admission to the hospital, cervicovaginal fluid was collected during speculum examination of the cervix using a sterile universal probe. The probe was inserted into the cervical canal and left in the cervical os for 10–15 s to absorb a sufficient amount of discharge. The tip of the probe was placed in an Eppendorf tube containing a saline solution. Venous blood samples were collected in tubes containing a coagulation activator. The blood was then centrifuged, and the resulting plasma was collected in 0.7 ml Eppendorf tubes. The plasma and cervical fluid were frozen until the study.
After hospitalization, all pregnant women received tocolytic therapy with hexoprenaline, pathogenetic therapy with micronized progesterone, and prevention of fetal respiratory distress syndrome with dexamethasone. Cervicovaginal fluid and blood plasma were collected again at the end of tocolytic therapy with hexoprenaline to assess changes in MMP-9 levels after tocolytic therapy. Pregnant women in the control group underwent a single collection at hospital admission.
An enzyme-linked immunosorbent assay was performed to determine the concentration of MMP-9 protein. Standard FineTest enzyme-linked immunosorbent assay kits "Human MMP-9 (Matrix Metalloproteinase 9) ELISA Kit" were used according to the manufacturer’s instructions. Protein concentrations were evaluated in the blood plasma and cervical lavage.
The study was reviewed and approved by the Research Ethics Committee of the I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University) (Ref. No: 22-22 of November 3, 2022) and the Research Ethics Committee of the S.S. Yudin Clinical Hospital (Ref. No: 9 of December 13, 2022).
Statistical analysis
Statistical analysis was performed using Microsoft Office Excel 2016 and StatTech v. 4.1.2 (Stattech LLC, Russia). Data distributions were tested for normality using the Shapiro–Wilk test. Continuous variables with a normal distribution are expressed as mean (M) and standard deviation (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) is reported. Categorical variables are reported as frequencies and percentages. Differences between groups for continuous variables were assessed using the nonparametric Mann–Whitney U test. Comparisons of percentages in the analysis of 2×2 contingency tables were performed using the Pearson chi-square test (when the expected frequency was >10) or Fisher's exact test (when the expected frequency was <10). Differences were considered statistically significant at p<0.05.
Results
The study group included 45 pregnant women at 28 weeks of gestation with signs of threatened PB. The control group comprised 40 women with healthy pregnancies. The baseline evaluation was based on medical history and laboratory and instrumental studies. The medical history data of patients in both groups are presented in the Table.
The median age of patients in both groups did not differ statistically and was 31 (26; 37) years and 29.5 (25;36) years, respectively (Mann–Whitney U test). In pregnant women with threatened PB, various somatic diseases were identified 2.5 times more often including lower extremity varicose veins (8.89% and 5%; p=0.67), genetic thrombophilia (4.44% and 0%; p=0.49), and gestational diabetes mellitus (17.5% and 5%; p=0.16) (Table).
A history of PB was reported in only 7/45 patients in the study group (15.6 %). Complicated obstetric history was 2.22 times more common in pregnant women in the study group (44.4% and 20%, respectively; p=0.001) (Table).
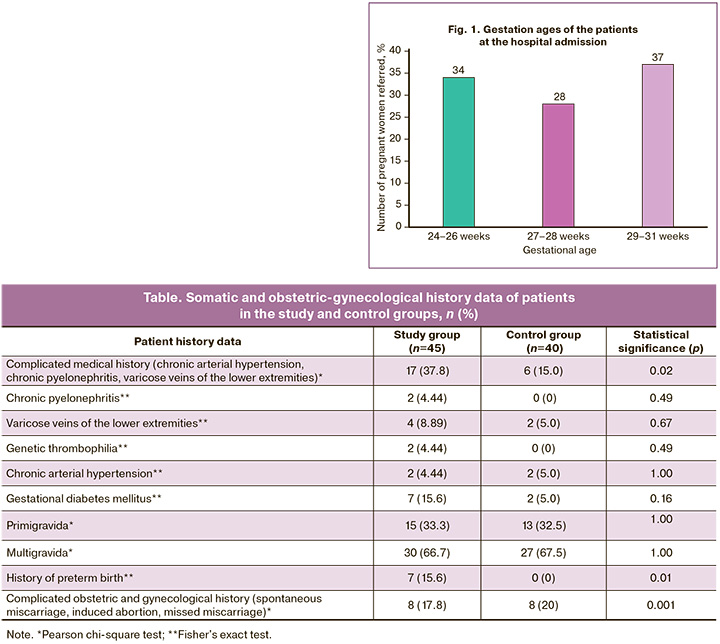
The distribution of patients by gestational age at hospital admission is presented in Figure 1: 29/85 (34%) women were admitted at 24–26 weeks of pregnancy, 24/85 (28%) at 27–28 weeks, and 32/85 (37%) patients at 29–31 weeks.
We found no differences in the mean blood plasma concentrations of MMP-9 between women in the study and control groups. The mean blood plasma concentration of MMP-9 in women with threatened PB was 116.9 ng/ml (1.63) and 120.14 ng/ml (26.9) in the control group (Fig. 2).
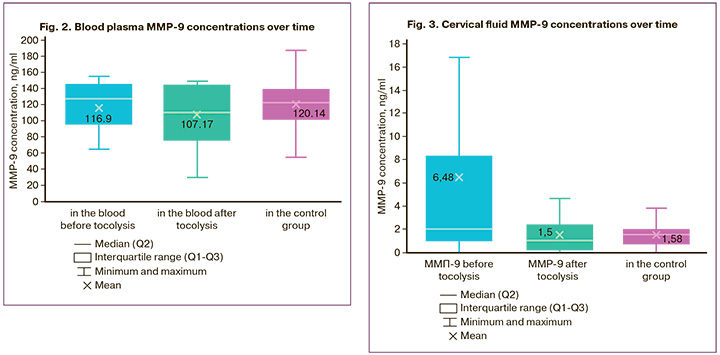
After tocolytic therapy in patients with threatened PB, there was no change in the mean blood plasma MMP-9 concentration, it was 107.17 ng/ml (34.1). The cervical fluid MMP-9 concentrations are shown in Figure 3.
When studying the cervical fluid MMP-9 concentration, we found statistically significant differences between women in the study and control groups. Thus, in patients with threatened PB, the mean cervical fluid MMP-9 concentration was 4.3 times higher than in women with a normal pregnancy: 6.48 (12.23) pc/g and 1.58 (1.05) pc/g, respectively (p=0.02). We also assessed the dynamics of changes in the cervical fluid MMP-9 concentration after tocolysis with hexoprenaline. A statistically significant more than 4-fold decrease in the mean cervical fluid MMP-9 concentration was revealed – from 6.48 pc/g to 1.5 pc/g after completion of tocolytic therapy. And the concentration of MMP-9 after tocolysis was comparable to the concentration of MMP-9 in pregnant women with an uncomplicated pregnancy: 1.5 (1.63) pc/g and 1.58 (1.05) pc/g, respectively (Fig. 3).
Discussion
Predicting preterm birth is a crucial task in modern obstetrics as it allows for timely intervention to prevent the birth of a preterm baby. The search for reliable biochemical markers that can accurately predict PB is a priority in this field. However, to date, no well-studied biochemical marker has been identified. Therefore, exploration of new biochemical markers is a promising avenue for research in modern obstetrics.
The objective of our study was to investigate the role of MMP-9 in the development of PB and to assess its potential as a marker of threatened PB. We measured the concentrations of MMP-9 in the blood plasma and cervicovaginal fluid of pregnant women with threatened PB and compared these concentrations with those in women experiencing a normal pregnancy. We also analyzed changes in MMP-9 concentrations before and after tocolytic therapy with hexoprenaline.
We chose to study MMP-9 because previous research has shown that its concentration is low during an uncomplicated pregnancy but significantly increases during a threatened pregnancy.
Our study revealed that blood plasma MMP-9 concentrations did not differ significantly between patients with uncomplicated pregnancies and those with threatened PB. Therefore, we concluded that blood plasma MMP-9 concentration cannot serve as a marker for PB.
In contrast, we found statistically significant differences in the mean MMP-9 concentration in cervical fluid between the study and control groups. Specifically, the concentration of MMP-9 in women with threatened PB was 4.3 times higher than that in women with normal pregnancy (6.48 pc/g and 1.58 pc/g; p=0.02). After tocolytic therapy, the concentration of MMP-9 significantly decreased and became comparable to that in women with normal pregnancy.
Conclusion
Our study demonstrated that the concentration of MMP-9 in blood plasma cannot serve as a marker for PB, as it remains relatively unchanged during PB and normal pregnancy. However, the cervical fluid MMP-9 concentration increases during threatened PB and decreases to levels observed in normal pregnancy following tocolytic therapy with hexoprenaline. Therefore, the assessment of MMP-9 concentration in cervicovaginal fluid shows promise as a potential marker of PB.
References
- Mandy G.T., Weisman L.E., Kim M.S. Incidence and mortality of the preterm infant. UpToDate. Available at: http://www.uptodate.com/contents/incidence-and-mortality-of-the-preterm-infant?search=Incidence%20and%20mortality%20of%20the%20preterm%20infant&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Преждевременные роды. 2020. [Ministry of Health of the Russian Federation. Clinical guidelines. Preterm birth. 2020. (in Russian)].
- Ting H.S., Chin P.S., Yeo G.S., Kwek K. Comparison of bedside test kits for prediction of preterm delivery: phosphorylated insulin-like growth factor binding protein-1 (pIGFBP-1) test and fetal fibronectin test. Ann. Acad. Med. Singap. 2007; 36(6): 399-402.
- Eleje G.U., Ezugwu E.C., Eke A.C., Eleje L.I., Ikechebelu J.I., Ezebialu I.U. et al. Accuracy of a combined insulin-like growth factor-binding protein-1/interleukin-6 test (Premaquick) in predicting delivery in women with threatened preterm labor. J. Perinat. Med. 2017; 45(8): 915-24. https://dx.doi.org/10.1515/jpm-2016-0339.
- Дикке Г.Б. Диагностика высокого риска преждевременных родов на основании биохимических тестов. Акушерство и гинекология. 2018; 7: 108-13. [Dikke G.B. Biochemical tests for the diagnosis of high risk of preterm birth. Obstetrics and Gynecology. 2018; (7): 108-13. (in Russian)].https://dx.doi.org/10.18565/aig.2018.7.108-113.
- Socha M.W., Flis W., Pietrus M., Wartęga M., Stankiewicz M. Signaling pathways regulating human cervical ripening in preterm and term delivery. Cells. 2022; 11(22): 3690. https://dx.doi.org/10.3390/cells11223690.
- Hulboy D.L., Rudolph L.A., Matrisian L.M. Matrix metalloproteinases as mediators of reproductive function. Mol. Hum. Reprod. 1997; 3(1): 27-45. https://dx.doi.org/10.1093/molehr/3.1.27.
- Dymanowska-Dyjak I., Stupak A., Kondracka A., Gęca T., Krzyżanowski A., Kwaśniewska A. Elastography and metalloproteinases in patients at high risk of preterm labor. J. Clin. Med. 2021; 10(17): 3886. https://dx.doi.org/10.3390/jcm10173886.
Received 20.03.2024
Accepted 27.06.2024
About the Authors
Oksana V. Skorobogatova, PhD student at the Department of Obstetrics, Gynecology and Perinatology of the Institute of Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), 119048, Russia, Moscow, Trubetskaya str., 8-2, +7(915)682-85-03, aisha27_sum@mail.ru,https://orcid.org/0009-0009-8664-3536
Vera S. Belousova, Dr. Med. Sci., Professor at the Department of Obstetrics, Gynecology and Perinatology of the Institute of Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), 119048, Russia, Moscow, Trubetskaya str., 8-2, +7(903)715-45-02, belousova_v_s@staff.sechenov.ru,
https://orcid.org/0000-0001-8332-7073
Irina V. Ignatko, Dr. Med. Sci., Corresponding Member of the RAS, Professor, Head of the Department of Obstetrics, Gynecology and Perinatology of the Institute of
Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), 119048, Russia, Moscow, Trubetskaya str., 8-2, +7(903)715-45-02,
ignatko_i_v@staff.sechenov.ru, https://orcid.org/0000-0002-9945-3848
Evdokia A. Zarova, Student at the I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), 119048, Russia, Moscow, Trubetskaya str., 8-2,
+7(903)715-45-02, zarovaea@mail.ru, https://orcid.org/0000-0003-4693-6886
Irina M. Bogomazova, PhD, Associate Professor at the Department of Obstetrics, Gynecology and Perinatology of the Institute of Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), 119048, Russia, Moscow, Trubetskaya str., 8-2, +7(903)715-45-02, bogomazova_i_m@staff.sechenov.ru,
https://orcid.org/0000-0003-1156-7726
Svetlana V. Pesegova, PhD, Teaching Assistant at the Department of Obstetrics, Gynecology and Perinatology of the Institute of Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), 119048, Russia, Moscow, Trubetskaya str., 8-2, +7(903)715-45-02, pesegova_s_v@staff.sechenov.ru,
https://orcid.org/0000-0002-1339-5422
Madina A. Kardanova, PhD, Associate Professor at the Department of Obstetrics, Gynecology and Perinatology of the Institute of Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), 119048, Russia, Moscow, Trubetskaya str., 8-2, kardanova_m_a@staff.sechenov.ru,
https://orcid.org/0000-0002-4315-0717
Tatyana E. Kuzmina, PhD, Associate Professor at the Department of Obstetrics, Gynecology and Perinatology of the Institute of Clinical Medicine, I.M. Sechenov First MSMU, Ministry of Health of Russia (Sechenov University), 119048, Russia, Moscow, Trubetskaya str., 8-2, +7(903)715-45-02, kuzmina_t_e@staff.sechenov.ru,
https://orcid.org/0000-0001-9649-5383
Corresponding author: Vera S. Belousova, belousova_v_s@staff.sechenov.ru



