The role of magnetic resonance imaging in evaluating the effectiveness of neoadjuvant chemotherapy in patients with locally advanced cervical cancer
Aim. To investigate the role of magnetic resonance imaging (MRI) in evaluating the effectiveness of neoadjuvant chemotherapy in patients with locally advanced cervical cancer.Ovodenko D.L., Bychenko V.G., Khabas G.N., Akinfiev D.M., Makarova A.S., Golitsyna Yu.S., Seregin A.A., Ashrafyan L.A.
Materials and methods. The study comprised 67 patients with locally advanced cervical cancer undergoing neoadjuvant chemotherapy followed by radical hysterectomy in those, who achieved a response. Clinical evaluation included general clinical examination and MRI.
Results. The reduction of the tumor volume in patients with different cancer stages ranged from 23.2 to 52.1% according to clinical examination and MRI. Fifty-nine patients (88.1%) underwent surgery after neoadjuvant chemotherapy, including all 17patients with stages IB2/IIA2, and 31 (91.2%) and 11 (68.8%) patients with stage IIB and IIIB cervical cancer, respectively.
Conclusion. Neoadjuvant chemotherapy for locally advanced cervical cancer results in the tumor response that allows radical surgery to be undertaken. MRI can be used to obtain objective data on changes in the cervical tumor volume during neoadjuvant chemotherapy.
Keywords
Cervical cancer is one of the most common female malignancies encountered by a gynecologic oncologist [1]. The diagnosis is made using a complex of clinical and morphological methods according to the FIGO guidelines, including a pelvic examination, colposcopy, cervical smear and biopsy cytology, and endocervical curettage. Clinical staging is performed using only clinical criteria according to the FIGO classification system [2]. Currently, in patients with early-stage cervical cancer, good outcomes may be achieved using both surgery and radiation therapy with the five-year overall survival rate about 93% [3-5]. In locally advanced disease, long-term outcomes are not fully satisfactory; the recurrence rates increase as the stage advances, reaching, according to various authors, 54–91% [6-8].
Over the past few decades, numerous studies have demonstrated survival benefits of neoadjuvant chemotherapy, followed by radical surgery in locally advanced cervical cancer. [9-11]. This approach requires careful patient monitoring by obtaining objectively documented information at all treatment stages. Only physical examination does not always provide an accurate assessment of the tumor volume. Thus, in some cases, the clinician faces difficulties in determining the true tumor size of cervical neoplasms, the degree of parametrial involvement, and the presence of pelvic node metastases [12].
To date, several studies have been performed to investigate the use of magnetic resonance imaging (MRI) for assessing the local spread of cervical cancer. Interest in this type of diagnostic imaging is due to several advantages over other imaging modalities including high tissue specificity and topographic accuracy, non-invasiveness and safety (due to the absence of ionizing radiation), the possibility of simultaneous multi‐slice acquisition of any organ of concern and obtaining images in various planes. As a result, it becomes possible to identify with sufficient accuracy the boundaries between normal and pathological tissues and, therefore, to obtain tumor images of many anatomical locations. According to the literature, the sensitivity and specificity of MRI in assessing parametrial invasion ranges from 44 to 92%, and the overall accuracy in staging invasive cervical cancer is 77–90% [13-15].
This study aimed to investigate the role of MRI in assessing the effectiveness of neoadjuvant chemotherapy in patients with locally advanced cervical cancer.
Materials and methods
 In this study, we analyzed medical records of 67 patients with locally advanced cervical cancer, who were treated at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia from 2012 to 2017.
In this study, we analyzed medical records of 67 patients with locally advanced cervical cancer, who were treated at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia from 2012 to 2017.
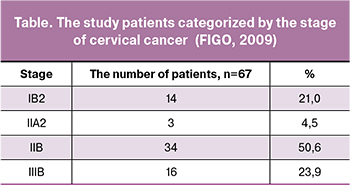
Morphological variants of neoplasms were represented by keratinizing (52.0%) and non-keratinizing (48.0%) subtypes of squamous cell carcinoma. The study cohort categorized by the disease stages is presented in the table.
Before treatment initiation and at its all stages, patients underwent clinical diagnostic evaluation and MRI. Multiparametric MRI included T2-weighted imaging (T2WI), T1-weighted imaging (cT1WI), and dynamic contrast-enhanced imaging. The parametrial invasion was determined by the tumor spread beyond the cervix on DWI and post-contrast scans.
All patients received neoadjuvant chemotherapy consisted of intravenous paclitaxel 175 mg/m2 and carboplatin ½AUC 6 with the supportive treatment, including standard sedation and antiemetics. On day two, they were given an intra-arterial injection of the remaining ½ carboplatin AUC 6 in with concurrent uterine artery embolization.
The manipulation was performed under spinal-epidural anesthesia in the interventional radiology operating room of the radiology department. Patients were catheterized through the right femoral artery using the Seldinger technique, and a fluoroscopy-guided catheter was placed directly into the aorta at the bifurcation. Before injecting cytostatic agents and embolic material, the patients underwent diagnostic pelvic angiography. After studying the pelvic vascular anatomy, the left and the right uterine artery were sequentially catheterized followed by injection of carboplatin ½ AUC 6 and 100-μm PVA particles as an embolic agent. Immediately after completion of the procedure, the patients underwent control angiography.
The treatment response was evaluated on day 14 after the administration of cytostatic agents. Patients with a sufficient reduction in total tumor size underwent radical surgery. A tumor measuring less than 50 cm3 was considered resectable.
The response to chemotherapy was determined by the change in the tumor volume, which was calculated by the formula V = A × B × C × 0.52, where A, B, C are the tumor sizes in three orthogonal planes obtained by physical examination or MRI. Since parametrial infiltrates are considered a part of the primary tumor, they were taken into account when calculating the cervical tumor volume. The response to neoadjuvant chemotherapy was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [16].
Statistical analysis. Continuous variables are presented as mean (M) and the standard deviation (SD) using M (SD) format. The relation between tumor volumes obtained using clinical examination and MRI was assessed with a nonparametric Spearman correlation coefficient. Statistical significance was assumed for p < 0.05. All analyses were performed using Statistica 10 (StatSoft) statistical software
Results
The cervical tumor volume, determined by physical examination, ranged from 28.1 to 131.0 [46.5 (19.4)], 24.2 to 152.9 [61.8 (27.8)], and 35.1 to 152.9 [70.5 (23.6)] cm3 in patients with cervical cancer stage IB2/IIA2, IIB, and IIIB, respectively.
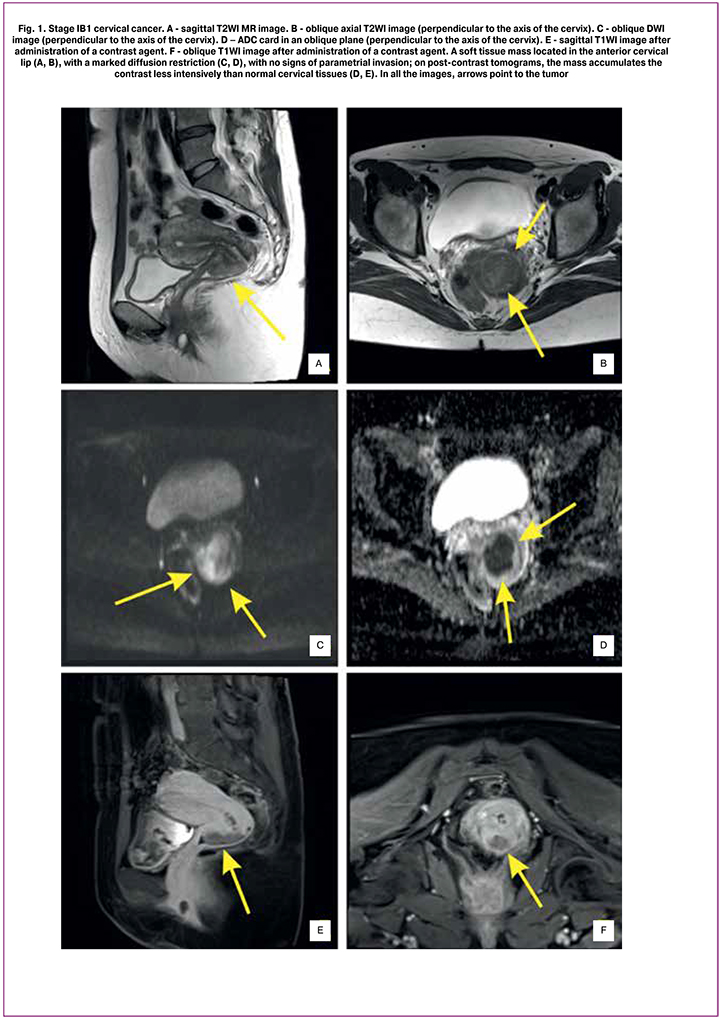
MRI-measured tumor volume before treatment initiation was from 3.0 to 73.3 [36.6 (17.0)], 32.2 to 103.1 [64.9 (19.9)], and 32.2 to 145.1 [72.3 (23.6)] cm3 in patients with cervical cancer stage IB2/IIA2, IIB, and IIIB, respectively (Fig. 1).
Baseline angiography showed a pathological blood vessel network around the cervix, which is indicative of cervical malignancy. In some cases, the abnormal vascular network was visualized outside the cervix, probably due to loco-regional cancer spread. After chemotherapy and tumor embolization, MRI showed a marked blood flow reduction in this area.
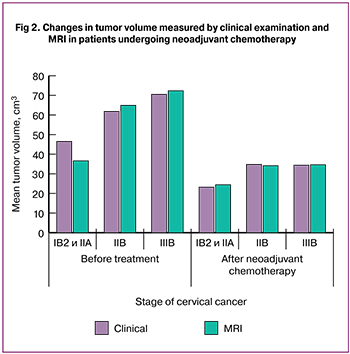 At day 14 after chemotherapy, a physical examination showed that in patients with cervical cancer stages IB2 and IIA2, the tumor volume decreased to 23.2 (16.6) cm3 (a 50.1% decrease). In stages IIB and IIIВ, the tumor volume decreased to 34.8 (18.0) cm3 (a 43.7% decrease) and 34.4 (12.4) cm3 (a 51.2% decrease).
At day 14 after chemotherapy, a physical examination showed that in patients with cervical cancer stages IB2 and IIA2, the tumor volume decreased to 23.2 (16.6) cm3 (a 50.1% decrease). In stages IIB and IIIВ, the tumor volume decreased to 34.8 (18.0) cm3 (a 43.7% decrease) and 34.4 (12.4) cm3 (a 51.2% decrease).
The post-chemotherapy MRI measurements showed that in patients with stages IB2 and IIA2, the tumor volume decreased to 24.4 (19.7) cm3 (a 33.3% decrease). In stages IIB and IIIВ, the tumor volume decreased to 34.1 (16.5) cm3 (a 47.5% decrease) and 34.6 (11.5) cm3 (a 52.1% decrease) (Figures 2–3).
There was a strong direct correlation between the tumor volume, as determined by clinical examination and by MRI in patients with locally advanced cervical cancer before and after neoadjuvant chemotherapy (r = 0.735382).
Evaluation of response by RECIST 1.1criteria showed that 6 (35.3%), 9 (52.9%), and 2 (11.8%) patients with stage IB2 and IIA2 cervical cancer achieved complete, partial, and no response, respectively. Among patients with cervical cancer stage IIB, 7 (20.6%), 23 (67.6%), and 4 (11.7%) had complete, partial, and no response, respectively. None of the patients with stage IIIB cervical cancer achieved complete response; partial and no response was observed in 13 (81.3%) and 3 (18.7%) patients, respectively. We did not observe the disease progression during neoadjuvant chemotherapy.
Patients whose tumor volume after neoadjuvant chemotherapy decreased to less than 50 cm3 underwent type III (C2) radical hysterectomy [17, 18]. A total of 59 patients with locally advanced cervical cancer (88.1%) underwent surgery after neoadjuvant chemotherapy including 17, 31 (91.2%), and 11 (68.8%) patients with stage IB2/IIA2, IIB, and IIIB cervical cancer.

Conclusion
The main criterion for assessment of the tumor resectability in patients with cervical cancer is the tumor volume and parametrial infiltration (if any). The results of the clinical examination of the patient are crucial. Adding MRI to traditional physical examination allows an objective and documented estimate of changes in the cervical tumor volume during chemotherapy. This diagnostic imaging modality plays a very strong supportive role in clarifying the cancer spread in locally advanced cervical cancer, although it cannot be used for early detection purposes in such patients.
Besides, MRI is a modern reliable and relatively accessible diagnostic technique that allows an accurate determination and documented fixation of changes in the cervical tumor volume, which can help assess the effectiveness of neoadjuvant chemotherapy for locally advanced cervical cancer.
Summary:
- The main criterion for the effectiveness of neoadjuvant chemotherapy in patients with locally advanced cervical cancer is the reduction of tumor volume and parametrial infiltration;
- MRI is a modern and informative diagnostic imaging modality for specifying diagnosis in patients with cervical cancer that provides the possibility to assess loco-regional invasion and monitor the tumor volume during neoadjuvant chemotherapy.
References
- Бохман Я.В. Руководство по онкогинекологии. СПб.: 2002. [Bohman Ya.V. Guide oncologic gynecology. SPb; 2002. (in Russian)]
- Wiebe E., Denny L., Thomas G. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 2012; 119(Suppl. 2): S100-9.
- Хохлова С.В., Коломиец Л.А., Кравец О.А., Крикунова Л.И., Морхов К.Ю., Нечушкина В.М., Новикова Е.Г., Телетаева Г.М., Урманчеева А.Ф., Тюляндина А.С. Практические рекомендации по лекарственному лечению рака шейки матки. Практические рекомендации по лечению злокачественных опухолей Российского общества клинической онкологии. М.: RUSSCO; 2016. 4. NCCN News. J. Natl. Compr. Canc. Netw. 2017; 15(1): XXVI-XXVII. [Khokhlova S.V., Kolomiets L.A., Kravets O.A., Krikunova L.I., Morkhov K.Yu., Nechushkina V.M., Novikova E.G., Teletaeva G.M., Urmancheeva A.F., Tyulyandina AS Practical recommendations for the treatment of cervical cancer. Practical recommendations for the treatment of malignant tumors of the Russian Society of Clinical Oncology. M.: RUSSCO; 2016. 4. NCCN News. J. Natl. Compr. Canc. Netw. 2017; 15 (1): XXVI-XXVII. (in Russian)]
- Colombo N., Carinelli S., Colombo A., Marini C., Rollo D., Sessa C., Group E. G.W. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012; 23(Suppl. 7): VII27-32.
- Винокуров В.Л. Рак шейки матки, тела матки и яичников: итоги и перспективы исследований в ЦНИРРИ МИНЗДРАВА РФ. Вопросы онкологии. 2003; 5: 656-62. [Vinokurov V.L. Cancer of the cervix, body of the uterus and ovaries: results and prospects of research in the cnerry of the RF Ministry of Health and Social Development. Questions oncology. 2003; 5:656-62. (in Russian)]
- Горбунова В.В. Оптимизация сочетанной лучевой терапии местнораспространенного рака шейки матки и рецидивов рака яичников с химиотерапией в терапевтическом режиме: дисс. … д-ра мед. наук. М.; 2002. [Gorbunova V.V. Optimization of combined radiation therapy for locally advanced cervical cancer and relapse of ovarian cancer with chemotherapy in a therapeutic regimen: Diss. ... Dr. med sciences. M.; 2002. (in Russian)]
- Limbergen V. Научно-обоснованные рекомендации по проведению лучевой терапии при раке шейки матки. М.: Европейская школа онкологии, Семинар на Красной площади «Современные аспекты онкогинекологии»; 2009: 11-27.
- Benedetti Panici P., Bellati F., Manci N., Pernice M., Plotti F., Di Donato V. et al. Neoadjuvant chemotherapy followed by radical surgery in patients affected by FIGO stage IVA cervical cancer. Ann. Surg. Oncol. 2007; 14(9): 2643-8.
- Ашрафян Л.А., Антонова И.Б., Алешикова О.И., Добровольская Н.Ю., Чазова Н.Л. Диагностические критерии и факторы прогноза эффективности неоадъювантной химиотерапии местно-распространенного рака шейки матки (IIb–IIIb стадии). Опухоли женской репродуктивной системы. 2007; 4: 63-71. [Ashrafyan L.A., Antonova I.B., Aleshikova O.I., Dobrovolskaya N.Yu., Chazova N.L. Diagnostic criteria and predictors of the effectiveness of neoadjuvated chemotherapy for locally advanced cervical cancer (stage IIb–IIIb). Tumors of the female reproductive system. 2007; 4: 63-71. (in Russian)]
- Ашрафян Л.А., Антонова И.Б., Алешикова О.И., Добровольская Н.Ю. Хирургический этап как один из основных компонентов в лечении рака шейки матки IIb—IIIb стадий. Российский онкологический журнал. 2007; 3: 21-5. [Ashrafyan L.A., Antonova I.B., Aleshikova O.I., Dobrovolskaya N.Yu. Surgical stage as one of the main components in the treatment of cervical cancer IIb — IIIb stages. Russian Oncology Journal. 2007; 3: 21-5. (in Russian)]
- Тарачкова Е.В., Стрельцова О.Н., Базаева И.Я., Ахвердиева Г.И., Панов В.О., Кравец О.А., Тюрин И.Е. Возможности магнитно-резонансной томографии в диагностике рака шейки матки. Опухоли женской репродуктивной системы. 2014; 3: 78-85. [Tarachkova E.V., Streltsova ON, Bazayeva I.Ya., Akhverdiyeva G.I., Panov V.O., Kravets O.A., Tyurin I.Ye. The possibilities of magnetic resonance imaging in the diagnosis of cervical cancer. Tumors of the female reproductive system. 2014; 3: 78-85. (in Russian)]
- Bipat S., Glas A.S., van der Velden J., Zwinderman A.H., Bossuyt P.M., Stoker J. Computed tomography and magnetic resonance imaging in staging of uterine cervical carcinoma: a systematic review. Gynecol. Oncol. 2003; 91(1): 59-66.
- Рубцова Н.А., Пузаков К.Б. Роль МРТ в диагностике, планировании и оценке эффективности лечения рака прямой кишки. Российский онкологический журнал. 2012; 3: 42-50. [Rubtsova N.A., Puzakov K.B. The role of MRI in the diagnosis, planning and evaluation of the effectiveness of treatment of colorectal cancer. Russian Oncology Journal. 2012; 3: 42-50. (in Russian.)]
- Kim M., Suh D.H., Kim K., Lee H.J., Kim Y.B., No J.H. Magnetic resonance imaging as a valuable tool for predicting parametrial invasion in stage IB1 to IIA2 cervical cancer. Int. J. Gynecol. Cancer. 2017; 27(2): 332-8.
- Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer. 2009; 45(2): 228-47.
- Piver M.S., Rutledge F., Smith J.P. Five classes of extended hysterectomy for women with cervical cancer. Obstet. Gynecol. 1974; 44(2): 265-72.
- Querleu D., Morrow C.P. Classification of radical hysterectomy. Gynecol. Oncol. 2009; 115(2): 314-5; author reply 315-6.
Received 03.12.2018
Accepted 22.02.2019
About the Authors
Ovodenko Dmitry L., Ph.D., Clinical Care Supervisor at the Department of Innovative Oncology and Gynecology, Oncologist, V.I. Kulakov NMRC for OG&P of Minzdravof Russia. Address: 117997, Russia, Moscow, Academician Oparin St., 4. E-mail: d_ovodenko@oparina4.ru Tel. +7 (495) 531-4444, +79035366886
Bychenko Vladimir G., Ph.D., Head of the Department of Radiology, Radiologist, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Address: 117997, Russia, Moscow, Academician Oparin St., 4. Tel. +7 (495) 438-76-47; E-mail: v_bychenko@oparina4.ru
Khabas Grigoriy N., Ph.D., Head of the Department of Innovative Oncology and Gynecology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Address: 117997, Russia, Moscow, Academician Oparin St., 4. Tel. + 7 (495) 531-4444; E-mail: g_khabas@oparina4.ru
Akinfiev Dmitry M., Interventional Radiologist at the Department of Radiology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Address: 117997, Russia, Moscow, Academician Oparin St., 4. Tel. +7 (495) 531-4444; E-mail: g_khabas@oparina4.ru
Makarova Anna S., Obstetrician-Gynecologist at the Department of Innovative Oncology and Gynecology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Address: 117997, Russia, Moscow, Academician Oparin St., 4. Tel. +7 (495) 531-4444; E-mail: a_makarova@oparina4.ru
Golitsyna Julia S., Obstetrician-Gynecologist at the Department of Innovative Oncology and Gynecology, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Address: 117997, Russia, Moscow, Academician Oparin St., 4. E-mail: yu_golitsyna@oparina4.ru +7 (495) 531-4444
Seregin Alexander A., Ph.D. Student at the V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Address: 117997, Russia, Moscow, Academician Oparin St., 4. Tel. +7 (495) 531-4444; E-mail: ggk32@ya.ru
Ashrafyan Lev A., Academician of the RAS, Dr.Med.Sci., Professor, Deputy Director, V.I. Kulakov NMRC for OG&P of Minzdrav of Russia.
Address: 117997, Russia, Moscow, Academician Oparin St., 4. +7 (495) 531-4444; Email: Levaa2004@yahoo.com
For citation: Ovodenko D.L., Bychenko V.G., Khabas G.N., Akinfiev D.M., Makarova A.S., Golitsyna Yu.S., Seregin A.A., Ashrafyan L.A. The role of magnetic resonance imaging in evaluating the effectiveness of neoadjuvant chemotherapy in patients with locally advanced cervical cancer. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019;7: 85-91(in Russian)
https://dx.doi.org/10.18565/aig.2019.7.85-91



