Treatment and prevention of recurrent genital lichen sclerosus in women
Objective: To compare the efficacy of topical glucocorticoids (GCs) monotherapy and combined hormonal therapy with low molecular weight hyaluronic acid in the treatment of genital lichen sclerosus (GLS) in women. Materials and methods: 66 women with GLS were divided into Group 1 (n=20) and Group 2 (n=46). Group 1 patients were treated with topical GCs (mometasone) for 3 months. Group 2 patients additionally received vaginal and vulvar Estrogial cream and gel for 6 months. The efficacy was assessed at 2, 6, 12 weeks and 6 months after the start of treatment by changes in symptoms and the severity of the most frequent (itching, atrophy and paleness of the vulva). Results: In Group 1, many symptoms had regressed by the 3rd month of treatment (p<0.001), but by the 6th month most symptoms relapsed. Group 2, on the other hand, showed an improvement in all GLS symptoms during the treatment course (p<0.001) and no relapse. The most pronounced effect by the 6th month of follow-up was noted between the groups regarding atrophy, paleness, burning, and sclerosis. Analysis of the severity of the leading GLS symptoms showed a significant reduction in the severity of all symptoms at month 6 of follow-up in Group 2 patients compared to Group 1 and at all time points of atrophy and paleness except for baseline (p<0.01). The greatest effect size was observed at the end of 6 months of follow-up for atrophy with between-group median difference between groups of 3.0 (-3.0–0), p<0.001. Conclusion: The use of vaginal and vulvar Estrogial cream and gel as part of complex therapy with topical GCs led to better treatment results and lower recurrence rate, compared to GCs monotherapy. Estrogial cream and gel for local application can be considered an addition to the baseline GLS therapy.Shperling N.V., Shperling M.I.
Keywords
Lichen sclerosus is a chronic inflammatory disorder of the skin and mucous membranes, most commonly affecting the genital area (genital lichen sclerosus, GLS) and manifested by itching, pain, atrophy, and scarring due to loss of vulvar tissue [1, 2].
The pathogenesis of GLS is related to vascular and immune disorders that include impaired microcirculation and deficiency of moisture-retaining substances together with immune system dysregulation resulting in impaired collagen and fibroblast metabolism (glycation, fiber reduction, elastosis). These changes lead to an increase in extensibility and thinning of vulvar skin with loss of tissue elasticity, while hydration, skin thickness, and keratinocyte activity decrease, and melanogenesis is impaired. These pathological changes cause epidermal wrinkling and atrophy, dryness, and altered pigmentation.
Patients with GLS most often complain of severe itching, pain in the anogenital area, cracks, and dryness of vulvar and vaginal skin and mucous membranes. In the event of a late start of GLS treatment and progression of the disease, hyperkeratosis, erosions, localized hemorrhages, cracks, and progressive scarring appear due to scratching.
The course of the disease can be divided into 3 stages. The initial manifestations (stage 1) are accompanied by edema and reddening of the genitals, caused by impaired microcirculation and tissue hypoxia. As the disease progresses (stage 2), the skin becomes atrophic. As a result, it can lead to reduced labia minora, narrowing of the vaginal introitus, impaired urination, dyspareunia, as well as perianal stenosis and painful defecation. Pigmentation disorders, dryness, and scarring appear. Stage 3 is characterized by severe scarring and external genitalia [3, 4]. The disease is characterized by a tendency to frequent recurrence and progression with deterioration of quality of life.
Often the course of the disease is complicated by dysbiosis and inflammatory processes in the form of vulvovaginitis [5]. GLS with chronic hyperkeratosis and erosion has a risk of neoplastic transformation to squamous cell carcinoma [6].
Treatment options for GLS include prolonged therapy with topical glucocorticoids (GCs) and calcineurin inhibitors [7, 8]. However, long-term use of topical GCs (clobetasol propionate, mometasone furoate) causes epithelial atrophy, which can lead to disease relapse [9] or side effects of topical GCs associated with increased atrophy due to long-term use.
Therefore, it is appropriate to use GCs together with drugs from other pharmacological groups to moisten the skin and mucous membranes and prevent relapse [8, 10].
The appearance on the market of moisturizers containing low-molecular-weight hyaluronic acid capable of penetrating into deep skin layers with moisturizing effect substantiated the development of treatment schemes for GLS to prevent atrophic processes of multilayered squamous epithelium of genitals and prevent recurrence of the disease.
The present study aimed to compare the efficacy of topical glucocorticoids monotherapy and combined hormonal therapy with low molecular weight hyaluronic acid (single dose Estrogial cream and Estrogial gel) in the treatment of genital lichen sclerosus in women.
Materials and methods
This prospective randomized controlled comparative study included 66 women with GLS aged 26–70 years, who were randomly divided into Group 1 (n=20) and Group 2 (n=46). Group 1 patients were treated with topical GCs (mometasone furoate 0.1%) for 3 months administered according to the following scheme: 1 month – 2 times a day, 2 month – 1 time a day, 3 month – 2 times a week.
Patients in Group 2 were also given a single-dose Estrogial cream intravaginally at bedtime once daily for 10 days every month for 6 months and Estrogial gel on the vulva area twice daily for 6 months. The efficacy of treatment was assessed after weeks 2, 6, 12, and 6 months by changes from baseline of symptoms and severity of the most frequent ones (pruritus, atrophy, and paleness of the vulva).
Statistical analysis
Statistical analysis was performed using Microsoft Office Excel 2016, Statistica 12, and GraphPad Prism 8.0 software. Based on the normality of the data assessed by the Shapiro–Wilk test, continuous variables showing normal distribution were expressed as means (M) and standard deviation (SD); otherwise, the median (Me) with interquartile range (Q1; Q3) were reported. For multiple comparisons of continuous paired variables, we used Friedman one-way repeated measure analysis of variance by ranks with post hoc comparisons using Dunn's test and calculation of effect size using Kendall's W-criterion. Dichotomous data of paired samples were compared using the Q-Cochran test, followed by paired comparison using the McNemar chi-square (χ2) with Edwards' adjustment. Comparisons of quantitative variables with normal distribution were performed using Student's t-test; with nonnormal distribution, Mann–Whitney U test with calculation of effect size by calculating the median difference (Hodges-Lehman estimate) and 95% confidence interval (CI). Unpaired categorical variables were compared using Fisher's exact test to calculate relative risk (RR) and CI. P<0.05 for paired comparisons and p<0.005 for multiple comparisons (with Bonferroni correction) were considered to be the critical level of significance.
Results
Patients with significant comorbidities (severe obesity, decompensated diabetes mellitus and arterial hypertension, autoimmune diseases), sexually transmitted infections and pelvic inflammatory diseases, as well as those receiving GLS therapy or with a history of such therapy (use of GC for more than 5 days) were excluded from the study.
At baseline, the study groups did not differ in age, mean disease duration, and comorbidities (uterine fibroids, chronic cystitis, arterial hypertension). The groups also did not differ at baseline in the presence of GLS symptoms and the severity of the main symptoms (pruritus, atrophy, paleness) (Table 1).
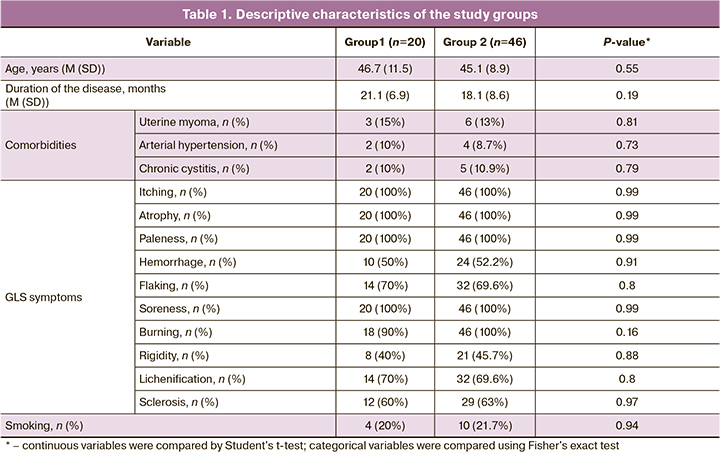
At baseline, the main vulval manifestations of GLS in all patients in both study groups included itching, atrophy, and paleness detected by external examination. In addition, most of patients in both groups complained of soreness and burning.
The evaluation of the changes in symptoms of GLS in women in both groups showed that the majority of symptoms (itching, atrophy, paleness, hemorrhages, peeling, tenderness, burning, lichenification) regressed during treatment in Group 1 patients who received only topical GCs (p<0.001). However, when evaluating the effectiveness of this therapy on the dynamics of symptoms such as rigidity and sclerosis, no statistical significance was found (p=0.06 and 0.03, respectively). At the same time, Group 2 patients showed an improvement in all GLS symptoms during therapy (p<0.001) (Table 2).
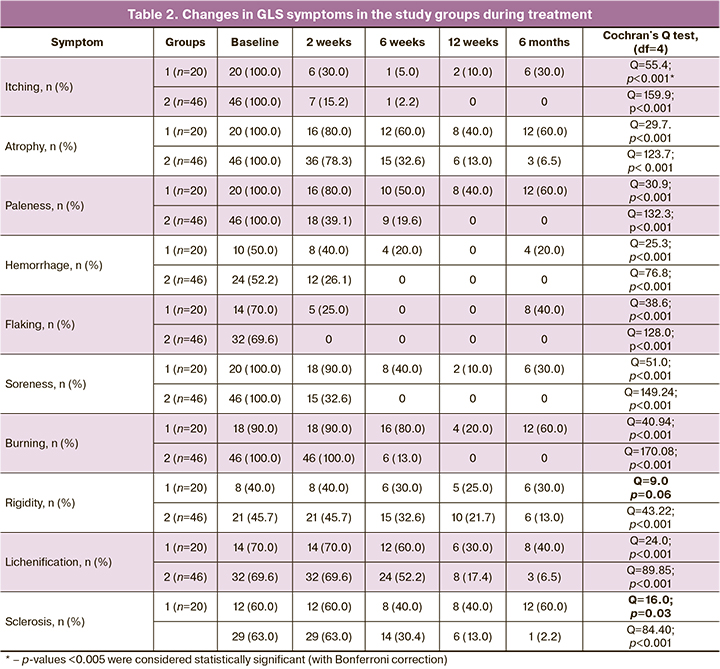
According to Table 3, we can conclude that the main symptoms of GLS (itching, atrophy, paleness, and peeling) are effectively eliminated already in the first 2 weeks of treatment, regardless of the chosen treatment modality. At the same time, burning, rigidity, lichenification, and sclerosis persist in patients for quite a long time and are eliminated only after several months of treatment. It should also be noted that by the end of the follow-up period (6 months), patients treated only with topical GCs (group 1) noted a return of several symptoms, such as hemorrhage, peeling, burning, stiffness, lichenification, and sclerosis, although these symptoms were effectively eliminated in the early treatment period. This may indicate a relapse of the disease due to discontinuation of therapy (3 months according to the Clinical Guidelines), as well as an adverse effect of long-term use of GCs due to its ability to cause atrophic changes in the skin and mucosa. In contrast, a combined use of hyaluronic acid agents with topical GCs (group 2) for 3 months with the continuation of Estrogial successfully and stably stopped the above symptoms. At the end of 6 months of treatment, statistically significant changes were observed from baseline in all symptoms of GLS without recurrence of the disease (Table 3).
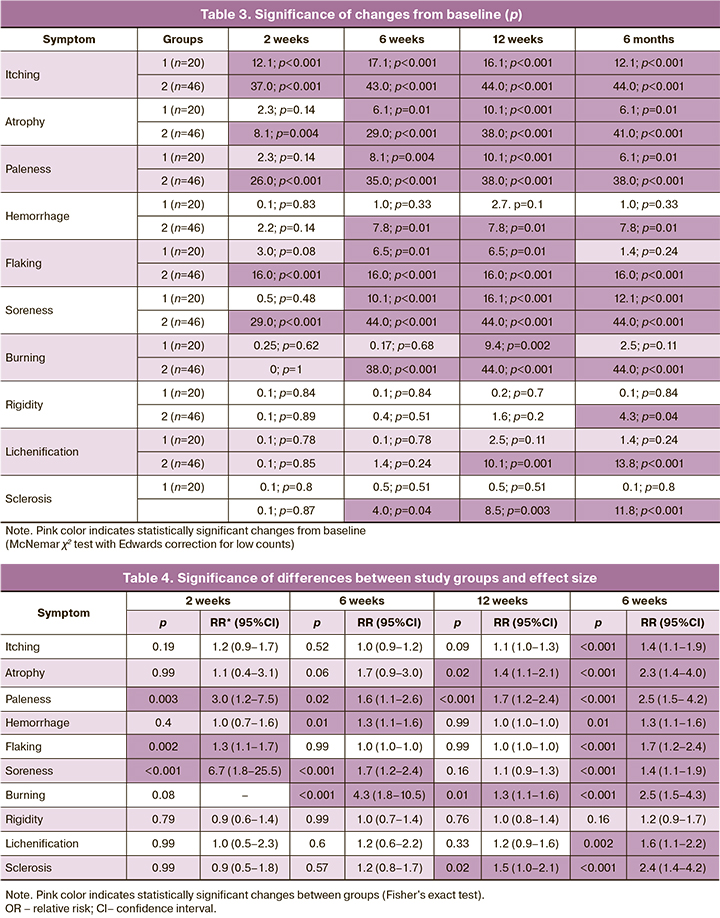
Analysis of changes from baseline in GLS symptoms showed that the addition of single-dose Estrogial cream and gel to topical GCs was associated with the most persistent and long-lasting effect. Therefore, at the end of treatment, a statistically significant difference was found between the study groups regarding the presence of most symptoms of GLS (with the exception of stiffness). The most pronounced effect by 6 months of follow-up was noted between the groups with respect to atrophy, paleness, burning, and sclerosis. There were also several significant differences between the groups during therapy (2 weeks, 6 weeks, and 12 weeks) (Table 4).
The present study also investigated changes in the severity of the main symptoms of GLS (pruritus, atrophy, and paleness) in a point estimate (Table 5).
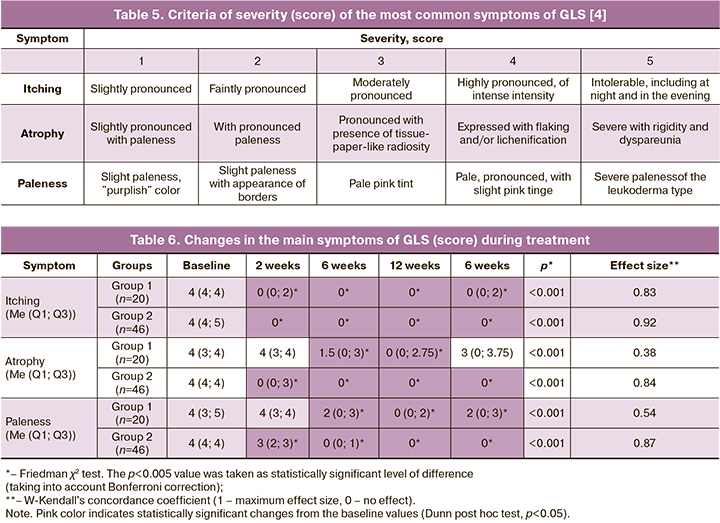
The nonparametric analysis of variance found that therapy with topical GCs, both isolated and together with hyaluronic acid agents led to an improvement in the dynamics of pruritus, atrophy, and paleness. But an a posteriori intragroup comparison revealed that Group 1 patients showed a reverse development of atrophic changes by the end of the 6th month of observation, which indicated a relapse of the disease against the background of the withdrawal of topical GCs and the absence of supportive therapy to restore the natural barrier of the skin and mucous membranes – no difference in the severity of these symptoms from the initial values was obtained (p=0.13). At the same time, Group 2 patients noted a persistent reduction in the severity of all GLS symptoms throughout the treatment period. The total treatment effect was also higher in group 2 patients in terms of pruritus, atrophy, and paleness (Table 6).
When evaluating the differences in the dynamics of itching, atrophy, and paleness, we found that the severity of itching during therapy did not differ significantly between the groups – only at the last control point (6 months) itching was significantly less pronounced in the group of patients receiving combined therapy (GCs + Estrogial). However, differences in the dynamics of atrophy and paleness were more significant – at each control point, the severity of these symptoms was statistically significantly lower in Group 2 patients than in the control group. The greatest effect size at the end of the 6th month of observation was observed for atrophy - the difference of medians between the groups was -3.0 (-3.0–0), p<0.001 (Table 7, Figure).
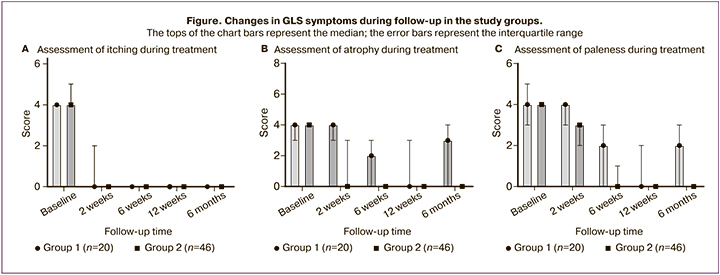
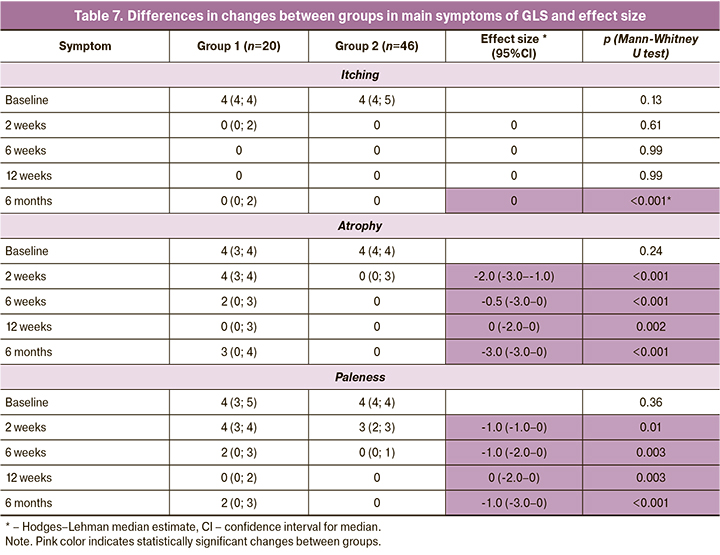
Discussion
According to current data and clinical guidelines, therapy with topical GCs is the basis of GLS therapy. In the uncomplicated course of the disease, this strategy is effective in the early stages of treatment in more than 70% of cases [2, 11]. However, long-term use of this group of drugs (more than 3 months) is not recommended due to the high risk of developing atrophic changes in the skin and mucosa. In this regard, even with high efficacy of therapy, the probability of recurrence of the disease without supportive or alternative therapy is high [12]. Taking into account the high propensity to recurrence of GLS, an important and urgent task at the moment is the use of means supplementing the basic therapy of GLS. The product line Estrogial (gel and two single-dose creams – Estrogial and Estrogial Plus) contains as active main components the sodium salt of hyaluronic acid and phyto-complex - extract from hop cones, clover grass, and calendula flowers.
Single-dose cream is available in the form of suppositories for ease of administration and packed in shaped cells. Single-dose cream Estrogial and Estrogial Plus differ in the composition of auxiliary components. Estrogial Plus has a fattier base and is recommended for more pronounced atrophy.
The extract of calendula flowers has an antioxidant, anti-inflammatory effect, improves the regeneration, of tissues, promotes regeneration and formation of the epithelium in the damaged area. Clover herb extract (phytoestrogen containing 4 isoflavones) is an antioxidant, stimulates collagen and hyaluronic acid, promotes hydration of mucous membranes, has a pronounced soothing effect on irritated skin and mucosa, and bactericidal properties to eliminate itching and discomfort. Hop cone extract has anti-inflammatory, antibacterial, and tonic action, activating the production of elastin and collagen. The following moisturizers and emollients are recommended for all patients with GLS: sodium hyaluronate in combination with phyto complex in the form of a single-dose cream - in the vaginal fornix 1 dose 1–2 times a day for 20–30 days, then 2–3 times a week for 3–4 weeks, then 1–2 times a week (duration of use is not limited); and (or) sodium hyaluronate in combination with phyto-complex in the form of gel for external use - 1-2 applications a day, duration of use is not limited [13]. If necessary, it is possible to combine both forms to enhance the effect.
In this study, the high efficacy of combined therapy of GLS in the form of a three-month course of therapy with topical GCs along with long-term use of agents based on low-molecular-weight hyaluronic acid (Estrogial), which has high absorption capacity in the layers of the skin and mucous membranes to hydrate tissues and maintain the natural protective barrier was shown. According to the instructions for use, Estrogial is used topically for complaints of itching, dryness, dyspareunia, discomfort of various genesis in the genital area, both as a complex and in monotherapy. The components included in Estrogial contribute to the elimination of most atrophic symptoms in the anogenital area associated with itching, dryness, irritation, and pain. In addition, Estrogial has a pronounced additive effect on potent GCs, which has been demonstrated both in this study and in other works [14].
Conclusion
Long-term therapy with topical GCs (mometasone furoate 0.1% once daily for 3 months) leads to improvement in the early stages of vulvar lichen sclerosus, but does not prevent relapse of the disease. The use of single-dose Estrogial cream and gel as part of complex therapy with topical GCs led to better treatment results during the first 3 months of observation, in comparison with GCs monotherapy. Monotherapy with low molecular weight hyaluronic acid in the next 3 months led to a significant decrease in the recurrence rate of the disease. Topical application of single-dose Estrogial cream and gel can be considered a complimentary therapy to the first-line GLS therapy.
References
- Krapf J.M., Mitchell L., Holton M.A., Goldstein A.T. Vulvar lichen sclerosus: current perspectives. Int. J. Womens Health. 2020; 12: 11-20.https://dx.doi.org/10.2147/IJWH.S191200.
- Singh N., Ghatage P. Etiology, clinical features, and diagnosis of vulvar lichen sclerosus: a scoping review. Obstet. Gynecol. Int. 2020; 2020: 7480754.https://dx.doi.org/10.1155/2020/7480754.
- Higgins C.A., Cruickshank M.E. A population-based case-control study of aetiological factors associated with vulval lichen sclerosus. J. Obstet. Gynaecol. 2012; 32(3): 271-5. https://dx.doi.org/10.3109/01443615.2011.649320.
- Шперлинг Н.В., Воронина Н.Б., Шперлинг И.А. Терапия склероатрофического лихена половых органов у женщин. Фарматека. 2018; 6: 29-35. https://dx.doi.org/10.18565/pharmateca.2018.6.29-35. [Shperling N.V., Voronina N.B., Shperling I.A. Therapy of lichen sclerosus et atrophicus in women. Farmateka. 2018; 6: 29-35. (in Russian)]. https://dx.doi.org/10.18565/pharmateca.2018.6.29-35.
- Chattopadhyay S., Arnold J.D., Malayil L., Hittle L., Mongodin E.F., Marathe K.S. et al. Potential role of the skin and gut microbiota in premenarchal vulvar lichen sclerosus: a pilot case-control study. PLoS One. 2021; 16(1): e0245243.https://dx.doi.org/10.1371/journal.pone.0245243.
- Spekreijse J.J., Streng B.M.M., Vermeulen R.F.M., Voss F.O., Vermaat H., van Beurden M. The risk of developing squamous cell carcinoma in patients with anogenital lichen sclerosis: a systematic review. Gynecol. Oncol. 2020; 157(3): 671-7. https://dx.doi.org/10.1016/j.ygyno.2020.02.020.
- Lee A., Fischer G. Diagnosis and treatment of vulvar lichen aclerosus: An update for dermatologists. Am. J. Clin. Dermatol. 2018; 19(5): 695-706.https://dx.doi.org/10.1007/s40257-018-0364-7.
- Российское общество дерматовенерологов и косметологов, Российское общество акушеров-гинекологов. Клинические рекомендации. Лишай склеротический и атрофический. М.; 2020. 30с. [Russian Society of Dermatovenerologists and Cosmetologists. Russian Society of Obstetricians and Gynecologists. Clinical guidelines. Lichen sclerosus and atrophicus. М.; 2020. 30 p. (in Russian)].
- Morrel B., van Eersel R., Burger C.W., Bramer W.M., Kate-Booij T., van der Avoort I.A.M. et al. The long-term clinical consequences of juvenile vulvar lichen sclerosus: a systematic review. J. Am. Acad. Dermatol. 2020; 82(2): 469-77. https://dx.doi.org/10.1016/j.jaad.2019.08.030.
- Corazza M., Schettini N., Zedde P., Borghi A. Vulvar lichen sclerosus from pathophysiology to therapeutic approaches: evidence and prospects. Biomedicines. 2021; 9(8): 950. https://dx.doi.org/10.3390/biomedicines9080950.
- Lewis F.M., Tatnall F.M., Velangi S.S., Bunker C.B., Kumar A., Brackenbury F. et al. British Association of Dermatologists guidelines for the management of lichen sclerosus. Br. J. Dermatol. 2018; 178(4): 839-53.https://dx.doi.org/10.1111/bjd.16241.
- Mautz T.T., Krapf J.M., Goldstein A.T. Topical corticosteroids in the treatment of vulvar lichen sclerosus: a review of pharmacokinetics and recommended dosing frequencies. Sex. Med. Rev. 2022; 10(1): 42-52. https://dx.doi.org/10.1016/j.sxmr.2021.03.006.
- Потекаев Н.Н., Доля О.В., Чернова Н.И. Аногенитальный склеротический и атрофический лишай: клиника, диагностика, лечение, профилактика. Методические рекомендации. М.: ГБУЗ «Московский Центр дерматовенерологии и косметологии»; 2021. 31с. [Potekaev N.N., Dolya O.V., Chernova N.I. Anogenital lichen sclerosus and atrophicus: clinic, diagnosis, treatment, prevention. Guidelines. Moscow: Moscow Center for Dermatovenerology and Cosmetology; 2021. 31 p. (ib Russian)].
- Чернова Н.И., Арутюнян Э. Современные аспекты терапии пациенток с дистрофией и атрофией интимной зоны. Что нового? Российский вестник акушера-гинеколога. 2018; 18(3): 95‑8. https://dx.doi.org/10.17116/rosakush201818295-98. [Chernova N.I., Arutyunyan E. Current aspects of treatment in patients with dystrophy and atrophy of the intimate area. What is new? Russian Bulletin of Obstetrician-Gynecologist. 2018; 18(3): 958. (in Russian)]. https://dx.doi.org/10.17116/rosakush201818295-98.
Received 25.11.2022
Accepted 01.12.2022
About the Authors
Natalia V. Shperling, Dr. Med. Sci., Professor, Department of Clinical Medicine, Reaviz University, shperling2@yandex.ru, https://orcid.org/0000-0002-7865-486X, 199098, Russia, St. Petersburg, Kalinina str. 8, k. 2, lit. A.Maxim I. Shperling, Senior Specialist (internist), 1472 Naval Clinical Hospital, mersisaid@yandex.ru, https://orcid.org/0000-0002-3274-2290,
299001, Russia, Republic of Crimea, Sevastopol, Gospitalny Descent, 1.
Corresponding author: Natalia V. Shperling, shperling2@yandex.ru
Authors’ contributions: Sperling N.V. – conception and design of the study, manuscript drafting; Sperling M.I. – material collection and processing, statistical analysis, manuscript editing.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors’ Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Shperling N.V., Shperling M.I. Treatment and prevention
of recurrent genital lichen sclerosus in women.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2022; 12: 122-130 (in Russian)
https://dx.doi.org/10.18565/aig.2022.280



