На сегодняшний день бесплодие является одной из основных проблем современной медицины и достигает 10–15% среди супружеских пар [1]. Согласно исследованиям последних лет, мужской фактор занимает около 50% в структуре причин бесплодия [2]. Многие исследователи сходятся во мнении, что за последние 50 лет отмечается тенденция к ухудшению показателей спермограммы, что проявляется в сокращении объема эякулята, снижении концентрации и подвижности сперматозоидов и увеличении числа их патологических форм [3].
На сегодняшний день имеется достаточное количество данных, демонстрирующих, что при оплодотворении сперматозоид выполняет не только функцию «доставки мужского генома», но также содержит множество важных для дальнейшего нормального развития эмбриона морфологических ультраструктур. Следовательно, успех оплодотворения и последующего развития эмбриона зависит от параметров развития, ядерной и цитоплазматической компетентности мужской половой клетки.
Одним из важных факторов нарушения мужской фертильности является фрагментация ДНК сперматозоидов. Хотя патофизиологические механизмы, приводящие к повреждению ДНК сперматозоидов, изучены не до конца, предполагается, что их причиной могут быть дефекты ремоделирования хроматина, апоптоз и окислительные процессы. Сохраняя способность к оплодотворению, генетически неполноценные мужские половые клетки могут нарушать процессы эмбрионального развития и приводить к прерыванию беременности [4, 5].
С внедрением такой методики оплодотворения ооцитов, как интрацитоплазматическая инъекция сперматозоида в ооцит (ИКСИ), появилась перспектива решения проблемы мужского бесплодия. Однако визуальная оценка лишь по таким критериям, как морфология и подвижность, может допустить отбор единичного сперматозоида, несущего разного рода патологии, что подчеркивает особую важность селекции сперматозоидов для ИКСИ.
Современной модификацией ИКСИ стал метод отбора сперматозоидов по их способности связываться с гиалуроновой кислотой (ГК) – основным компонентом ооцит-кумулюсного комплекса (физиологическая ИКСИ, ПИКСИ). Автором данной методики является Доктор Габор Хазар, профессор Йельской школы медицины.
ГК в качестве «физиологического селектора» применима на практике in vitro и может служить для улучшения вклада отцовского генома в развитие эмбриона при искусственном оплодотворении и, таким образом, оказать положительное влияние на клинические исходы при лечении бесплодия.
В ряде крупных исследований была отмечена положительная тенденция относительно улучшения качества полученных эмбрионов [6, 7] и частоты наступления беременности при применении методики ПИКСИ. В недавних исследованиях было продемонстрировано статистически значимое снижение частоты самопроизвольных абортов после проведения ИКСИ связанными с ГК сперматозоидами в сравнении с теми, что были отобраны лишь посредством визуальной оценки. Данные клинические результаты подтверждают выводы, полученные в проведенных ранее исследованиях биохимических и молекулярных маркеров функциональной зрелости сперматозоидов человека [7]. Кроме того, в работе A. Yagci и соавт. было показано, что использование для ИКСИ сперматозоидов, отобранных при помощи ГК, увеличивает показатель частоты имплантации [6].
Результаты представленных исследований свидетельствуют об отсутствии негативных последствий, оказываемых на ооциты и преимплантационный эмбриогенез при использовании ГК для селекции сперматозоидов в циклах ИКСИ [6, 7]. Лучшее качество сперматозоидов, способных связываться с ГК, подтверждено рядом исследований. Так, было продемонстрировано отсутствие фрагментации ДНК в ядре сперматозоидов, связавшихся с ГК [6–8]. Кроме того, была доказана эффективность селекции сперматозоидов, связывающихся с ГК, в снижении частоты анеуплоидий как при олигозооспермии, так и при нормальной концентрации половых клеток. При этом наблюдалось существенное уменьшение частоты дисомий и диплоидий хромосом [9].
Для предотвращения передачи генетических дефектов и повышения эффективности методов ВРТ, особенно при применении методики ИКСИ, необходима правильная оценка целостности генома мужских половых клеток [10, 11]. Однако полноценная оценка не всегда возможна при использовании стандартного анализа спермы, рекомендованного ВОЗ [12]. Сперматозоид, морфологически расцененный как нормальный, в ряде случаев может иметь дефекты в целостности структуры ДНК [13].
Во многих исследованиях установлено негативное влияние высокого процента поврежденной ДНК в сперматозоидах на частоту наступления беременности [14, 15]. Авторы этих работ полагают, что повреждения ДНК могут служить основными причинами негативного отцовского вклада в отношении преимплантационного развития эмбриона.
Другие исследователи доказывают, что фрагментация ДНК не оказывает влияния на оплодотворение, но играет важную роль в формировании бластоцист и в процессе имплантации [10, 16]. Необходимо отметить, что ни один из рекомендованных ВОЗ параметров спермы, таких как объем, число сперматозоидов, подвижность, морфология не дают прогноза вероятности развития эмбриона до стадии бластоцисты [7].
Необходимо изучение в нашей стране эффективности применения технологий селекции при наличии патозооспермии и высокого процента фрагментации ДНК сперматозоидов для поиска способов повышения эффективности лечения бесплодия в когорте супружеских пар с подобными отклонениями.
Цель исследования: определить связь между целостностью ДНК сперматозоидов в эякуляте, эмбриологическими параметрами (процент оплодотворения ооцитов; процент бластуляции), клиническими исходами программ ВРТ и различными методиками отбора сперматозоидов.

Материал и методы исследования
На базе отделения вспомогательных технологий в лечении бесплодия и лаборатории цитологии ФГБУ Научный центр акушерства, гинекологии и перинатологии им. академика В.И. Кулакова Минздрава России было проведено проспективное исследование случай-контроль.
В исследование были включены 67 супружеских пар, соответствовавших критериям включения (возраст пациентки 18–39 лет, возраст супруга 18–50 лет, регулярный менструальный цикл, женское бесплодие трубного происхождения и/или мужской фактор бесплодия при отсутствии тяжелой патозооспермии, наличие высокого процента фрагментации ДНК сперматозоидов в эякуляте партнера (15% и выше)).
Критериями не включения в исследование были: эндокринный фактор бесплодия, эндометриоз III–IV степени распространения, генетические аномалии, пороки развития половых органов, некрозооспермия, необходимость выполнения TESA, MESA, PESA для получения сперматозоидов, необходимость использования донорской спермы и другие. Супружеские пары были разделены на 2 группы в зависимости от типа оплодотворения (ИКСИ, ПИКСИ): I группа – 37 пар, которым производилось ИКСИ, II группа – 30 пар, которым производилось ПИКСИ.
Фрагментацию ДНК определяли методом TUNEL на мазках эякулята. Образцы спермы наносили на адгезивные стекла (HistoBond) и обрабатывали по протоколу фирмы-изготовителя (Dеad End TM Fluorometric TUNEL System PROMEGA). Препараты окрашивали DAPI (4,6-diamino-2-phenylidole, Sigma) для специфической окраски ДНК. Визуальную оценку осуществляли с помощью микроскопа Axiovert 200V (CarlZeiss). Проводился подсчет клеток с фрагментацией и без фрагментации ДНК.
Пациенткам проводилась стимуляция суперовуляции препаратами гонадотропинов по стандартному протоколу с антагонистами гонадотропин-рилизинг гормона со 2–3-го дня менструального цикла. При достижении фолликулами размеров 18–20 мм в диаметре с целью финального дозревания ооцитов в качестве триггера овуляции вводился препарат хорионического гонадотропина человека в дозе 10 000 ЕД однократно за 35–36 часов до планируемой трансвагинальной пункции яичников. Забор ооцитов проводился под внутривенной анестезией и ультразвуковым контролем с использованием одноразовых игл (Vitrolife Inc, США) в условиях малой операционной. Аспирация фолликулярной жидкости производилась под отрицательным давлением 190 мм водного столба в заранее подогретые стерильные пробирки, которые немедленно передавались эмбриологу для последующей идентификации ооцит-кумулюсного комплекса и оценки степени зрелости полученных ооцитов. Сразу же после аспирации фолликулярной жидкости производилась идентификация ооцит-кумулюсных комплексов и оценка степени зрелости ооцитов под стереомикроскопом на нагретой поверхности стерильного ламинарного бокса. Во время производимых манипуляций постоянно поддерживали стабильную температуру (+37°С) и показатель рН среды (7,5). Ооцит-кумулюсные комплексы отмывали от фолликулярной жидкости и крови и помещали в стерильные планшеты Nunc с культуральной средой (Fertilization medium, COOK, Австралия) на 2–3 часа с целью предварительной инкубации при температуре +37°С и атмосфере с 5% СО2.
Отбор сперматозоидов для ИКСИ производился по морфологическим параметрам (стандартное ИКСИ), либо согласно их способности связываться с гиалуроновой кислотой (ПИКСИ) по протоколу. Для проведения ПИКСИ используются чашки Петри – культуральная чашка из полистирола с нанесенными на верхнюю часть чашки тремя микрокаплями гиалуроновой кислоты. Чашки стерильны, не содержат эндотоксина и нетоксичны для эмбрионов. Спустя 5 минут после проведения гидратирования микрокапель и добавления суспензии сперматозоидов происходит набухание гиалуроната и начинается связывание сперматозоидов. Связавшиеся с гиалуроновой кислотой сперматозоиды легко отличимы: они активно бьют хвостом, но при этом лишены прогрессивной подвижности.
После проведения оплодотворения методом ИКСИ/ПИКСИ ооциты переносили в культуральную среду с целью дальнейшего культивирования. Оценка формирования пронуклеусов проводилась через 14–16 часов после оплодотворения. Оплодотворение расценивали как нормальное при наличии двух пронуклеусов в цитоплазме.
Оценку качества эмбрионов производили на 3-и и 5-е сутки после оплодотворения на основании совокупности их морфологических характеристик [17].
Перенос эмбрионов в полость матки осуществлялся в асептических условиях под ультразвуковым контролем посредством применения одноразовых гибких катетеров на 5-е сутки культивирования in vitro. В цикле производился селективный перенос 1 эмбриона с лучшими морфологическими характеристиками. Поддержка лютеиновой фазы и посттрансферного периода осуществлялись по схеме: через 24 часа после трансвагинальной пункции яичников назначался препарат дидрогестерон (дюфастон) в дозе 20 мг/сут. После переноса эмбрионов в полость матки дозировку увеличивали в два раза.
Диагностика наступления беременности производилась путем определения в сыворотке крови концентрации β-субъединицы хорионического гонадотропина через 14 дней после переноса эмбриона в полость матки.
Исследование было одобрено комитетом по этике ФГБУ НЦАГиП им. В.И. Кулакова МЗ РФ. Статистическая обработка данных выполнена при помощи пакета прикладных программ SPSS Statistics 22.0. Для оценки значимости межгрупповых различий по данным, имеющим нормальное распределение, применяли тест Стьюдента для 2 независимых выборок. При непараметрическом распределении данных сравнение между двумя группами проводилось методом Манна–Уитни. Достоверность различий по частоте встречаемости качественных признаков определяли по критерию χ2. Статистически значимыми считались различия при р<0,05. Отношение шансов (ОШ) приведено с 95% доверительным интервалом.
Результаты и обсуждение
Анализ возрастных характеристик включенных в исследование пациенток и их половых партнеров не выявил статистически значимых межгрупповых различий. Средний возраст женщин в I группе составил 31,9±4,6 года; во II группе – 32,4±4,2 года (критерий Манна–Уитни, р=0,672). Средний возраст мужчин в I группе составил 30,7±3,05 года; во II группе – 34,8±5,9 года (t-критерий Cтьюдента, р=0,648).
Пациентки в исследуемых группах были сопоставимы по параметрам овариального резерва (уровень ФСГ, АМГ, количество антральных фолликулов в раннюю фолликулярную фазу по данным ультразвукового исследования органов малого таза). Также не было выявлено статистически значимых различий по частоте распространения гинекологических и экстрагенитальных заболеваний между группами.
При оценке репродуктивной функции женщин было выявлено, что случаи первичного и вторичного бесплодия встречались с одинаковой частотой в обеих группах.
Клинико-анамнестические данные и гормональный профиль пациенток представлен в табл. 1 и 2 соответственно. Проведенный анализ анамнестических и клинических данных описан в табл. 1 и свидетельствует об однородности исследуемых групп.
Уровень фрагментации ДНК в сперматозоидах у обследованных нами мужчин с нарушением репродуктивной функции варьировал от 15,6 до 47%. Средний уровень данного показателя составил 22,67%.
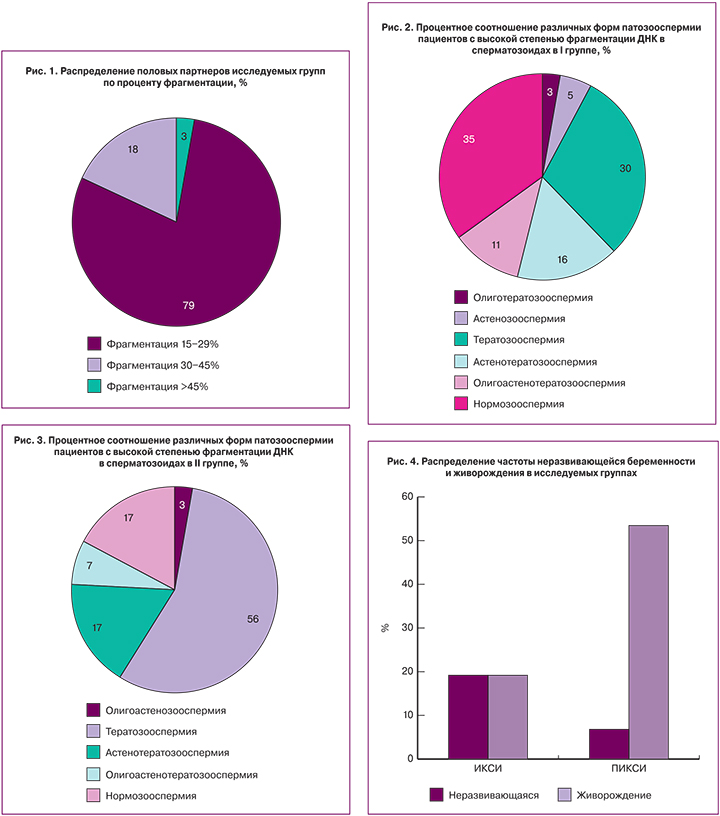
Нами был проведен анализ данных по частоте встречаемости различной степени фрагментации ДНК сперматозоидов среди мужчин в обеих группах. Обнаружено, что фрагментация ДНК в диапазоне от 15 до 30% встречается у 79% (53 мужчин), в диапазоне от 30 до 45% – у 18% (12 мужчин), выше 45% – у 3% (2 пациентов) (рис. 1).
В обеих исследуемых группах среди мужчин с высоким процентом фрагментация ДНК в сперматозоидах встречались различные формы патозооспермии (рис. 2,3).
В ходе проведенного исследования была отмечена явная тенденция к увеличению частоты наступления клинической беременности в группе, где перед проведением процедуры ИКСИ производилась селекция сперматозоидов по способности к связыванию с гиалуронатом (р=0,059). Отсутствие статистически значимой ассоциации типа оплодотворения и частоты наступления клинической беременности может быть обусловлено малой выборкой пациентов (30 и 37 соответственно), участвовавших в данном исследовании (табл. 3).
Результаты же ранее проведенных исследований продемонстрировали существенную связь методики селекции мужских половых гамет для ИКСИ и клинической эффективности программы ЭКО/ИКСИ [6–11]. Были приведены убедительные доказательства того, что «физиологический» отбор сперматозоидов для ИКСИ значительно улучшает качество эмбрионов, существенно уменьшая степень фрагментации эмбрионов 3-го дня, а также способствуя формированию бластоцист хорошего качества, что увеличивает частоту имплантации и частоту наступления клинической беременности [18, 19].
Согласно полученным нами данным, имеются достоверные различия в группе ИКСИ и ПИКСИ по частоте имплантации (χ2=4,249; р=0,039). В первой группе частота имплантации составила 32,56%, во второй группе – 55%, что демонстрирует прямую зависимость качества эмбрионов от генетической полноценности гамет.
Далее мы проводили анализ частоты ранних репродуктивных потерь и процента живорождения у супружеских пар после проведения программы ЭКО/ИКСИ и ЭКО/ПИКСИ. Были обнаружены значимые различия по проценту живорождения в пользу применения методики «физиологический» селекции мужских гамет для ИКСИ (χ2=8,704; р=0,003). В 1-й группе пациентов (стандартное ИКСИ) показатель живорождения составил 18,92% из расчета на 1 эмбрион, тогда как во 2-й группе с выбором сперматозоидов по способности связываться с ГК данный показатель составил 53,30%, что представлено на рис. 4.
В систематическом обзоре 11 исследований, включающих 1549 циклов ЭКО и ИКСИ, было показано, что повреждение спермальной ДНК обладает статистически достоверной прямой корреляцией с частотой самопроизвольных абортов [20].
Также влияние фрагментации ДНК сперматозоидов на привычное невынашивание беременности исследовалось группой иранских ученых. В 1-ю группу были включены 30 пар с привычным невынашиванием беременности, во 2-ю (контроль) – 30 фертильных пар. В 1-й группе уровень фрагментации ДНК сперматозоидов составил 43,3%, что достоверно превышало показатель в группе контроля – 16,7% (p=0,024). Сперма мужчин в парах с невынашиванием беременности имела более высокий уровень фрагментации ДНК, чем в контрольной группе, что указывает на возможную связь между идиопатическими репродуктивными потерями и фрагментацией ДНК сперматозоидов [8]. Сходные результаты были получены и в работах отечественных авторов [21, 22].
В недавно опубликованном метаанализе, в основу которого были положены результаты 16 когортных и 14 проспективных исследований (n=2969), также было показано, что фрагментация ДНК сперматозоидов увеличивает риск невынашивания беременности после ИКСИ [15]. Согласно нашим результатам, частота неразвивающейся беременности в группах была статистически не значима, что, вероятно, связано с малым объемом выборки пациентов (χ2=2,139; P=0,144).
Заключение
Таким образом, жизненно важная роль передачи генетически полноценного материала заставила ученых сосредоточиться на исследовании эффективности различных методик селекции мужских половых клеток для оплодотворения in vitro. В связи с тем, что ни один из рекомендованных ВОЗ параметров спермы, таких как объем, число сперматозоидов, подвижность, морфология не дают полной информации по целостности мужского генома, сперматозоид, расцененный по итогам микроскопического анализа как нормальный, в ряде случаев может иметь дефекты в целостности ДНК. Физиологическая селекция сперматозоида для ИКСИ (ПИКСИ) снижает риск передачи генетической патологии. При наличии анеуплодии использование феномена связывания сперматозоидов с ГК способствует селекции единичного зрелого, функционально компетентного сперматозоида с целостной ДНК, снижая, таким образом, потенциальные риски возникновения побочных эффектов, обусловленных вкладом отцовского генома в развитие эмбриона.
По результатам нашего исследования, у супружеских пар с высоким процентом фрагментации ДНК сперматозоидов определяется существенная связь методики селекции мужских половых гамет для ИКСИ по способности к связыванию с ГК и клинической эффективности программы ЭКО/ИКСИ. Обнаружены значимые различия по проценту живорождения в пользу применения методики «физиологический» селекции мужских гамет для ИКСИ (18,92 и 53,30% соответственно). Имеются достоверные различия в группе ИКСИ и ПИКСИ по частоте имплантации (32,56 и 55% соответственно). Фрагментация ДНК сперматозоидов увеличивает риск невынашивания беременности после ИКСИ. Частота неразвивающейся беременности в группах была статистически не значима, что, вероятно, связано с малым объемом выборки пациенток. Благодаря установленному полному отсутствию токсичности ГК, а также возможности снижать частоту возникновения генетических осложнений, применение методики ПИКСИ в качестве альтернативы традиционному ИКСИ позволит существенно улучшить характеристики преимплантационного эмбриогенеза и, в конечном счете, увеличить количество благоприятных исходов ВРТ.



