Терминология и эпидемиология
Акне (аcne vulgaris) – хроническое воспалительное заболевание кожи, проявляющееся открытыми или закрытыми комедонами и воспалительными поражениями кожи в виде папул, пустул, узлов, а также различной степени выраженности процессами рубцевания [1, 2]. С учетом клинической картины заболевания выделяют: комедональные акне, папуло-пустулезные акне легко-средней степени тяжести, тяжелые папуло-пустулезные акне, узловатые акне умеренной степени тяжести, узловатые акне тяжелой степени, конглобатные акне. Локализация акне обусловлена расположением сальных желез в коже: лицо, шея, зона декольте, плечи, спина [1].
Акне являются одним из самых распространенных дерматозов. Принято считать, что акне – чисто юношеское заболевание: первые проявления акне обычно начинаются в возрасте 12–14 лет у девочек, отмечаются почти у 90%, у 20% из них – тяжелая форма течения заболевания [2]. Известно, что акне страдают 85% лиц в возрасте от 12 до 24 лет, 8% лиц в возрасте от 25 до 34 лет и 3% лиц в возрасте от 35 до 44 лет [1]. К сожалению, у 20–50% пациенток остаются рубцы постакне или очаги пигментации [3].
В подростковом возрасте мальчики и девочки болеют акне практически в равных соотношениях, в то время как при поздних акне заболеваемость у женщин существенно выше, чем у мужчин. В последние годы появляется все больше данных о высокой частоте этой патологии у женщин репродуктивного возраста. Распространенность акне по данным анкетирования в репродуктивном возрасте достигает 25–35% и 15% у женщин старше 50 лет [4, 5].
У женщин выделяют 3 подтипа акне: стойкие (персистирующие) акне, акне с поздним началом и рецидивирующие акне. Для стойких (персистирующих) акне, наблюдающихся у 80% женщин, характерно начало заболевания в подростковом периоде с постепенным переходом во взрослый возраст; для акне с поздним началом характерна манифестация после 25 лет. Рецидивирующие акне развиваются у женщин, имевших в анамнезе акне в подростковом возрасте, разрешившиеся в течение нескольких лет [1, 3].
Закономерно изменения и поражения кожи лица вызывают ряд психологических проблем: неуверенность, тревожность, депрессию, снижение самооценки, агрессивность и социальную дезадаптацию, что снижает качество жизни в целом [2, 6]. При этом степень тяжести акне не коррелирует с серьезностью психо-эмоциональных нарушений. В связи с вышеизложенным знание гинекологами методов эффективной коррекции акне у женщин репродуктивного возраста более, чем актуально.
Патофизиологические основы возникновения акне.
Роль половых гормонов
Согласно определению Российского общества дерматовенерологов и косметологов, акне – это мультифакториальный дерматоз, в патогенезе которого большую роль играют генетически обусловленная гиперандрогения и генетически детерминированный тип секреции сальных желез [1]. Однако факторы риска и гены, ассоциирующиеся с прогнозом и лечением акне, не уточнены [2]. Курение может усилить тяжесть проявлений акне [7].
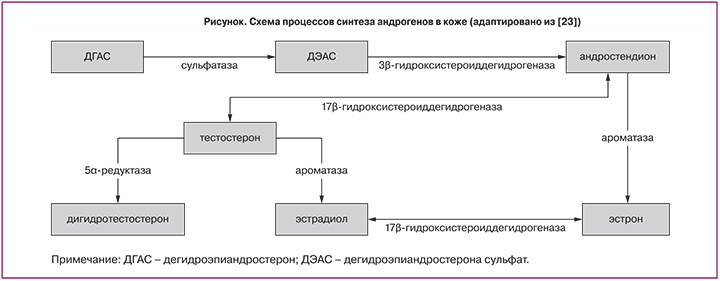
Выделяют четыре основных звена патогенеза акне: увеличение продукции кожного сала, избыточный фолликулярный гиперкератоз, размножение Propionibacterium acnes (P.acnes) и воспаление [8, 9]. Воспаление при акне первично и предшествует фолликулярному гиперкератозу, а бактерии Р. acnes принимают активное участие в формировании микрокомедонов. Регуляция активности сальных желез и кератинизации происходит при участии андрогенов, рецепторы к которым есть на базальном слое сальных желез и кератиноцитах. В коже присутствует достаточная концентрация андрогенов, действующих интракринно и паракринно, а также все необходимые ферментные системы для локальной продукции их активных фракций – 3β-гидроксистероид-дегидрогеназа, 17β-гидроксистероиддегидрогеназа и 5α-редуктаза (рисунок).
Под воздействием андрогенов стимулируется выработка секрета сальных желез, при этом его качественный состав значительно меняется. Так, в кожном сале снижается уровень α-линоленовой кислоты, участвующей в регуляции дифференцировки фолликулярных кератиноцитов, что приводит к повышению кератинизации выводных протоков сальных желез и, как следствие, к формированию комедонов и затруднению оттока кожного сала. Так создается благоприятная среда для активного размножения резидентной анаэробной липофильной микрофлоры, что, в свою очередь, стимулирует миграцию нейтрофилов в патологический очаг и формирование воспалительных высыпаний. Наряду с Р. acnes воспаление инициируют: провоспалительные цитокины, выделяемые эпителиальными и иммунными клетками, разрыв стенки протока сальной железы с выходом содержимого в дерму, вторичное инфицирование аэробной микрофлорой (стрепто- и стафилококками) [10–12].
Понимая участие андрогенов в патогенезе акне, у пациенток с данной жалобой в первую очередь необходимо уточнить наличие других проявлений системной гиперандрогении (гирсутизм, нарушения менструального цикла, алопеция, себорея, клиторомегалия, барифония, признаки инсулинорезистентности), провести обследование с целью установки правильного диагноза и назначения патогенетической терапии. Наиболее частыми причинами гиперандрогении являются синдром поликистозных яичников (СПКЯ) и врожденная дисфункция коры надпочечников (ВДКН) [13]. Для дифдиагностики рекомендуется определение уровня общего и свободного тестостерона, дегидроэпиандростерона сульфата (ДЭАС), 17-оксипрогестерона, лютеинизирующего гормона, фолликулостимулирующего гормона; проведение теста на толерантность к глюкозе [1, 14]. В частности, при СПКЯ диагностическое значение имеют уровни общего и свободного тестостерона, уровни андростендиона и ДЭАС имеют вспомогательное значение. Диагностически значимым для постановки диагноза ВДКН является повышение уровня 17-оксипрогестерона выше 13–15 нмоль/л и подтверждение мутации гена 21-гидроксилазы [14]. Резистентность акне к проводимой терапии также указывает на необходимость пересмотра данных обследования пациентки и уточнение наличия эндокринной патологии или вирилизирующих опухолей [14].
При этом у женщин без гирсутизма и других проявлений гиперандрогенизма не выявлено корреляции степени тяжести акне с уровнями андрогенов. Клинически у большинства пациенток с акне уровни андрогенов находятся в нормальных пределах, однако, при сравнении с женщинами без акне их уровни андрогенов выше [15]. По всей видимости, имеет место гиперэкспрессия андрогеновых рецепторов на кератиноцитах и себоцитах или гиперфункция ферментов локального превращения андрогенов [3]. Поэтому определение уровней андрогенов у пациенток с акне, но регулярным овуляторным менструальным циклом не имеет диагностического значения.
Влияние эстрогенов на продукцию кожного сала окончательно не определено [16]. Считается, что при определенных концентрациях эстрогены подавляют активность сальных желез, однако, этот уровень индивидуален. Известны случаи усиления акне в период высоких концентраций эстрогенов – во время беременности. Скорее всего, эстрогены влияют на активность сальных желез посредством нескольких механизмов: прямой антагонизм с андрогенами, ингибирование продукции андрогенов по механизму отрицательной обратной связи через гипофиз, регуляция активности генов, отвечающих за пролиферацию сальных желез и липогенез [17].
По данным исследований, ведущие причины акне в репродуктивном возрасте – стрессы и дефицит сна [9, 18]. Также известно, что у 39–85% женщин репродуктивного возраста отмечается ухудшение акне в предменструальные дни [3], при этом распространенность напрямую коррелирует с возрастом. Наличие клеща Demodex увеличивает риск акне втрое (P=2,80 (95% CI 2,34–3,36) [19]. Обсуждается роль окружающей среды и личной гигиены, при этом необходимость соблюдения диеты не доказана [2].
Лечение акне: приоритет выбора у женщин репродуктивного возраста
Особенности патогенетических механизмов развития акне, а также вариабельность клинических форм заболевания диктуют многообразие методов лечения, представленных в различных клинических руководствах [1, 20, 21]. Очевидно, что для успешной терапии акне требуется индивидуальный подход к подбору как системных (при более тяжелых или осложненных формах акне), так и топических препаратов. Назначение специальных вспомогательных средств для ухода за кожей дает возможность во время наружного или системного лечения уменьшить дозы и кратность применения лекарственных средств, обеспечить более комфортное состояние кожи пациента.
Одним из ведущих методов лечения акне является длительное назначение антибактериальных препаратов тетрациклиновой группы (уровень доказательности С) [1]. Однако следует отметить, что до 80% женщин репродуктивного возраста с акне рефрактерны к проводимой антибактериальной терапии, а также учесть неблагоприятный профиль побочных эффектов антибиотиков при длительном применении [22].
Основными показаниями для назначения изотретиноина для перорального приема являются тяжелые формы акне (узловатые, конглобатные акне или акне с риском образования рубцов); акне, не поддающиеся другим видам терапии; акне в сочетании с выраженными психоэмоциональными расстройствами по поводу заболевания. Препарат является потенциальным тератогеном и обладает побочными эффектами (наиболее часто отмечаются хейлит, сухость кожи, шелушение, реже – алопеция, конъюнктивит, головная боль, артралгии и др.). На фоне проводимой терапии необходимо определять исходные показатели функции печени и липидного обмена, а затем повторить их через 2–4 недели; контрацептивный период после лечения составляет 1 месяц. В дерматологии крайне остро стоит вопрос контрацепции на фоне приема ретиноидов, в этом отношении лидируют комбинированные гормональные контрацептивы [1, 16, 20].
Применение топических препаратов ретиноидов или азелаиновой кислоты эффективно в коррекции акне, но не всегда оказывают желаемый эффект у женщин репродуктивного возраста [24].
В идеале назначенный метод лечения должен воздействовать на несколько звеньев цепи развития акне. Принимая во внимание ведущую патогенетическую роль половых гормонов, применение гормональной терапии обосновано. Необходимо добиться снижения синтеза яичниковых и надпочечниковых андрогенов, блокады как самих андрогеновых рецепторов, так и ферментов их локального синтеза в коже. С учетом вышесказанного патогенетически обоснованным является применение комбинированных гормональных контрацептивов (КОК). Согласно клиническим рекомендациям The Global Alliance Guidelines, назначение КОК является наилучшим выбором лечения акне для женщин с или без эндокринной патологии, нуждающихся в контрацепции [20].
Изолированное назначение препаратов антиандрогенов (блокаторов андрогеновых рецепторов или ферментных систем) ограничено ввиду необходимости жесткой контрацепции в связи с тератогенным действием и профилем побочных эффектов при длительном применении, а также меньшей эффективностью в сравнении с КОК (уровень доказательности С) [1, 14]. Дополнительно комбинированные оральные контрацептивы обеспечивают множество неконтрацептивных эффектов, таких как регуляция менструального цикла, коррекция анемии, снижение риска внематочной беременности и эпизодов ВЗОМТ, а также снижение риска рака яичников, эндометрия и колоректального рака [25].
КОК обладают комплексным воздействием: снижают уровень продукции андрогенов в яичниках, повышают уровень глобулина, связывающего половые стероиды, а, значит, снижают концентрацию свободных андрогенов и их связывание со своими рецепторами. Прогестагены в составе КОК снижают активность 5α-редуктазы, тем самым также уменьшая активность работы сальных желез, а гестагены с антиандрогенной активностью блокируют андрогеновые рецепторы и в коже [26].
Дроспиренон, прогестаген 4-го поколения, является производным 17α-спиронолактона и объединяет в себе антиандрогенные и антиминералокортикоидные свойства. Дроспиренон-содержащие КОК (ДРСП-КОК) доказали свою эффективность в купировании предменструального дисфорического расстройства [27], о психо-эмоциональных нарушениях пациенток с акне говорилось выше.
По результатам Кохрановского обзора от 2012 г., в котором были проанализированы данные 31 исследования с участием 12 579 пациенток, КОК эффективны в коррекции акне, однако, четких данных о преимуществах того или иного КОК по данным обзора выявлено не было [28, 29].
В 2010 г. на рынке был представлен новый ДРСП-КОК с микродозой этинилэстрадиола (20 мкг) и инновационным режимом приема 24+4. Отсутствие перерывов в приеме препарата способствовало лучшей приверженности к этому методу контрацепции, а три дополнительных дня приема активных таблеток усилили дополнительные эффекты за счет снижения гормональных колебаний в безгормональный интервал. Проведен ряд рандомизированных плацебо-контролируемых исследований, подтверждающих эффективность и преимущество именно ДРСП-КОК с режимом 24+4 в лечении акне [26, 30–32]. Так, в исследовании Maloney и соавт. наблюдались 538 женщин в возрасте 14–45 лет с умеренными акне, прием ДРСП-КОК 24+4 способствовал снижению выраженности акне на 46,3% в сравнении с 30,6% в группе плацебо (p<0,001), помогал добиваться чистой или почти чистой кожи в сравнении с плацебо (OR 3,02; 95% CI 1,99–4,59) [30]. На фоне приема ДРСП-КОК 24+4 отмечено исчезновение большего числа элементов (MD 29,08; 95% CI 3,13–55,03), как воспалительных, так и невоспалительных, папул и закрытых комедонов. Отмечен лучший косметический результат ДРСП-КОК 24+4 в сравнении с КОК с 17β-эстрадиолом с диеногестом или номегестрола ацетатом [27]. Следует напомнить, что 3 мг дроспиренона в составе КОК эквивалентно 25 мг спиронолактона, нередко назначаемого в виде монотерапии для коррекции акне с доказанной эффективностью [33]. На основании проведенных исследований лечение умеренных акне было внесено отдельным показанием в инструкцию с ДРСП-КОК 24+4 (джес).
При назначении КОК учитываются медицинские критерии методов контрацепции ВОЗ от 2015 г. [25]. В отношении успешности в лечении акне пациентку следует предупредить, что минимальный срок для оценки эффективности назначенной терапии – 3 месяца. В этот период назначение топических ретиноидов или системной антибактериальной терапии может ускорить терапевтический эффект. КОК являются препаратом выбора у женщин с акне и жирностью кожи и волос, при зависимости акне от фазы цикла, нарушениях менструального цикла, неэффективности применения негормональных методов коррекции [34]. Важно, что КОК не влияют на фертильность пациентки в будущем [14].
Однако известно, что эндогенные эстрогены и синтетический этинилэстрадиол (входящий в состав КОК) обладают фотосенсибилизирующим действием, провоцируя появление очагов гиперпигментаций на открытых участках кожного покрова в весенне-летний период, что ограничивает применение КОК как с контрацептивной, так и с противоугревой целью у отдельных пациенток, особенно со светлым фототипом кожи, веснушками, а также у любительниц загорать на солнце и посещать солярий [12]. Кроме того, известно о фотосенсибилизирующем эффекте актибактериальных препаратов из группы фторхинолонов и тетрациклинов. Нивелировать данную проблему можно благодаря фотопротективному потенциалу фолатов: достаточный уровень фолатов в организме способствует лучшей переносимости лечения в весенне-летний период, а также у пациенток со светлым фототипом кожи [12].
В состав препарата джес плюс, помимо уникального гестагена – дроспиренона (3 мг) и микродозированного этинилэстрадиола (0,02 мг), входит стабильная матаболически активная форма фолиевой кислоты – левомефолат кальция (метафолин) в количестве (451 мкг), эквивалентном суточной потребности организма в фолиевой кислоте (400 мкг). Фолиевая кислота практически не синтезируется в организме человека, и ее суточная норма должна восполняться алиментарным путем с пищевыми продуктами, содержащими фолаты, продуктами питания, обогащенными фолатами или с медикаментозными добавками. В дополнение к возможным сложностям, связанным с соблюдением вышеуказанного пищевого рациона для обеспечения организма суточной нормой фолиевой кислоты, это вещество практически полностью (до 90%) инактивируется под воздействием УФ-излучения и после термической обработки продуктов питания (выше 40°С). Трудно переоценить значение фолатов в физиологии организма человека [35]. Исключительно важна их роль в течение первых недель развития эмбриона, способствуя формированию нервной трубки эмбриона. Фолатный дефицит приводит к врожденным порокам развития, несовместимым с жизнью (анэнцефалия) или приводящим к инвалидности (расщелина позвоночника), а также высокому риску спонтанного выкидыша [36]. Следовательно, женщинам детородного периода, принимающим КОК и планирующим беременность в ближайшее время, потребление фолатов необходимо начинать заблаговременно до зачатия [35]. В исследовании Lamers и соавт. была доказана эффективность левомефолата кальция аналогичная таковой фолиевой кислоты в снижении плазменных уровней гомоцистеина – фактора риска задержки внутриутробного развития плода, преждевременных родов, преэклампсии и дефектов нервной трубки [37].
Назначение лечения при СПКЯ, как классического примера гиперандрогенизма с яркими клиническими проявлениями, обусловлено необходимостью коррекции андрогензависимых дермопатий (гирсутизм, акне), метаболических нарушений и нормализации массы тела, восстановления менструального цикла и фертильности и профилактикой поздних метаболических осложнений. Повышенный уровень гомоцистеина, столь характерный для пациенток с СПКЯ, также требует применения фолатов для снижения метаболических рисков и сердечно-сосудистых заболеваний.
Фолатный дефицит может проявляться усталостью, бессонницей, чувством беспокойства, потерей аппетита, а также сопряжен с риском развития эндогенной депрессии, манифестации шизофрении и болезни Альцгеймера. Со стороны кожи и ее придатков хроническая фолатная недостаточность ведет к снижению регенеративной функции кожи и слизистых, поседению и поредению волос, ломкости ногтей, нарушению пигментации. Установлена ключевая роль фолиевой кислоты в процессах репарации ДНК кератиноцитов и фибробластов кожи, в частности, после фотолиза, индуцированного УФ-излучением (загар). Фолаты участвуют в обмене пуринов, гомоцистеина, холина, гистидина, осуществляют синтез аминокислот, нуклеиновых кислот, пиримидинов, эссенциальных фосфолипидов, нейротрансмиттеров (серотонин, мелатонин, адреналин, дофамин), а также клеточных рецепторов. Экспериментальные исследования установили, что антидепрессивный эффект фолиевой кислоты объясняется ее взаимодействием с норадренергическими (α1 и α2) и серотонинергическими рецепторами (5-гидрокситриптаминовыми 5-HT1A и 5-HT2A/2C) [37]. Таким образом, применение КОК с фолатами способствует нормализации психоэмоциональной сферы пациенток с акне.
Особенно ценно использование препарата джес плюс в качестве контрацепции у женщин детородного периода, получающих терапию по поводу акне системным изотретиноином на протяжении всего срока лечения (8 мес.) и 1–2 мес. по его окончании, благодаря метаболической нейтральности дроспиренона (не вмешиваясь в метаболизм триглицеридов и холестерина, в отличие от прочих гестагенов, входящих в состав КОК, не потенцирует неизбежное негативное влияние изотретиноина на липидно-холестериновый профиль) [12].
Заключение
Резюмируя данные литературы, можно заключить, что в настоящее время назначение дроспиренон-содержащих комбинированных гормональных контрацептивов с фолатами является патофизиологической основой лечения акне у женщин репродуктивного возраста. Эффективность подтверждена уже через 3 месяца применения, поэтому лечение умеренных акне внесено как дополнительное показание в инструкцию к применению препарата. Дополнительное содержание фолатов способствует нивелированию возможной фотосенсибилизации на фоне синтетических эстрогенов, а также поддержанию оптимального уровня фолиевой кислоты в организме женщины для планирования последующей беременности. Именно дроспиренон-содержащие КОК с фолатами могут по праву называться патогенетической терапией акне у женщин репродуктивного возраста.



