Principles of early diagnosis of endometriosis based on the assessment of comorbidity and clinical manifestations
Objective: To analyze the clinical characteristics and concomitant diseases in patients with endometriosis. Materials and methods: The cross-sectional study was conducted at the National Medical Research Center for Obstetrics, Gynecology and Perinatology in Moscow, Russia in the period from 2021 to 2022. The main group included 110 patients (mean age 29 (25; 36) years) with extragenital endometriosis confirmed by ultrasound and MRI assessment; the control group consisted of 110 women who were comparable in age to the patients of the main group (mean age 27 (26; 32) years) without echographic signs of endometriosis. The patients of both study groups were interviewed and surveyed for a comprehensive analysis of clinical and anamnestic data; the presence of comorbid conditions was determined on the basis of laboratory and instrumental studies which were carried out in accordance with the clinical recommendations of the Ministry of Health of the Russian Federation. Results: Not only the severity of dysmenorrhea, dyspareunia and chronic pelvic pain, assessed by the visual analog scale (VAS), are significant, but also the combination of the symptoms is of importance, as it demonstrates a two-fold increase in the risk of endometriosis. It was found that lower body mass index (BMI) is characteristic of the patients of the main group (p=0.001). Every fifth patient with endometriosis had a body weight deficit (BMI<18.5 kg/m2), every second patient had a BMI<21 kg/m2. The patients with endometriosis, in comparison with the patients of the control group, had a higher incidence of acne (39.5 and 13.6%), fibrocystic disease (29.5 and 12%), human papillomavirus (41.7% and 22.7%), endometrial polyps (20.9 and 5.5%), uterine fibroids (23.6 and 8.2%, respectively). The presence of recurrent functional cysts (OR 2.11, 95% CI 1.66–2.69) and ovarian apoplexy (OR 1.99, 95% CI 1.62–2.45) had the greatest diagnostic significance. The combination of several comorbid conditions greatly increased the likelihood of endometriosis. Conclusion: At the initial stage of the examination of a patient with suspected endometriosis, it is advisable to listen to the patient’s complaints and also to conduct a cumulative assessment of their severity counting the points according to the VAS score. Thus, precise anamnesis and clinical examination can be considered effective and available methods of identifying women at risk for the development of endometriosis. Authors’ contributions: Chernukha G.E., Pronina V.A. – developing the concept of the study; Pronina V.A. – obtaining and analyzing actual data, writing and editing the text of the article; Chernukha G.E., Dumanovskaya M.R. – editing and approval of the text of the article. Conflicts of interest: The authors declare that there are no conflicts of interest. Funding: The study was carried without sponsorship. Acknowledgements: We express our gratitude to PhD N.D. Simich-Lafitsky for statistical processing and mathematical description of the data obtained in the course of the study. Ethical Approval: The study was approved by the Ethical Review Board of Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia. Patient Consent for Publication: The patients provided an informed consent for the publication of their data. Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator. For citation: Pronina V.A., Dumanovskaya M.R., Chernukha G.E. Principles of early diagnosis of endometriosis based on the assessment of comorbidity and clinical manifestations. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (4): 87-96 (in Russian) https://dx.doi.org/10.18565/aig.2023.9Pronina V.A., Dumanovskaya M.R., Chernukha G.E.
Keywords
The improvement of the principles of diagnosing endometriosis is one of the main tasks of gynecology at the present time, as there is a consistent trend towards an increase in the frequency of endometriosis, difficulties in diagnosis and delay in prescribing therapy [1, 2]. There is no doubt that the diagnosis of endometriosis should be complex and include a survey of patients, clinical examination and the use of visual methods such as transvaginal ultrasound and magnetic resonance imaging (MRI). Many authors agree that an adequate assessment of complaints and taking a patient’s history are important, since they can allow the clinicians to assume a correct diagnosis at the initial stage of the examination [3–6]. According to the 2022 clinical guidelines, laparoscopy with histological verification of the diagnosis is an informative examination method, but it is not absolutely necessary for diagnostic search; therefore, non-invasive examination methods come to the fore [7]. It should be noted that the classic symptoms of endometriosis such as dysmenorrhea, dyspareunia and chronic pelvic pain may be absent in patients with an asymptomatic course of the disease [8, 9]. Thus, it is necessary to continue the search for phenotypic markers of the disease and comorbid conditions which can help to identify women at high risk for the development of endometriosis. There are some studies demonstrating the connection of endometriosis with a number of diseases. For example, the frequency of detection of chronic endometritis is significantly higher in the group of patients with endometriosis (52.94%), regardless of its form in comparison with the control group (27.02%) (p=0.0311) [10]. A retrospective cohort study involving 141,460 women showed that there was a 3-fold increased risk of endometriosis in patients with pelvic inflammatory diseases (PID) (RR 3.02 [95% CI: 2.85;3.2]) [11]. Other studies revealed the relationship of endometriosis with irritable bowel syndrome (IBS) (RR 2.39 [95% CI: 1.83;3.11]) [12], interstitial cystitis (OR 3.74 [95% CI: 1.76;7.94]) [13], migraine (RR 2.62 [95% CI: 1.43;4.79]) [14], chronic autoimmune thyroiditis (RR 11.37 [95% CI: 6.0;21.5]) [15], as well as with a lower body mass index (BMI) [16]. A positive family history of endometriosis is also important; the risk of the disease increases if siblings experience this disease [17, 18]. Moreover, there is evidence that an increase in the number of symptoms raises the likelihood of diagnosis of the disease [19]. However, there is still no comprehensive approach to assessing the risks of endometriosis.
Thus, the objective of this study is to analyze the clinical characteristics and comorbidities in patients with endometriosis in order to determine the significance of comorbidity in detecting this disease.
Materials and methods
It was a cross-sectional study that included 220 women aged 18 to 45 years. The main group consisted of 110 patients (mean age 29 (25; 36) years, mean BMI 20.7 (19.37; 22.49) kg/m2) with extragenital endometriosis; the control group included 110 women (mean age 27 (26; 32) years, mean BMI 22.31(20.07; 23.92) kg/m2) who were comparable in age to the patients of the main group (р>0.05) without endometriosis. The inclusion criterion for the patients of the main group was the presence of extragenital endometriosis verified by the ultrasound assessment and MRI of the pelvic organs. The patients who had no signs of endometriosis according to ultrasound assessment, MRI, or the results of previous surgical intervention met the criterion for inclusion in the control group. The patients of both study groups were interviewed and surveyed for a comprehensive analysis of clinical and anamnestic data; the presence of comorbid conditions was determined on the basis of laboratory and instrumental studies which were carried out in accordance with the clinical recommendations of the Ministry of Health of the Russian Federation [20]. The exclusion criteria for both groups were current and previous oncological diseases of the female reproductive system, pregnancy and lactation, hormone therapy for three months prior to inclusion in the study. In order to reduce the likelihood of systematic error, patients with echographic signs of extragenital endometriosis as well as patients with adenomyosis of any degree were excluded from the control group. The family history of endometriosis was assessed as the presence of the disease in the first line of kinship, as well as in the maternal grandmother. IBS was determined according to the Rome IV criteria [21], migraine was assessed using the data from the ID Migraine questionnaire in case of two or more positive answers [22]. Clinical hyperandrogenism was confirmed when acne and/or hirsutism were detected. Testing for the presence of human papillomavirus (HPV) and herpes simplex virus was carried out only in sexually active women. The severity of dysmenorrhea, dyspareunia and chronic pelvic pain was assessed using an 11-score visual analog scale (VAS). In virgin patients, the indicator of dyspareunia was not taken into account in the calculations. Chronic pelvic pain was defined as an acyclic pain in the pelvic area, not associated with menstruation or sexual intercourse and lasting for 6 months or more. Before the analysis of the clinical material, all the data were completely depersonalized. The study was conducted at the National Medical Research Center for Obstetrics, Gynecology and Perinatology in Moscow, Russia in the period from 2021 to 2022 and it was approved by the local ethics committee. Given the relatively small sample size (the results of an interim analysis are presented), it was not possible to assess the dependence of clinical manifestations on the form of endometriosis; this analysis will be done in the future.
Statistical analysis
Statistical analysis was performed using the SPSS software (IBM Statistical Package for the Social Sciences, version 26). The sample size was calculated on the basis of the prevalence of dysmenorrhea as the most significant clinical manifestation of endometriosis [7, 19], that was 63 women if the significance level was 5% and CI was 95%. Taking into account the potentially smaller number of women who could be examined for a number of parameters (dyspareunia, HPV, etc.), this sample was sufficient to conduct the study. The control group was selected according to the ratio 1:1. The following parameters were calculated for quantitative indicators: mean value (M), standard deviation (SD), 95% CI, median (Me), interquartile interval (Q1; Q3). Frequency (%) was calculated for qualitative and ordinal indicators. All the obtained quantitative parameters were checked for compliance with the normal distribution using the Shapiro–Wilk test. Numerical parameters having a normal distribution are presented in the format M (SD), where M is the mean value, SD is the standard deviation of the mean value. The parameters which have a distribution other than normal are presented in the format Me (Q1; Q3). In order to find the differences between the groups of patients, we applied a pairwise comparison of the groups using the nonparametric method of the Mann–Whitney U-test (for quantitative parameters) and the Fisher’s exact test (for categorical parameters). To estimate the threshold value of the scores of dysmenorrhea, chronic pelvic pain and dyspareunia, we used a ROC analysis based on the maximum Yuden index (Se+Sp)-1 for each of the parameters. In order to obtain a numerical value of the clinical significance of the test, as well as to compare the two tests, the AUC parameter (Area Under Curve, or area under the curve) was used with an indication of the confidence interval; it is possible to estimate the quality of the test using AUC values scale. To compare the probability of an outcome depending on the presence of a risk factor, the relative risk indicator was used. When testing statistical hypotheses, the critical significance level was assumed to be 0.05.
Results
Among all the cases of extragenital endometriosis, isolated peritoneal endometriosis was diagnosed in 2/110 women (47.3%) and endometriomas were revealed in 22/110 women (20%). Deep endometriosis was verified in 36/110 women (32.7%); it was combined with two other forms in 22/36 (61.1%) of them; every fourth patient with extragenital endometriosis (8/36 (22.2%)) had colorectal endometriosis, and one patient (1/36 (2.8%)) had bladder endometriosis). Extragenital endometriosis was combined with diffuse adenomyosis in 89/110 cases (80.9%), and with diffuse nodular form in 8/110 women (7.3%). Previously, 65/110 women (56.4%) received hormone therapy, 36/110 patients (32.7%) were operated on for endometriosis, including 8/36 women (22.2%) who underwent it repeatedly.
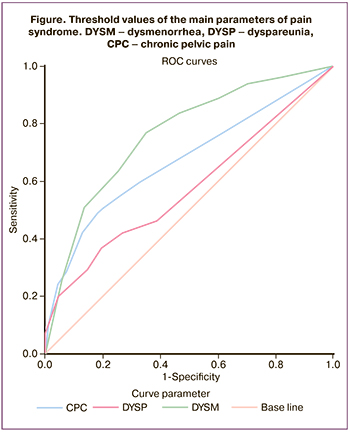
The analysis of clinical and anamnestic data showed that patients with endometriosis were characterized by a lower BMI than women in the control group (20.7 (19.37; 22.49) kg/m2 versus 22.31 (20.07; 23.92) kg/m2, p<0.01). Almost every fifth patient with endometriosis had a BMI of less than 18.5 kg/m2, and every second patient had a BMI of less than 21 kg/m2. The data presented in Table 1 shows that body weight deficiency was about twice as common in patients with endometriosis, and excess body weight was twice as rare (Table 1).
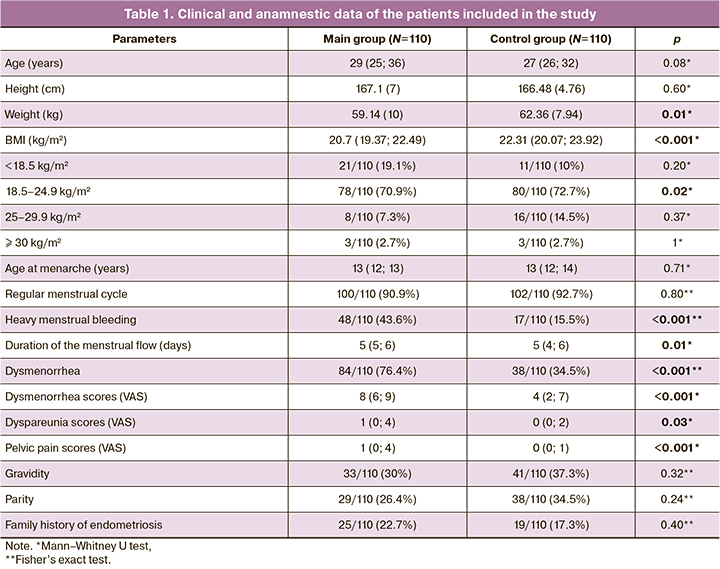
The analysis of the clinical data revealed that patients with endometriosis have heavier, more prolonged and painful menstruation. The intensity of dysmenorrhea, dyspareunia and pelvic pains differed significantly in the two study groups (Table 1). Taking into account the threshold values, it was found that this parameter for dysmenorrhea was 5.5 VAS scores (sensitivity 76.4%, specificity 65.5%, AUC 0.76 [95% CI: 0.70;0.83]); it was 1.5 scores for pelvic pains (sensitivity 49.1%, specificity 91.8%, AUC 0.67 [95% CI: 0.60;0.74]); it was 2.5 scores for dyspareunia (sensitivity 40.8%, specificity 88.4%, AUC 0.58 [95% CI: 0.50;0.65]) (p<0.001) (Figure).
Two groups of patients were analyzed in order to confirm the necessity of determining VAS scores for the diagnosis of endometriosis. The first group included women whose pain syndrome was determined when its intensity exceeded the estimated threshold values. The second group included women whose pains were assessed without VAS scores, but only due to the fact of the presence or absence of pain in their subjective evaluation. Thus, taking into account the estimated threshold values, dysmenorrhea was observed in 84/110 women with endometriosis (76.4%) and 38/110 women of the control group (34.5%); when threshold values were not considered, dysmenorrhea was observed in 108/110 (98.2%) and 101/110 (91.8%) women, respectively; dyspareunia was noted in 40/98 (40.8%) and 21/97 (21.6%) women taking into account threshold values, and it was noted in 50/98 (51%) and 42/97 (43.3%) patients without considering threshold values, respectively; pelvic pains were observed in 54/110 (49.1%) and 20/110 (18.2%) women taking into account threshold values, and in 63/110 (57.3%) and 32/110 (29.1%) patients without taking into account threshold values, respectively. Thus, determining the VAS scores allows clinicians not only to increase the probability of diagnosing endometriosis, but also to exclude possible false positive results in a subjective survey of patients. The greatest clinical significance was noted in the combined assessment of the characteristics of the pain syndrome taking into account the defined thresholds. The combination of dysmenorrhea and dyspareunia in patients with endometriosis was three times more common than in patients of the control group (33/98 (33.7%) and 13/97 (13.4%) (RR 1.64 [95% CI: 1.27;2.13]; the combination of dysmenorrhea with pelvic pains was noted more frequently by 3.5 times (24/98 (24.5%) and 7/97 (7.2%) (RR 1.72 [95% CI: 1.33;2.21]); the combination of dysmenorrhea with pelvic pains was five times more common (51/110 (46.4%) and 10/110 (9.1%) (RR 2.25 [95% CI: 1.79;2.84]); the presence of all three parameters of pain syndrome was six times more frequent (24/98 (24.5%) and 4/97 (4.1%), respectively) (RR 1.93 [95% CI: 1.54;2.43]), and the risk of endometriosis increased twice.
When extragenital pathology was assessed, a significant difference between the groups attracted attention (Table 2). Thus, fibrocystic disease (FCD) affected approximately every third patient with endometriosis which is 2.5 times more common than in the patients in the control group; migraine, IBS and chronic cystitis occurred in every fourth woman in the main group and twice as often, respectively; however, there was no statistically significant difference between the groups. Clinical hyperandrogenism was observed in 66/110 (60%) patients with endometriosis, and it was noted twice less often in the patients of the control group. Acne was three times more common in the patients in the main group (every third woman).
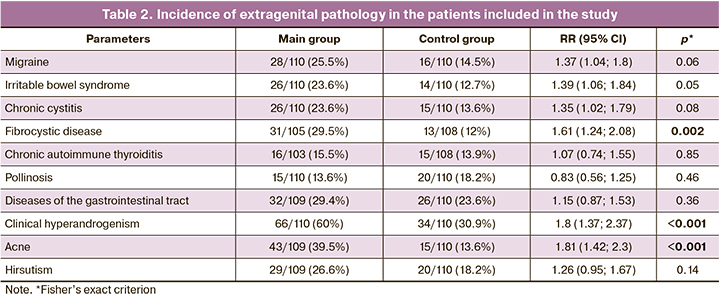
The data on the comparative assessment of the frequency of detecting concomitant gynecological diseases are presented in Table 3. We noticed a high incidence of HPV (40/96 (41.7%) in the main group and 22/97 (22.7%) in the comparison group), endometrial polyps (23/110 (20.9%) and 6/110 (5.5%) and uterine fibroids (26/110 (23.6%) and 9/110 (8.2%) in endometriosis. Recurrent functional ovarian cysts were found in every second patient with endometriosis, ovarian apoplexy was noted in every fifth woman which was much more common in comparison with the control group. Despite the fact that genital herpes, PID and paraovarian cysts were two or three times more common in patients with endometriosis, there was no statistically significant difference between the groups.
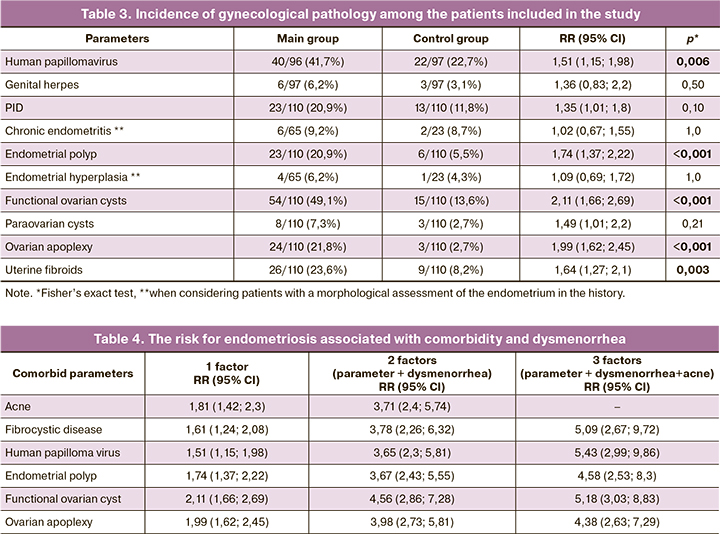
In order to improve the early diagnosis of endometriosis based on all available data on comorbidity, we created a table of disease risk stratification based on the data of six parameters with reliable statistical significance, dysmenorrhea being the most important clinical symptom of endometriosis (Table 4). To calculate the RR value, the presence of all factors was considered as the “presence” of a risk factor (a value of ≥5.5 was used for the dysmenorrhea score (see the results of the ROC analysis), the absence of all factors was considered as the “absence” of a risk factor (a value of <5.5 was used for the dysmenorrhea score). As one can see it in Table 4, a combination of comorbid conditions increases the risk of endometriosis. For example, HPV increases the probability of endometriosis by 1.5 times, when this disease is combined with dysmenorrhea, the risks increase up to 3.7 times, and the combination of these signs with acne raises the likelihood of endometriosis by up to 5.5 times. Comparable results were obtained for the parameters of endometrial polyp and apoplexy. However, the combination of more than three symptoms slightly increased the risks for endometriosis or the risks could not be calculated due to the small sample of patients.
Discussion
The present study which is devoted to the role of comorbidity in the diagnosis of endometriosis has shown that an adequate assessment of complaints and anamnesis data, as well as visual examination, makes it possible to identify a group of women at high risk for the development of this disease at the initial stage of the examination. The obtained data indicate that clinical hyperandrogenism, in particular the presence of acne, is quite simple to diagnose and at the same time it is a significant comorbid condition in endometriosis. Thus, according to the results of the study, clinical hyperandrogenism was revealed twice more often, and acne was noted three times more often in patients with endometriosis. The data of a large study conducted in Korea indicate a 5-fold increase in the frequency of acne in patients with endometriosis compared with the patients of the control group [23]. According to other publications, the risk of endometriosis increases twice in adolescents with severe acne [24, 25]. The frequency of acne is known to decrease progressively after 25 years. However, the results of the study showed that the incidence of acne remained quite high among patients with endometriosis aged 25 years and older: patients aged from 25 to 34 years old (15/47 (31.9%) versus 8% of general population, and from 35 to 44 years old (9/40 (22.5%) versus 3% of general population [26]). Despite the association of endometriosis and acne, the mechanisms of their relationship have not been totally identified. At the same time, it is known that the gene sequences responsible for the development of acne and endometriosis are located at the adjacent loci of chromosome 8q24. Therefore, it is possible to assume a linked inheritance of both conditions [27]. However, not only genetic factors are likely to play a role in the genesis of endometriosis and acne, but also chronic inflammation and changes in the microbiota; further research in this area is needed.
The results of the study showed that patients with endometriosis are characterized not only by acne, but also by lower BMI values. Thus, every fifth woman with endometriosis was found to have a body weight deficit; while an excess of body weight was observed twice less often in the patients of the main group than in the patients of the control group. A number of other studies also noted a statistically significant difference between women with endometriosis and the control group, both in BMI [28], including the puberty period [29], and in height and somatotype [30]. Nowadays, there are several hypotheses that may explain the lower BMI values in endometriosis. The most significant of them include the role of genetic factors. The expression of the Cyp2r1, Fabp4, Mrc1 and Rock2 genes associated with a decrease in body weight and the amount of adipose tissue was found to be increased in endometriosis, and the expression of the Igfbp1 and Mmd2genes associated with obesity was reduced [31]. The influence of adipokines is also highlighted in the literature, in particular, a higher level of leptin [32], which may be associated with the ability of endometrioid tissue to produce it [33]. Given the anorexigenic properties of leptin [34], it cannot be excluded that its additional production by the ectopic endometrium may contribute to a decrease in body weight and the amount of adipose tissue.
The association of endometriosis with a number of estrogen-dependent diseases is well known [19]. This relationship is also confirmed by the results of the study which showed the comorbidity of extragenital endometriosis fibrocystic disease, uterine fibroids, and endometrial polyps. Attention was drawn to a 4-fold increase in the frequency of endometrial polyps in patients with endometriosis in comparison with the control group (RR 1.74 [95% CI: 1.37;2.22]). According to a meta–analysis involving 2,896 women, the risk of endometrial polyps in women with endometriosis is increased by almost 3 times (RR 2.81 [95% CI: 2.48;3.18]), mainly with degree 2–4 of the disease prevalence [35]. The results of another study showed that histologically verified endometrial polyps occur 1.5 times more often in patients with endometriosis and infertility (38.8%), compared with women suffering only from infertility (24.21%) (RR 1,992, p<0.001) [36]. One of the possible explanations for comorbidity of endometriosis and endometrial polyps may be their connection with chronic inflammation [37, 38]. Moreover, these diseases belong to the category of proliferative processes of endometrium and myometrium; proliferation increase mediated by local influence of estrogens [39, 40] and aromatase [41, 42] plays a role in their genesis.
This study revealed a 2-fold increase in the frequency of HPV detection in patients with endometriosis. There are some studies demonstrating a higher incidence of HPV infection of high oncogenic risk in women with endometriomas [43]. Papillomavirus infection was also found to be associated with infertility (RR 1.39 [95% CI: 1.19;1.63]), the risk increased in the presence of concomitant endometriosis (RR 1.85 [95% CI: 1.33;2.58]) [44]. However, the association of these two diseases is denied by some scientists [45]. It should be noted that currently there is no pathogenetic explanation for comorbidity of HPV and endometriosis. Endometriosis-associated chronic inflammation may prolong the persistence of HPV, or, as suggested by Oppelt R. et al., viral infection may contribute to the implantation of endometrioid tissue in patients suffering from hyperestrogenism, chronic inflammation and immune imbalance [46].
According to the obtained data, there is the highest correlation between endometriosis and functional ovarian cyst, and especially between endometriosis and ovarian apoplexy. As the study showed, functional ovarian cyst occurred in 54/110 women (49.1%), ovarian apoplexy in 24/110 (21.8%) patients with extragenital endometriosis, which was 4 and 9 times more common than in the control group (15/110 (13.6%) and 3/110 (2.4%), respectively). The results of a number of other studies are consistent with these data and demonstrate that functional ovarian cysts occur 3-7 times more often in women with endometriosis [19, 23, 47]. However, none of these studies discussed the reason for their more frequent occurrence in patients with endometriosis. As for ovarian apoplexy, there are few data in the foreign literature presented mainly by clinical cases of hemoperitoneum in endometriosis due to apoplexy of endometriomas or corpus luteum cysts [48, 49]. The Russian scientists have noted that ovarian apoplexy occurs in every fifth patient with extragenital endometriosis [50]. In 70% of cases, apoplexy manifests in the luteal phase of the cycle characterized by a physiological increase in blood flow in the ovary [51]. The production of vascular endothelial growth factor (VEGF) increases in endometriosis and it enhances angiogenesis causing pathological neovascularization [52]. An increase in the expression of VEGF, as well as its receptors, VEGF-1 and VEGF-2, is found in the endothelium of the ovarian vessels and luteal cells of the corpus luteum [53]. Thus, atypical vascularization and increased permeability of the vascular wall may predispose to hemorrhage [54].
The most common complaints in endometriosis are dysmenorrhea, pelvic pains and dyspareunia. According to the results of this study, dysmenorrhea was observed in 84/110 (76.4%) patients with endometriosis and 38/110 (34.5%) women of the control group. It is also important that the frequency of dysmenorrhea was comparable in the cohort of patients in the control group, 8/23 (34.7%) women with laparoscopically excluded endometriosis. Given the relatively high frequency of primary dysmenorrhea (from 17% to 90%) [55], it is important to note that the frequency of dysmenorrhea in patients under 30 years of age was 46/56 (82.1%) in the main group and 32/74 (43.2%) in the control group; while its frequency remained quite high in women with endometriosis in the age group of 30 years and older, 38/54 (70.4%) women, and it was 2.5 times lower in the control group, 6/36 (16.7%) patients. In real clinical practice, it is important not only to identify dysmenorrhea which is assessed subjectively by the patient, but also to determine the degree of its severity, the absence of a tendency to decrease the intensity of pain with age and the combination with pelvic pain and dyspareunia which may twice increase the risk of endometriosis. Although dysmenorrhea is considered to be the main symptom of endometriosis, it is still not detected in 20-30% of women [9]. Therefore, a comprehensive assessment of comorbidity greatly increases the chances of timely diagnosis of endometriosis. The simplest symptoms which do not require additional research, in addition to dysmenorrhea, are the presence of acne and ovarian apoplexy in the anamnesis. The first two symptoms can be found out during the first visit to the gynecologist, and the last one can be revealed according to the patient’s medical records. The assessment of the combination of two parameters appeared to increase the risk of endometriosis from 1.5 to 2 times (only dysmenorrhea: RR 2.6 [95% CI: 1.83;3.68], dysmenorrhea in combination with apoplexy: RR 3.98 [95% CI: 2.73;5.81], dysmenorrhea in combination with functional ovarian cyst: RR 4.56 [95% CI: 2.86;7.28]). The detection of acne is essential in combination with dysmenorrhea and other comorbid conditions, such as functional ovarian cyst, fibrocystic disease, endometrial polyps and HPV. Similar data were obtained in a study by Ballard et al. where the probability of detecting endometriosis increased in the process of increasing the number of concomitant symptoms (one symptom: OR 5.0 [95% CI: 4.4;5.7]; three symptoms: OR 22.9 [95% CI: 19.9;26.4]) [19], which confirms the necessity of an integrated approach in the diagnosis of extragenital endometriosis.
Conclusion
Thus, the results of the study suggest that the assessment of comorbidity and characteristic clinical symptoms at the initial stage of the examination allows the clinicians to identify a group of women with a high risk for extragenital endometriosis and refer the patients for further examination. The use of this approach in clinical practice in the future may help to reduce the time before diagnosis and timely treatment of endometriosis.
References
- Nnoaham K.E., Hummelshoj L., Webster P., d'Hooghe T., de Cicco Nardone F., de Cicco Nardone C. et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil. Steril. 2011; 96(2): 366-73.e8. https://dx.doi.org/10.1016/j.fertnstert.2011.05.090.
- Morassutto C., Monasta L., Ricci G., Barbone F., Ronfani L. Incidence and estimated prevalence of endometriosis and adenomyosis in northeast Italy: a data linkage study. PLoS One. 2016; 11: e0154227.
- Signorile P.G., Cassano M., Viceconte R., Marcattilj V., Baldi A. Endometriosis: a retrospective analysis of clinical data from a cohort of 4,083 patients, with focus on symptoms. In Vivo. 2022; 36(2): 874-83. https://dx.doi.org/10.21873/invivo.12776.
- Eisenberg V.H., Decter D.H., Chodick G., Shalev V., Weil C. Burden of endometriosis: infertility, comorbidities, and healthcare resource utilization. J. Clin. Med. 2022; 11(4): 1133. https://dx.doi.org/10.3390/jcm11041133.
- Chapron C., Lafay-Pillet M.-C., Santulli P., Bourdon M., Maignien C., Gaudet-Chardonnet A. et al. A new validated screening method for endometriosis diagnosis based on patient questionnaires. EClinicalMedicine. 2022; 44: 101263. https://dx.doi.org/10.1016/j.eclinm.2021.101263.
- Fauconnier A., Drioueche H., Huchon C., Du Cheyron J., Indersie E., Candau Y. Early identification of women with endometriosis by means of a simple patient-completed questionnaire screening tool: a diagnostic study. Fertil. Steril. 2021; 116(6): 1580-9. https://dx.doi.org/10.1016/j.fertnstert.2021.07.1205.
- ESHRE Endometriosis Guideline Group. ESHRE guideline: endometriosis. Hum. Reprod. Open. 2022; 2022(2): hoac009. https://dx.doi.org/10.1093/hropen/hoac009.
- Singh S., Soliman A.M., Rahal Y., Robert C., Defoy I., Nisbet P., Leyland N. Prevalence, symptomati burden, and diagnosis of endometriosis in Canada: cross-sectional survey of 30 000 women. J. Obstet. Gynaecol. Canada. 2020; 42(7): 829-38. https://dx.doi.org/10.1016/j.jogc.2019.10.038.
- Moradi Y., Shams-Beyranvand M., Khateri S., Gharahjeh S., Tehrani S., Varse F. et al. A systematic review on the prevalence of endometriosis in women. Indian J. Med. Res. 2021; 154(3): 446-54. https://dx.doi.org/10.4103/ijmr.IJMR_817_18.
- Takebayashi A., Kimura F., Kishi Y., Ishida M., Takahashi A., Yamanaka A. et al. The association between endometriosis and chronic endometritis. PLoS One. 2014; 9(2): e88354. https://dx.doi.org/10.1371/journal.pone.0088354.
- Tai F.-W., Chang C., Chiang J.-H., Lin W.-C., Wan L. Association of pelvic inflammatory disease with risk of endometriosis: a nationwide cohort study involving 141,460 individuals. J. Clin. Med. 2018; 7(11): 379. https://dx.doi.org/10.3390/jcm7110379.
- Saidi K., Sharma S., Ohlsson B. A systematic review and meta-analysis of the associations between endometriosis and irritable bowel syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020; 246: 99-105. https://dx.doi.org/10.1016/j.ejogrb.2020.01.031.
- Wu C.-C., Chung S.-D., Lin H.-C. Endometriosis increased the risk of bladder pain syndrome/interstitial cystitis: a population-based study. Neurourol. Urodyn. 2018; 37(4): 1413-8. https://dx.doi.org/10.1002/nau.23462.
- Maitrot-Mantelet L., Hugon-Rodin J., Vatel M., Marcellin L., Santulli P., Chapron C., Plu-Bureau G. Migraine in relation with endometriosis phenotypes: results from a french case-control study. Cephalalgia. 2020; 40(6): 606-13. https://dx.doi.org/10.1177/0333102419893965.
- Porpora M.G., Scaramuzzino S., Sangiuliano C., Piacenti I., Bonanni V., Piccioni M.G. et al. High prevalence of autoimmune diseases in women with endometriosis: a case-control study. Gynecol. Endocrinol. 2020; 36(4): 356-9. https://dx.doi.org/10.1080/09513590.2019.1655727.
- Shah D.K., Correia K.F., Vitonis A.F., Missmer S.A. Body size and endometriosis: results from 20 years of follow-up within the Nurses’ Health Study II prospective cohort. Hum. Reprod. 2013; 28(7): 1783-92. https://dx.doi.org/10.1093/humrep/det120.
- Ashrafi M., Sadatmahalleh S.J., Akhoond M.R., Talebi M. Evaluation of risk factors associated with endometriosis in infertile women. Int. J. Fertil. Steril. 2016; 10(1): 11-21. https://dx.doi.org/10.22074/ijfs.2016.4763.
- Kim H.J., Lee H.S., Kazmi S.Z., Hann H.J., Kang T., Cha J. et al. Familial risk for endometriosis and its interaction with smoking, age at menarche and body mass index: a population-based cohort study among siblings. BJOG. 2021; 128(12): 1938-48. https://dx.doi.org/10.1111/1471-0528.16769.
- Ballard K., Seaman H., de Vries C., Wright J. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study-Part 1. BJOG. 2008; 115(11): 1382-91. https://dx.doi.org/10.1111/j.1471-0528.2008.01878.x.
- Министерство здравоохранения Российской Федерации. Клинические рекомендации. Эндометриоз. 2020. [Ministry of Health of the Russian Federation. Clinical guidelines. Endometriosis. 2020. (in Russian)]. Available at: https://cr.minzdrav.gov.ru/clin_recomend
- Drossman D.A., Hasler W.L. Rome IV—functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016; 150(6): 1257-61.https://dx.doi.org/10.1053/j.gastro.2016.03.035.
- Lipton R.B., Dodick D., Sadovsky R., Kolodner K., Endicott J., Hettiarachchi J., Harrison W.; ID migraine validation study. a self-administered screener for migraine in primary care: the ID migraine validation study. Neurology. 2003; 61(3): 375-82. https://dx.doi.org/10.1212/01.wnl.0000078940.53438.83.
- Choi E.J., Cho S.B., Lee S.R., Lim Y.M., Jeong K., Moon H.S., Chung H. Comorbidity of gynecological and non-gynecological diseases with adenomyosis and endometriosis. Obstet. Gynecol. Sci. 2017; 60(6): 579-86.https://dx.doi.org/10.5468/ogs.2017.60.6.579.
- Xie J., Kvaskoff M., Li Y., Zhang M., Qureshi A.A., Missmer S.A., Han J. Severe teenage acne and risk of endometriosis. Hum. Reprod. 2014; 29(11): 2592-9. https://dx.doi.org/10.1093/humrep/deu207.
- Ricci G., Castelpietra E., Romano F., Di Lorenzo G., Zito G., Ronfani L. et al. Case-control study to develop and validate a questionnaire for the secondary prevention of endometriosis. PLoS One. 2020; 15(3): e0230828.https://dx.doi.org/10.1371/journal.pone.0230828.
- Российское общество дерматовенерологов и космететологов. Акне вульгарные. Клинические рекомендации. 2020. [Russian Society of Dermatovenerologists and Cosmetologists. Acne vulgar. Clinical guidelines. 2020. (in Russian)].
- Zhang M., Qureshi A.A., Hunter D.J., Han J. A genome-wide association study of severe teenage acne in European Americans. Hum. Genet. 2014; 133(3): 259-64. https://dx.doi.org/10.1007/s00439-013-1374-4.
- Backonja U., Hediger M.L., Chen Z., Lauver D.R., Sun L., Peterson C.M., Buck Louis G.M. Beyond body mass index: using anthropometric measures and body composition indicators to assess odds of an endometriosis diagnosis. J. Womens Health. 2017; 26(9): 941-50. https://dx.doi.org/10.1089/jwh.2016.6128.
- Aarestrup J., Jensen B.W., Ulrich L.G., Hartwell D., Trabert B., Baker J.L. Birth weight, childhood body mass index and height and risks of endometriosis and adenomyosis. Ann. Hum. Biol. 2020; 47(2): 173-80. https://dx.doi.org/10.1080/03014460.2020.1727011.
- Farland L.V., Missmer S.A., Bijon A., Gusto G., Gelot A., Clavel-Chapelon F. et al. Associations among body size across the life course, adult height and endometriosis. Hum. Reprod. 2017; 32(8): 1732-42. https://dx.doi.org/10.1093/humrep/dex207.
- Goetz L.G., Mamillapalli R., Taylor H.S. Low body mass index in endometriosis is promoted by hepatic metabolic gene dysregulation in mice. Biol. Reprod. 2016; 95: 1-8. https://dx.doi.org/10.1095/biolreprod.116.142877.
- Hussein S.S., Farhan F.S., Ibrahim Ali A. Serum leptin as a marker for severity of endometriosis. Obstet. Gynecol. Int. 2020; 2020: 6290693.https://dx.doi.org/10.1155/2020/6290693.
- Choi Y.S., Oh H.K., Choi J.H. Expression of adiponectin, leptin, and their receptors in ovarian endometrioma. Fertil. Steril. 2013; 100(1): 135-41.e1-2. https://dx.doi.org/10.1016/j.fertnstert.2013.03.019.
- Picó C., Palou M., Pomar C.A., Rodríguez A.M., Palou A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2022; 23(1): 13-30.https://dx.doi.org/10.1007/s11154-021-09687-5.
- Zheng Q.M., Mao H.I., Zhao Y.J., Zhao J., Wei X., Liu P.S. Risk of endometrial polyps in women with endometriosis: a meta-analysis. Reprod. Biol. Endocrinol. 2015; 13: 103. https://dx.doi.org/10.1186/s12958-015-0092-2.
- Zhang Y., Zhang Y., Yu Q., Guo Z., Ma J., Yan L. Higher prevalence of endometrial polyps in infertile patients with endometriosis. Gynecol. Obstet. Invest. 2018; 83(6): 558-63. https://dx.doi.org/10.1159/000487946.
- Cicinelli E., Trojano G., Mastromauro M., Vimercati A., Marinaccio M., Mitola P.C. et al. Higher prevalence of chronic endometritis in women with endometriosis: a possible etiopathogenetic link. Fertil. Steril. 2017; 108(2): 289-95.e1. https://dx.doi.org/10.1016/j.fertnstert.2017.05.016.
- Huang L., Xiang M. Recent advances in endometrial polyps. J. Int. Obstet. Gynecol. 2014; 41: 43-6.
- Jones R.K., Bulmer J.N., Searle R.F. Immunohistochemical characterization of proliferation, oestrogen receptor and progesterone receptor expression in endometriosis: comparison of eutopic and ectopic endometrium with normal cycling endometrium. Hum. Reprod. 1995; 10(12): 3272-9.https://dx.doi.org/10.1093/oxfordjournals.humrep.a135901.
- Lopes R.G., Baracat E.C., de Albuquerque Neto L.C., Ramos J.F., Yatabe S., Depesr D.B., Lippi U.G. Analysis of estrogen- and progesterone-receptor expression in endometrial polyps. J. Minim. Invasive Gynecol. 2007; 14(3): 300-3. https://dx.doi.org/10.1016/j.jmig.2006.10.022.
- Noble L.S., Simpson E.R., Johns A., Bulun S.E. Aromatase expression in endometriosis. J. Clin. Endocrinol. Metab. 1996; 81(1): 174-9.https://dx.doi.org/10.1210/jcem.81.1.8550748.
- Maia H. Jr, Pimentel K., Silva T.M., Freitas L.A., Zausner B., Athayde C., Coutinho E.M. Aromatase and cyclooxygenase-2 expression in endometrial polyps during the menstrual cycle. Gynecol. Endocrinol. 2006; 22(4): 219-24. https://dx.doi.org/10.1080/09513590600585955.
- Heidarpour M., Derakhshan M., Derakhshan-Horeh M., Kheirollahi M., Dashti S. Prevalence of high-risk human papillomavirus infection in women with ovarian endometriosis. J. Obstet. Gynaecol. Res. 2017; 43(1): 135-9.https://dx.doi.org/10.1111/jog.13188.
- Hsu L.C., Tsui K.H., Wei J.C., Yip H.T., Hung Y.M., Chang R. Female human papillomavirus infection associated with increased risk of infertility: A Nationwide Population-Based Cohort Study. In.t J. Environ. Res. Public Health. 2020; 17(18): 6505. https://dx.doi.org/10.3390/ijerph17186505.
- Vestergaard A.L., Knudsen U.B., Munk T., Rosbach H., Bialasiewicz S., Sloots T.P. et al. Low prevalence of DNA viruses in the human endometrium and endometriosis. Arch. Virol. 2010; 155(5): 695-703. https://dx.doi.org/10.1007/s00705-010-0643-y.
- Oppelt P., Renner S.P., Strick R., Valletta D., Mehlhorn G., Fasching P.A. et al. Correlation of high-risk human papilloma viruses but not of herpes viruses or Chlamydia trachomatis with endometriosis lesions. Fertil. Steril. 2010; 93(6): 1778-86. https://dx.doi.org/10.1016/j.fertnstert.2008.12.061.
- Soliman A.M.S.E., Johnson S.J., Davis M., Castelli-Haley J., Snabes M.C.Incidence of comorbidities among women with endometriosis: a retrospective matched cohort study. Fertil. Steril. 2016; 106(3): e277-8.https://dx.doi.org/10.1016/j.fertnstert.2016.07.796.
- Wang L., Jiang Y.J. Rupture of ovarian endometriotic cyst complicated with endometriosis: a case report. World J. Clin. Cases. 2021; 9(28): 8524-30.https://dx.doi.org/10.12998/wjcc.v9.i28.8524.
- Cozzolino M., Corioni S., Maggio L., Sorbi F., Guaschino S., Fambrini M. Endometriosis-related hemoperitoneum in pregnancy: a diagnosis to keep in mind. Ochsner J. 2015; 15(3): 262-4.
- Елгина С.И., Лаврова Е.В. Экстрагенитальный эндометриоз: клинико-анамнестические особенности. Медицина в Кузбассе. 20214; 2: 47-9. [Elgina S.I., Lavrova E.V. Extragenital endometriosis: clinical and anamnestic features. Medicine in Kuzbass. 2021; (2): 47-9.(in Russian)].
- Кох Л.И. К вопросу этиопатогенеза апоплексии яичников. Мать и дитя в Кузбассе. 2014: 4: 15-8. [Kokh L.I. On pathogenesis of ovarian apoplexy. Mother and Child in Kuzbass. 2014: (4): 15-8. (in Russian)].
- Donnez J., Smoes P., Gillerot S., Casanas-Roux F., Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum. Reprod. 1998; 13(6): 1686-90. https://dx.doi.org/10.1093/humrep/13.6.1686.
- Стрижаков А.Н., Шахламова М.Н., Пирогова М.Н., Смирнов А.А. Апоплексия яичника: овариальный ангиогенез и прогностическая роль сосудисто-эндотелиального фактора роста. Вопросы гинекологии, акушерства и перинатологии. 2017; 16(1): 18-24. [Strizhakov A.N., Shakhlamova M.N., Pirogova M.N., Smimov A.A. Ovarian apoplexy: ovarian angiogenesis and the prognostic role of vascular-endothelial growth factor. Issues of Gynecology, Obstetrics and Perinatology. 2017; 16(1): 18-24. (in Russian)]. https://dx.doi.org/10.20953/1726-1678-2017-1-18-24.
- Buggio L., Aimi G., Vercellini P. Hemoperitoneum following sexual intercourse in a woman with deep infiltrating endometriosis. Gynecol. Obstet. Invest. 2016; 81(6): 559-62. https://dx.doi.org/10.1159/000447262.
- Ferries-Rowe E., Corey E., Archer J.S. Primary dysmenorrhea. Obstet. Gynecol. 2020; 136(5): 1047-58. https://dx.doi.org/10.1097/aog.0000000000004096.
Received 09.01.2023
Accepted 04.04.2023
About the Authors
Veronika A. Pronina, obstetrician-gynecologist, PhD student, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology,Ministry of Health of Russia, +7(916)025-86-26, ver22595@yandex.ru, https://orcid.org/0000-0003-4566-4065, 117997, Russia, Moscow, Ac. Oparin str., 4.
Madina R. Dumanovskaya, PhD, Researcher at the Department of Gynecological Endocrinology, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, +7(916)737-40-54, dumanovskaya@gmail.com, https://orcid.org/0000-0001-7286-6047,
117997, Russia, Moscow, Ac. Oparin str., 4.
Galina E. Chernukha, Dr. Med. Sci., Professor, Chief Researcher at the Department of Gynecological Endocrinology, Academician V.I. Kulakov National Medical Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Health of Russia, +7(985)999-60-00, c-galina1@yandex.ru, https://orcid.org/0000-0002-9065-5689,
117997, Russia, Moscow, Ac. Oparin str., 4.
Corresponding author: Galina E. Chernukha, c-galina1@yandex.ru



