Clinical and elastographic evaluation of methods for ripening an unripe cervix
Aim. To investigate the effectiveness of a combined use of DILAPAN-S expanders and oral mifepristone for cervical ripening and induction of labor.Brega E.S., Pekarev O.G., Gus A.I., Lun’kov S.S.
Material and methods. This prospective study comprised 200 pregnant women aged 23 to 38, who had an unfavorable cervix and indications for labor pre-induction. Fifty women (group 1) received four DILAPAN-S dilators for up to 12 hours co-administered with two 200 mg doses of oral mifepristone taken 24 hours apart. DILAPAN-S dilators were inserted simultaneously with the second mifepristone dose. DILAPAN-S dilators alone were used in 50 women (group 2). In group 3 (n=50), patients underwent cervical ripening with a Foley balloon catheter inserted into the cervix for up to 12 hours. Patients in group 4 (n=50) received cervical ripening with two doses of prostaglandin E2 gel 0.5 mg intracervically. Cervical ripening was assessed using the Bishop score and sonoelastography.
Results. DILAPAN-S in combination with mifepristone was more effective than other cervical ripening modalities when cervical maturation was assessed both by Bishop score and sonoelastography.
Conclusion The combined use of four DILAPAN-S expanders and two 200 mg doses of oral mifepristone (2 tablets) is effective in inducing cervical ripening for labor induction. The effectiveness of this method is confirmed by both a subjective assessment (Bishop score) and by a more objective sonoelastography with determining the color elastotype and calculating the strain ratio.
Keywords
In recent years, there has been a significant increase in the rates of labor induction and pre-induction [1–4]. The need for inducing cervical ripening and labor, is as a rule, associated with conditions such as post-term pregnancy, preeclampsia, isosensitization to Rhesus or AB0 system antigens, fetal macrosomia, premature rupture of membranes at full term, as well as a number of non-obstetric and oncological maternal comorbidities, requiring early delivery [5, 6].
In clinical practice, the most commonly used method to evaluate cervical ripening is the modified Bishop score because it is most predictive for successful labor induction [7–9]. A Bishop score between 8 and 12 is considered significant for cervical ripening and is one of the criteria for elective amniotomy, as well as a predictor of spontaneous onset of labor (table). A Bishop score from 0 to 5 suggests that the cervix is unripe, and labor is unlikely to start without induction.

An important parameter for assessing cervical ripening is cervical consistency (degree of softening); therefore, an objective assessment of cervical density (stiffness) by ultrasound-based cervical elastography can be useful for predicting the outcome of pre-induction and induction of labor [10–12]. According to a meta-analysis by Hatfield A. et al. [13], the sonographic cervical length was not an effective predictor of successful labor induction. In this regard, two approaches to cervical elastography have been developed for quantitative and objective assessment of cervical density: strain elastography and shear wave elasticity. In recent years, there has been an increasing amount of literature on the role of these methods in predicting preterm delivery and successful labor induction [14–17].
Today, there are two approaches to the elastographic assessment of cervical density: qualitative assessment (color-coded elasticity map), which is carried out visually, and quantitative assessment by calculating stiffness coefficient (strain ratio, StR – a ratio between the density of the examined and reference tissue) [18–20]. In most studies, strain ratio has been used as a more objective and operator-independent indicator [14–17].
This study aimed to investigate the effectiveness of a combined use of DILAPAN-S expanders and oral mifepristone for cervical ripening and induction of labor.
Material and methods
This prospective study comprised 200 pregnant women aged 23 to 38 (mean 29.6 ± 3.4 years) at 259 to 284 (mean 276.4 ± 6.2) days’ gestation. The inclusion criteria for the study patient selection were as follows: a singleton pregnancy, cephalic presentation, 37 or more weeks’ gestational age, and unripe cervix (Bishop score from 0 to 5). Indications for pre-induction of labor were a tendency to post-term pregnancy or giving birth to a large fetus; somatic diseases complicating the course of pregnancy (chronic arterial hypertension, kidney disease, chronic lung disease, antiphospholipid syndrome, diabetes mellitus); malignancies requiring early delivery; long-term moderate preeclampsia (PE), not amenable to medical correction; isosensitization to Rh factor or ABO system antigens (based on clinical and laboratory data).
Group 1 included 50 pregnant women who received four DILAPAN-S synthetic osmotic dilators for cervical ripening before labor induction. Osmotic dilators were inserted for up to 12 hours and were co-administered with two 200 mg doses of oral mifepristone taken 24 hours apart. In this case, the DILAPAN-S dilators were inserted simultaneously with the second mifepristone dose. In group 2 (n=50) cervical ripening was performed only with four DILAPAN-S synthetic osmotic dilators. In group 3 (n=50), patients underwent cervical ripening with a Foley balloon catheter inserted into the cervix for up to 12 hours. Patients in group 4 (n=50) received cervical ripening with two doses of prostaglandin E2 (Dinoprostone) gel 0.5 mg intracervically 6 hours apart.
Cervical ripening was assessed using the Bishop score and sonoelastography. During the study, patients of all groups were divided into three subgroups depending on the degree of cervical ripening (according to the Bishop score) before labor pre-induction. Patients in subgroups A, B, and C had a Bishop score from 0 to 2, 3, and from 4 to 5, respectively. Ten patients from each subgroup were randomly selected for elastographic measurement of cervical density before and after labor pre-induction. Transvaginal elastographic measurements were performed on a Hitachi Preirus ultrasound system with a 6 MHz transducer. A strain ratio was calculated, and a qualitative assessment was performed according to the following classification [12, 21–23]:
- elastotype 1 - has a single color green appearance, characterizing the tissue as elastic;
- elastotype 2 - has a mosaic-like pattern with green and blue colors, also characterizing the plotted area as elastic;
- elastotype 3 - has double colors, is mapped with blue in the central part and green at the periphery, characterizing the tissue as elastic with local areas of stiffness;
- elastotype 4 - has a uniform color pattern, is mapped in blue, characterizing the tissue as stiff.
Statistical analysis was performed using Microsoft Excel spreadsheets and the GraphPad Prism 6 software package (GraphPad Software, USA). Graphs were built using Microsoft Excel spreadsheets and the R and R Studio software version 1.1.463, (USA). The normality of the distribution was tested by the D’Agostino-Pearson test. Continuous are presented as mean and the standard deviation or median and interquartile range and compared with Student’s t-test or Wilcoxon test (for paired samples). The ANOVA and Kruskal-Wallis’ tests were used for comparing continuous data between three or more groups with post hoc tests for multiple comparisons, and a test for a linear trend in the group means. Correlation between two quantitative variables was analyzed using Spearman correlation coefficient. Correlation coefficients from 0 to 0.29 correspond to weak correlation, from 0.3 to 0.69 to moderate correlation, and from 0.7 to 1 to strong correlation. Differences between the groups were considered statistically significant at p<0.05.
Results and discussion
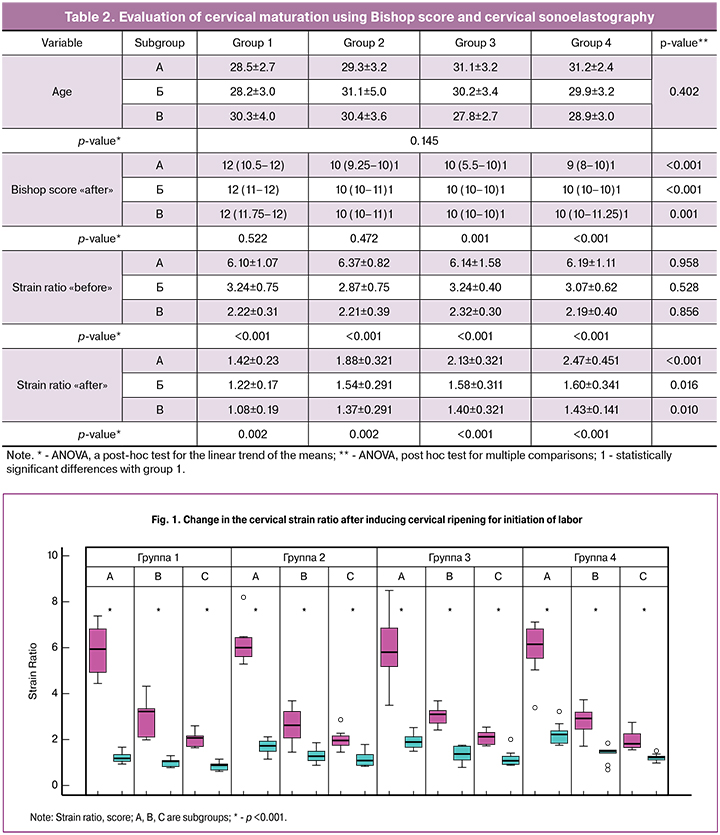
The mean age of the patients was 29.6 ± 3.38 years and did not differ between the study groups and subgroups (table 2). Patient parity also did not differ between the study groups. In group 1, 40 (80%) women were primiparous and 10 (20%) were multiparous; in group 2, 36 (72%) women were primiparous; in group 3, 43 (86%) women were primiparous; in group 4, 41 (82%) patients had no history of childbirth (p = 0.408). The Bishop’s cervical maturity score did not differ between the study groups and was 3 (2–3), 3 (2–4), 3 (2–4), and 3 (2–4), respectively (p = 0.865). The change in the strain ratio after inducing cervical ripening in all the study groups are presented in Fig. 1.
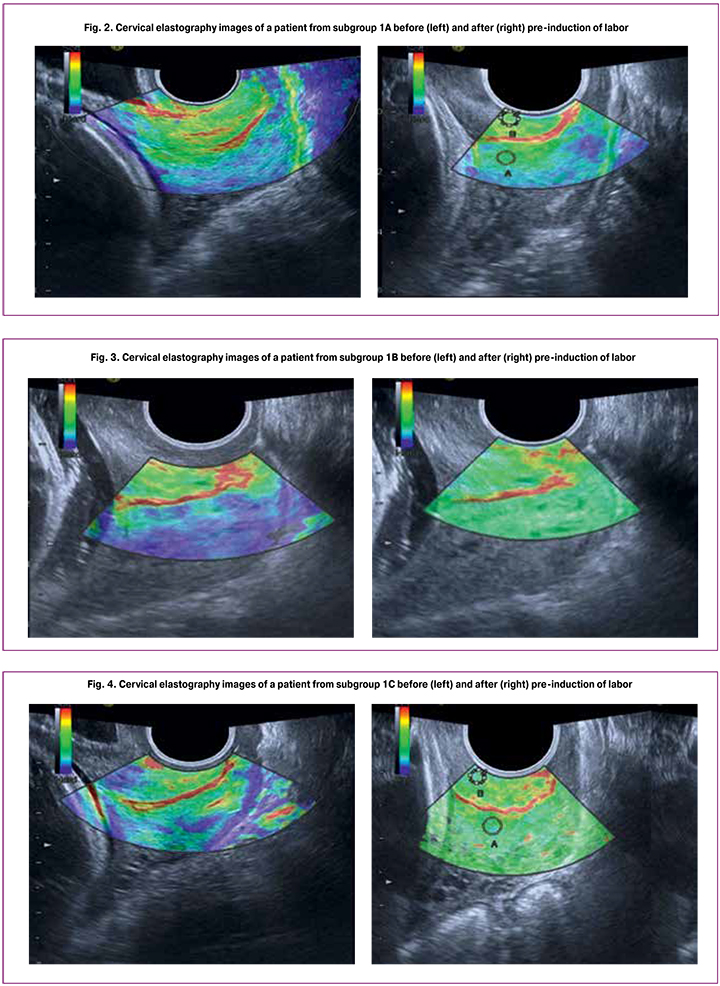
In patients of subgroup 1A (DILAPAN-S + Mifepristone; Bishop score 0–2), baseline sonoelastography showed low cervical maturity with elastography images dominated by blue color throughout the entire thickness of the tissue, which indicated a high level of stiffness (strain ratio 6, 10 ± 1.07). When the cervix was ripened by the combined method, ultrasound elastography revealed that the entire thickness of the tissue was dominated by green color that is characteristic of the soft, elastic cervix (strain ratio 1.42 ± 0.23, p < 0.001, Fig. 2).
In subgroup 1B (DILAPAN-S + Mifepristone; Bishop score 3), cervical elastographic images were dominated by green and blue colors, and only the area around the external cervical os was dark-blue indicating high stiffness of local tissues. The rest of the cervix had moderately stiff tissue (strain ratio 3.24 ± 0.75). When the cervix was ripened by the combined method, ultrasound elastography showed a prevalence of green color throughout the cervical length, including the internal and external cervical os that is characteristic of the soft, elastic cervix (strain ratio 1.22 ± 0.17, p < 0.001, Fig. 3).
Patients in subgroup 1C (DILAPAN-S + Mifepristone; Bishop score 4-5) had high baseline cervical scores with elastographic images dominated by mixed, mainly green and red colors, which indicated the soft, elastic cervix (strain ratio 2.22 ± 0.31), and only the area of the internal cervical os was blue, indicating an increased tissue stiffness in this area. When the cervix was ripened by the combined method, the tissues had mixed colors throughout the entire cervix length, including the internal os, which indicated its softness and elasticity (strain ratio 1.08 ± 0.13, p < 0.001, Fig. 4).
Patients in group 1had increased baseline Bishop’s scores, which was expectedly accompanied by a decrease in the strain ratio from 6.10 ± 1.07 to 2.22 ± 0.31 (p - for a trend <0.001). After pre-induction cervical ripening, Bishop scores in patients of group 1 did not significantly differ, making up 12 (10.5–12), 12 (11–12), and 12 (11.75–12), respectively (p = 0.522). These findings suggest high effectiveness of DILAPAN-S combined with mifepristone for cervical ripening, regardless of the baseline cervical maturation. At the same time, the higher was cervical maturation before pre-induction, the lower was the strain ratio after pre-induction (p - for a trend = 0.002).
In patients of subgroup 2A (DILAPAN-S; Bishop score 0–2), sonoelastography showed stiff tissues with a predominance of blue color throughout the entire cervix (strain ratio 6.37 ± 0.82). Following cervical ripening by the insertion of four DILAPAN-S expanders, sonoelastography revealed that the entire thickness of the tissue was dominated by green color, which indicated changes in the cervical maturation; however, areas of blue coloring indicated the presence of dense muscle fibers in the tissue structure (strain ratio 1.88 ± 0.32, p <0.001, Fig. 5).

In subgroup 2B (DILAPAN-S; Bishop score 3), cervical elastographic images were dominated by green and blue colors, and only the area around the external cervical os was dark-blue indicating high stiffness of local tissues. The rest of the cervix had moderately stiff tissue (strain ratio 2.87 ± 0.75). Following induced cervical ripening, ultrasound elastography showed a prevalence of green color that is characteristic of the elastic cervix (strain ratio 1.54±0.29, p<0.001, Fig. 6).
In patients of subgroup 2C (DILAPAN-S; Bishop score 4–5), cervical elastographic images were dominated by green and red colors, signifying soft tissues (strain ratio 2.21 ± 0.39), and only the area around the internal cervical os was blue indicating increased stiffness of local tissues. Repeat sonoelastography showed mixed colors throughout the entire length of the cervix and the internal cervical os, which is characteristic of soft and elastic tissues (strain ratio 1.15 ± 0.22, p <0.001, Fig. 7).

Patients in subgroups of group 2 showed an increase in Bishop scores over baseline values, which was expectedly accompanied by a decrease in strain ratio from 6.37 ± 0.82 to 2.21 ± 0.39 (pfor a trend <0.001). After the pre-induction of labor, Bishop score in patients of group 2 did not significantly differ and amounted to 10 (9.25-10), 10 (10-11), and 10 (10-11), respectively (p = 0.472). These findings suggest that DILAPAN-S is effective in inducing cervical ripening, regardless of the baseline cervical maturation. In this case, the higher was cervical maturation before pre-induction, the lower was the strain ratio after pre-induction (p-for a trend = 0.002).
In patients of subgroup 3A (Foley catheter; Bishop score 0–2), the sonoelastography image was characterized by dark-blue and blue-colored areas of cervical tissue, which is characteristic of stiff structures with low elasticity (strain ratio 6.14 ± 1.58). After achieving cervical ripening induced by a Foley catheter, about 2/3 of cervical length was dominated by green color (strain ratio 2.13 ± 0.32, p <0.001, Fig. 8).
Cervical elastographic images of patients in subgroup 3B (Foley catheter; Bishop score 3) were dominated by green color with dark blue and blue inclusions (strain ratio 3.24 ± 0.40). Repeat examination showed a shift to the domination of green color and the disappearance of dark blue and blue colors (strain ratio 1.58 ± 0.31, p < 0.001, Fig. 9).

In subgroup 3C (Foley catheter; Bishop score 4-5), elastographic images were dominated by green color with red inclusions (strain ratio 2.32 ± 0.30). After achieving cervical ripening, the colors acquired a more even distribution of red areas among green areas, which indicated an increase in tissue elasticity (strain ratio 1.40 ± 0.32, p < 0.001, Fig. 10).
Patients in subgroups of group 3 showed an increase in Bishop scores over baseline values, which was expectedly accompanied by a decrease in strain ratio from 6.14 ± 1.58 to 2.32 ± 0.30 (p- for a trend <0.001). Among patients of group 3, Bishop’s score after the pre-induction of labor was significantly lower in subgroup A, making up10 (5.5-10), 10 (10-10), and 10 (10-10), respectively (p = 0.001). These findings suggest that the Foley catheter is effective in inducing cervical ripening in patients, whose Bishop score ranges from 3 to 5 and not very effective in patients with Bishop scores from 0 to 2. Moreover, the higher was cervical maturation before pre-induction, the lower was the strain ratio after pre-induction (p-for a trend <0.001).
In patients of subgroup 4A (prostaglandin E2; Bishop score 0–2), the sonoelastography images were dominated by dark-blue-colored areas, which is indicative of stiff tissues (strain ratio 6.19 ± 1.11). Repeat sonoelastography following prostaglandin E2 induced cervical ripening showed that the color of the tissues changed to green, however, blue-colored areas were still present, which characterized the cervix as stiff-elastic (strain ratio 2.47 ± 0.45, p < 0.001, Fig. 11). The change in the strain ratio was the smallest compared with the other subgroups, which indicates the low effectiveness of prostaglandin E2 in cervical ripening in patients with unripe cervix (Bishop 0–2).
In patients of subgroup 4B (prostaglandin E2; Bishop score 3), the sonoelastography images were characterized by green color and combinations of dark-blue and blue elements, which indicates the presence of local tissue stiffness (strain ratio 3.07 ± 0.62). Repeat examination following prostaglandin E2 induced cervical ripening showed that the color of the tissues changed to green, which is characteristic of elastic tissues (strain ratio 1.60 ± 0.34, p < 0.001, Fig. 12). However, the change in the strain ratio was also the smallest as compared with all other subgroups.

In patients of subgroup 4C (prostaglandin E2; Bishop score 4-5), the cervical sonoelastography images had mixed colors with a predominance of green and red (strain ratio 2.19 ± 0.40). Repeat examination showed mixed colors over the entire cervical length, including the region of the internal os, which indicated an increase in tissue elasticity (strain ratio 1.43 ± 0.14, p < 0.001, Fig. 13).
Patients in subgroups of group 4 initially had increased Bishop scores, which was expectedly accompanied by a decrease in the strain ratio from 6.19 ± 1.11 to 2.19 ± 0.40 (p- for a trend <0.001). Among patients of group 4, Bishop’s scores after the pre-induction of labor were directly related to the scores before pre-induction and amounted to 9 (8–10), 10 (10–10), and 10 (10–11.25) points, respectively (p = 0.001). These findings suggest high effectiveness of prostaglandin E2 in patients with Bishop score of 3 and not very effective in patients with Bishop score of 0 to 2. In patients with an initial Bishop score of 4 to 5, the effectiveness of prostaglandin E2 can be rated as high by Bishop score and as high enough when based on Strain Ratio. At the same time, the higher was cervical maturation before pre-induction, the lower was the strain ratio after pre-induction (p-for a trend <0.001).
At the next stage of the study, we compared the combined method for cervical ripening in each subgroup. Before pre-induction, the groups did not differ either in terms of cervical maturation by Bishop score or by strain ratio (table 2). After pre-induction of labor in subgroups A, B and C, the most effective in inducing cervical ripening was a combination of DILAPAN-S and mifepristone, as estimated both by Bishop score (p <0.001, p <0.001 and p = 0.001, respectively), and by strain ratio (p <0.001, p = 0.016 and p = 0.010, respectively). Mean Bishop scores and strain ratio values for all subgroups are shown above and are presented in Table 2.
The analysis of the correlation between Bishop score and strain ratio before inducing cervical ripening resulted in the correlation coefficient 0.882 (p <0.001), which corresponds to strong negative correlation. After pre-induction of labor, the correlation coefficient was -0.443, which corresponds to moderate negative correlation. Therefore, despite the high similarity of these indicators before inducing cervical ripening, there are some differences after the pre-induction, which implies the possibility of using the combination of these criteria in clinical practice.
Conclusion
The combined use of four DILAPAN-S expanders and two 200 mg doses of oral mifepristone (2 tablets) is effective in inducing cervical ripening for labor induction, as assessed subjectively by Bishop score and by a more objective sonoelastography with determining the color elastotype and calculating the Strain Ratio. This method for cervical ripening was highly effective regardless of the initial cervical Bishop score, whereas, in group 3 (Foley catheter) and group 4 (prostaglandin E2), the effectiveness was directly related to the initial state of the cervix.
References
- Vogel J.P., Betrán A.P., Vindevoghel N., Souza J.P., Torloni M.R., Zhang J., et al. Use of the Robson classification to assess caesarean section trends in 21 countries: a secondary analysis of two WHO multicountry surveys. Lancet Glob Heal. 2015; 3(5):e260–70. https://doi.org/10.1016/S2214-109X(15)70094-X
- Брега Е.С., Сахарова Г.В., Пекарев О.Г. Результаты применения комбинированного метода подготовки шейки матки к родам. Акушерство и гинекология. 2017; (11): 37-43. [Brega E.S., Sakharova G.V., Pekarev O.G. Results of using a combined method of cervical preparation for childbirth. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; (11): 37-43. (In Russian)]. https://dx.doi.org/10.18565/aig.2017.11.37-43
- Брега Е.С., Пекарев О.Г. Варианты подготовки шейки матки к родам. Акушерство и гинекология. 2017; (7): 136-9. [Brega E.S., Pekarev O.G. Options for preparing the cervix uteri for childbirth. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; (7): 136-9. (In Russian)] http://dx.doi.org/10.18565/aig.2017.7.136-9
- Baev O.R., Rumyantseva V.P., Tysyachnyu O. V, Kozlova O.A., Sukhikh G.T. Outcomes of mifepristone usage for cervical ripening and induction of labour in full-term pregnancy. Randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2017; 217:144–9.
- Баев О.Р., Румянцева В.П., Кан Н.Е., Тетруашвили Н.К., Тютюнник В.Л., Ходжаева З.С., Шмаков Р.Г. Медикаментозная подготовка шейки матки к родам и родовозбуждение. Клинический протокол. М.: Планида, 2013. 24 с.[Baev O.R., Rumjanceva V.P., Kan N.E., Tetruashvili N.K., Tjutjunnik V.L., Hodzhaeva Z.S., Shmakov R.G. Medikamentoznaja podgotovka shejki matki k rodam i rodovozbuzhdenie. Klinicheskij protokol. M.: Planida, 2013. 24 s. (In Russian)]
- Chen W., Xue J., Peprah M.K., Wen S.W., Walker M., Gao Y., et al. A systematic review and network meta-analysis comparing the use of Foley catheters, misoprostol, and dinoprostone for cervical ripening in the induction of labour. BJOG. 2016; 123(3): 346-54. doi: 10.1111/1471-0528.13456.
- Bishop E.H. Pelvic scoring for elective induction. Obstet Gynecol. 1964; 24: 266-8. PMID: 14199536
- Wormer K.C., Williford A.E. Bishop Score. StatPearls. 2018.
- Induction of labour. Evidence-based Clinical Guideline. 2nd ed. London: RCOG Press, 2008.
- Londero A.P., Schmitz R., Bertozzi S., Driul L., Fruscalzo A. Diagnostic accuracy of cervical elastography in predicting labor induction success: a systematic review and meta-analysis. J. Perinat. Med. 2016; 44(2): 167-78. DOI: 10.1515/jpm-2015-0035
- Fruscalzo A., Mazza E., Feltovich H., Schmitz R. Cervical elastography during pregnancy: a critical review of current approaches with a focus on controversies and limitations. J Med Ultrason (2001). 2016; 43(4): 493-504. DOI: 10.1007/s10396-016-0723-z
- Зыкин Б.И., Постнова Н.А., Медведев М.Е. Ультразвуковая эластография. Медицинский алфавит. 2013; 1-2 (10): 14-9. [Zykin B.I., Postnova N.A., Medvedev M.E. Ul’trazvukovaja jelastografija. Medicinskij alfavit. 2013; 1-2(10): 14-9.(In Russian)] eLIBRARY ID: 20787906
- Hatfield A.S., Sanchez-Ramos L., Kaunitz A.M. Sonographic cervical assessment to predict the success of labor induction: a systematic review with metaanalysis. Am J Obstet Gynecol. 2007; 197(2): 186-92. DOI: 10.1007/s00404-015-3769-z
- Hernandez-Andrade E., Romero R., Korzeniewski S.J., Ahn H., Aurioles-Garibay A., Garcia M., et al. Cervical strain determined by ultrasound elastography and its association with spontaneous preterm delivery. J Perinat Med. 2014; 42(2): 159-69. doi: 10.1515/jpm-2013-0277
- Sabiani L., Haumonte J.-B., Loundou A., Caro A.-S., Brunet J., Cocallemen J.-F., et al. Cervical HI-RTE elastography and pregnancy outcome: a prospective study. Eur J Obstet Gynecol Reprod Biol. 2015; 186: 80-4. DOI: 10.1016/j.ejogrb.2015.01.016
- Fruscalzo A., Londero A.P., Fröhlich C., Meyer-Wittkopf M., Schmitz R. Quantitative elastography of the cervix for predicting labor induction success. Ultraschall Med. 2015; 36(1): 65-73. doi: 10.1055/s-0033-1355572
- Hee L., Rasmussen C.K., Schlütter J.M., Sandager P., Uldbjerg N. Quantitative sonoelastography of the uterine cervix prior to induction of labor as a predictor of cervical dilation time. Acta Obstet Gynecol Scand. 2014; 93(7): 684-90. doi: 10.1111/aogs.12389
- Осипов Л.В. Ультразвуковые диагностические приборы: режимы, методы и технологии. М.: Изомед, 2011. 316 c. [Osipov L.V. Ul’trazvukovye diagnosticheskie pribory: rezhimy, metody i tehnologii. M.: Izomed, 2011. 316 c. (In Russian)].
- Doyley M.M., Meaney P.M., Bamber J.C. Evaluation of an iterative reconstruction method for quantitative elastography. Phys Med Biol. 2000; 45(6): 1521-40.
- Doherty J.R., Trahey G.E., Nightingale K.R., Palmeri M.L. Acoustic radiation force elasticity imaging in diagnostic ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 2013; 60(4): 685-701. DOI: 10.12691/jbet-1-2-1
- Varghese T. Quasi-Static Ultrasound Elastography. Ultrasound Clin. 2009; 4(3): 323-38. DOI: 10.1016/j.cult.2009.10.009
- Papadacci C., Bunting E.A., Konofagou E.E. 3D Quasi-Static Ultrasound Elastography With Plane Wave In Vivo. IEEE Trans Med Imaging. 2017; 36(2): 357-65. http://dx.doi.org/10.1109/TMI.2016.2596706
- Ophir J., Alam S.K., Garra B.S., Kallel F., Konofagou E.E., Krouskop T., et al. Elastography: Imaging the elastic properties of soft tissues with ultrasound. J Med Ultrason (2001). 2002; 29(4): 155. https://doi.org/10.1007/BF02480847
Received 27.05.2019
Accepted 21.06.2109
About the Authors
Tel. +7 (929) 521-45-90. E-mail: e_brega@oparina4.ru117997, Russia, Moscow, Ac. Oparina str. 4
Oleg G. Pekarev, MD, professor, deputy director of institution of obstetrics, National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare of Russian Federation. E-mail: o_pekarev@oparina4.ru
117997, Russia, Moscow, Ac. Oparina str. 4
Aleksander I. Gus, MD, professor, the leader of the department of functional diagnostics department of diagnostic imaging, National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare of Russian Federation. Tel. +7 (495) 438-11-77. E-mail: a_gus@oparina4.ru
117997, Russia, Moscow, Ac. Oparina str. 4.
Stanislav S. Lunkov, doctor of the department of functional diagnostics department of diagnostic imaging, National Medical Research Center for Obstetrics, Gynecology and Perinatology Ministry of Healthcare of Russian Federation. Tel. +7 (495) 438-11-77. E-mail: s_lunkov@oparina4.ru
117997, Russia, Moscow, Ac. Oparina str. 4.
For citation: Brega E.S., Pekarev O.G., Gus A.I., Lun’kov S.S. Clinical and elastographic evaluation of methods for ripening an unripe cervix
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 10: 81-91.(In Russian).
https://dx.doi.org/10.18565/aig.2019.10.81-91



