Наиболее часто встречающаяся доброкачественная опухоль матки – миома. Частота диагностики миомы матки (ММ) в репродуктивном возрасте колеблется от 35 до 75% [1–7]. При этом чаще всего ММ квалифицируется как хирургическая патология, что приводит к высокой частоте гистерэктомий (1 млн в мире в год) [8].
С изменением социализации общества, тенденцией к позднему планированию беременности и увеличением частоты встречаемости ММ среди женщин репродуктивного возраста актуальность проблемы лечения данного заболевания значительно возрастает и имеет социальную значимость. В России в 2018 г. почти каждый третий ребенок был рожден женщиной старше 35 лет, а пик рождаемости составил 30 лет жизни женщины [9].
ММ представляет собой первично-множественную моноклональную опухоль, митогенно и пролиферативно чувствительную к действию прогестерона. Рецептор прогестерона представляет собой димер, состоящий из двух рецепторных белков PRА и PRВ, каждый из которых способен связываться с прогестероном, но активирует транскрипцию гена только PRВ [2, 10–14].
Иммуногистохимические (ИГХ) исследования показали, что до 90% клеток пролиферирующих лейомиом матки содержат активные рецепторы прогестерона, который стимулирует выработку эпидермального фактора роста (epidermal growth factor – EGF), инсулиноподобного фактора роста 1 (insulinlike growth factor 1 – IGF-1), главных митогенов миомы, и антиапоптического протоонкогена Bcl-2 [4, 10, 15].
В настоящее время ведение пациенток с ММ представляет яркое отражение персонифицированного подхода в медицине. Выбор тактики лечения зависит от возраста пациентки, желания сохранить репродуктивную функцию, клинической картины заболевания, локализации миоматозных узлов, их размеров, темпов роста, информированности врача и пациента о необходимости активной тактики ведения, возможностях органосохраняющих операций и технологий, широкого применения медикаментозного лечения.
Варианты лечения пациенток с ММ включают терапевтическое, хирургическое и радиологическое вмешательства [2, 4, 5].
Консервативное воздействие чаще применяют при необходимости сохранения фертильности, при предоперационной подготовке, в пременопаузальном периоде, при наличии противопоказаний к оперативному вмешательству и в качестве противорецидивной терапии [7, 16–19].
Увеличивается актуальность медикаментозного лечения миомы матки, а также предоперационной подготовки больных [2, 12, 14, 16, 18, 20, 21]. Такая терапия является непродолжительной, дискретной.
Семейство модуляторов рецепторов прогестерона (МРП) представлено различными лигандами этих рецепторов и включает в себя:
- агонисты прогестерона (сам прогестерон и прогестины);
- вещества со смешанным действием агонистов и антагонистов прогестерона (селективные МРП – СМРП);
- антагонисты прогестерона (антипрогестины или антигестагены, например, мифепристон) [22].
В нашей стране мифепристон в дозе 50 мг зарегистрирован под торговым названием Гинестрил. Применение в длительном режиме эффективно в качестве самостоятельной медикаментозной терапии ММ [22].
Молекулярно-биологические механизмы влияния улипристала на ММ изучены главным образом in vitro. СМРП обеспечивают снижение пролиферации и повышение апоптоза, активацию металлопротеиназ, подавление их ингибиторов и продукции факторов роста [23–26].
Возможные изменения эндометрия, связанные с действием улипристала, носят доброкачественный и обратимый характер, не требуют принятия мер и самостоятельно разрешаются после завершения курса лечения [21, 23].
В мире сегодня используется 12 препаратов группы аналогов гонадотропин-рилизинг-гормона (ГнРГ), из которых 4 зарегистрированы в России [https://www.rlsnet.ru].
Целью нашего исследования было проведение оценки сравнительной эффективности препаратов мифепристона (Гинестрил), СМПР (Эсмия) и агониста ГнРГ (аГнРГ – Бусерелин-депо) в лечении женщин с ММ и внутренним эндометриозом.
Материалы и методы
Были отобраны 150 женщин с диагнозом «ММ и внутренний эндометриоз тела матки». Критериями включения являлись наличие симптомной ММ, отказ от хирургического лечения, желание сохранить репродуктивную функцию, подготовка к планируемой беременности.
К критериям невключения относили большие размеры миоматозно измененной матки (общая величина соответствует матке 14-недельного срока беременности), обильное маточное кровотечение в момент начала исследования в сочетании с анемией средней и тяжелой степени, быстрый рост опухоли, острое нарушение питания миомы (перекрут ножки субсерозного узла, некроз опухоли), сочетание ММ с рецидивирующей или атипической гиперплазией эндометрия, опухолью яичника, наличие миоматозного узла в области трубного угла матки, который является причиной бесплодия, возраст старше 45 лет.
Все пациентки были случайным образом разделены на три группы по 50 человек в каждой в зависимости от назначаемого препарата (мифепристон, улипристала ацетат, аГнРГ) и с целью удобства статистического анализа. Терапию назначали на протяжении 3 месяцев. Мифепристон (Гинестрил) рекомендовали по 1 таблетке (50 мг) в сутки в непрерывном режиме. Улипристала ацетат (Эсмия) назначали по 1 таблетке (5 мг) в непрерывном режиме. Агонист ГнРГ (Бусерелин-депо) вводили внутримышечно по 3,75 мг каждые 4 недели. Все обследованные находились в репродуктивном возрасте. Средний возраст пациенток во всех группах не отличался и составил 35,6±4,75 года с колебаниями от 28 до 44 лет.
Использовалась классификация ММ, предложенная И.С. Сидоровой и С.А. Леваковым (2002) [1], проводящая выделение простой (непролиферирующей) и пролиферирующей формы, и классификация FIGO 2011 (по локализации узлов).
Выбранные пациенты имели клинические проявления ММ и внутреннего эндометриоза. К ним относили следующие жалобы: аномальные маточные кровотечения, тазовую боль, тяжесть внизу живота, увеличение живота, нарушение функции мочевого пузыря (дизурию), нарушение функции кишечника (дисхезию), бесплодие.
Значимыми аспектами анамнеза являлись: отсутствие беременности и родов, раннее менархе, увеличение частоты менструации, длительность дисменореи, отягощенную наследственность, повышенную массу тела, артериальную гипертензию, сахарный диабет, возраст старше 30–35 лет, аденомиоз, частые аборты (более 3) и использование внутриматочных контрацептивов.
Проводили обследование, обязательно включавшее физикальное исследование, клинический анализ крови, определение уровня ферритина и эхографию. Ультразвуковое исследование (УЗИ) (трансвагинальное, трансабдоминальное) проводили во всех наблюдениях с цветным и энергетическим допплеровским картированием, которое, при необходимости, дополняли трансвагинальной соногистерографией с контрастированием (дифференцирование подслизистой ММ и полипа эндометрия) и магнитно-резонансной томографией (МРТ) (при наличии атипичных форм образований малого таза и брюшной полости). Объем миоматозных узлов и объем матки вычисляли по формуле: V матки (см3) = (А + В + С) × 0,4186, где А – продольный, В – переднезадний, С – поперечный размеры радиуса миоматозного узла или матки (см), коэффициент 0,4186 – π4/3, применяемый для вычисления объема эллипсоидных объектов [26].
Следует отметить, что существует формула, использующая коэффициент 0,523.
Кроме того, доступно большое количество онлайн-калькуляторов вычисления уменьшения объема миоматозного узла (рис. 1). На рис. 1 видно, что при 38% снижении объема миоматозного узла его диаметр уменьшился лишь на 1,4 см. При этом акцент делается именно на процент уменьшения объема, что не всегда представляет адекватную статистику и клинический эффект.
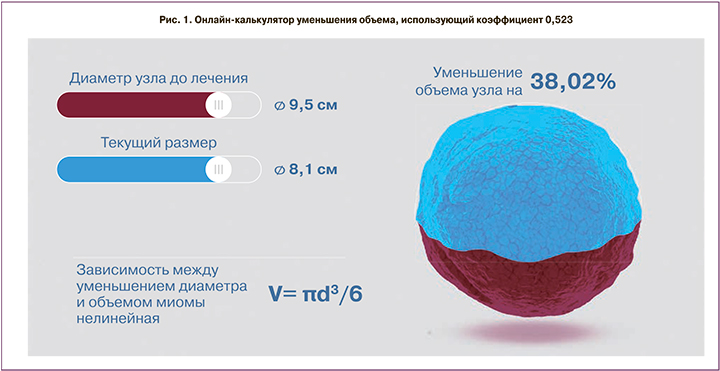
Применяемая формула вычисления объема миоматозного узла не столь важна, как устранение клинических проявлений, темпов роста и уменьшения абсолютных размеров миомы и матки.
Оценку эффективности терапии проводили через 1, 3 и 6 месяцев от начала лечения. Принимали во внимание наступление аменореи, уменьшение объема менструальной кровопотери, восстановление уровня гемоглобина и ликвидацию латентного дефицита железа, уменьшение болевого синдрома, наступление беременности (после завершения лечения), объем миоматозных узлов, общий объем матки, результаты допплеровского картирования. Интенсивность болевого синдрома оценивали с помощью визуально-аналоговой шкалы (ВАШ) с количеством баллов от 0 до 10.
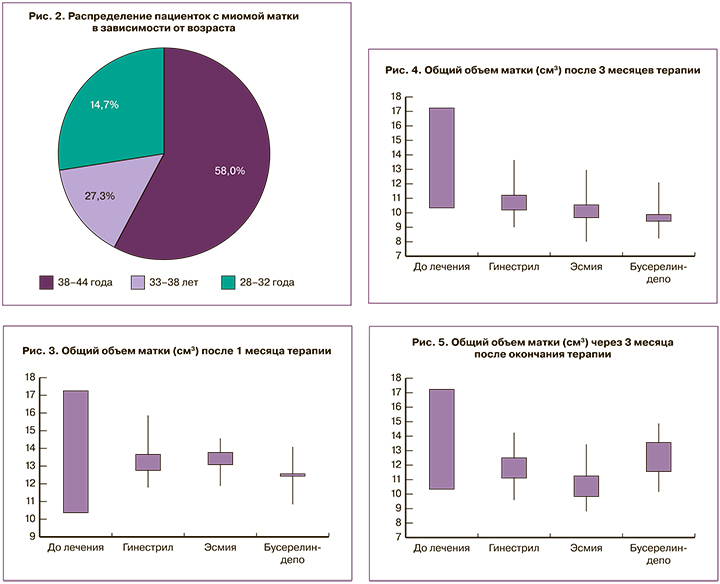
Среди обследованных пациенток выявлена устойчивая тенденция к увеличению частоты ММ с возрастом (рис. 2).
При морфологических исследованиях очаги пролиферации опухолевых миоцитов чаще всего локализуются в периваскулярных пространствах вокруг сосудов. В отдельных случаях вся миома состоит из пролиферирующих миоцитов с синусоидальными сосудами.
Для дифференциальной диагностики простой и пролиферирующей миомы использовали допплеровское исследование. ММ считали пролиферирующей, если в динамике имелся рост образования и при визуализации во время цветного и энергетического (низкорезистентные сосуды) допплеровского картирования обнаруживали пери- и внутринодулярный кровоток.
Частота встречаемости простой (28,7%; n=43) и пролиферирующей (71,3%; n=107) ММ достоверно не отличалась в зависимости от группы. Высокая частота встречаемости пролиферирующего типа миомы в исследовании объяснима выбором пациенток с наличием симптомов.
Принимая во внимание одинаковую клиническую картину, отсутствие специфических симптомов и кровотока в ЦДК-режиме при внутреннем эндометриозе тела матки, возможное сочетание двух заболеваний признавали по умолчанию.
Результаты и обсуждение
Через 1 месяц после начала медикаментозной терапии с целью первичной оценки эффективности лечения выполняли УЗИ. Все пациентки продемонстрировали адекватный эффект. Уменьшился объем менструальной кровопотери, в 88% наблюдений наступила аменорея. На 34% снизилась частота дизурии и дисхезии, при оценке болевого синдрома по ВАШ 18,7% женщин отметили отсутствие/почти отсутствие тазовых болей (не выше 3–4 баллов).
Общее уменьшение размеров матки наблюдалось во всех группах (рис. 3). Статистически достоверных различий в снижении объема матки в зависимости от использованного препарата выявлено не было. Эти результаты позволили продолжить ведение пациенток согласно дизайну исследования.
Наиболее важным результатом было достоверное уменьшение объема матки через 3 месяца (рис. 4). Наиболее выраженно (76,91%) объем матки снизился у пациенток, получавших аГнРГ, что, вероятно, связано с его воздействием не только на ММ, но и на эндометриоидные гетеротопии.
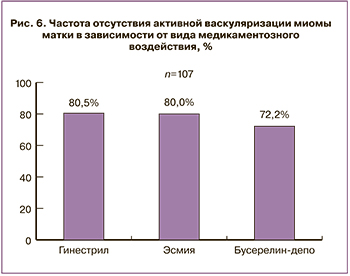
Наибольшее количество наблюдений с отсутствием активного пери- и внутринодулярного кровотока в области миоматозного узла после 3 месяцев лечения было зарегистрировано в группе пациенток, получавших мифепристон (62%; n=31).
 Через 6 месяцев от начала проведения исследования (3 месяца после завершения терапии) повторно оценили объем матки (рис. 5). На данном этапе из исследования были исключены 6 (4%) женщин, у которых наступила беременность.
Через 6 месяцев от начала проведения исследования (3 месяца после завершения терапии) повторно оценили объем матки (рис. 5). На данном этапе из исследования были исключены 6 (4%) женщин, у которых наступила беременность.
При применении мифепристона и улипристала отмечена наибольшая частота «деваскуляризации» миоматозных узлов по данным допплеровского исследования. Из 36 пациенток, получавших мифепристон, у 29 ММ трансформировалась из пролиферирующей в простую. В группе с улипристалом были схожие данные (28 из 35), а при назначении аГнРГ – 26 из 36 (рис. 6).
Во всех группах обследованных имелись пациентки, которым была показана миомэктомия даже после завершения медикаментозной терапии.
Во всех 38 (25,33%) наблюдениях миомэктомия выполнялась лапароскопическим доступом, не потребовалось проведения органоуносящих операций. В обязательном порядке проводили полноценное ушивание стенки матки, используя как нити противоскольжения, так и узловые швы.
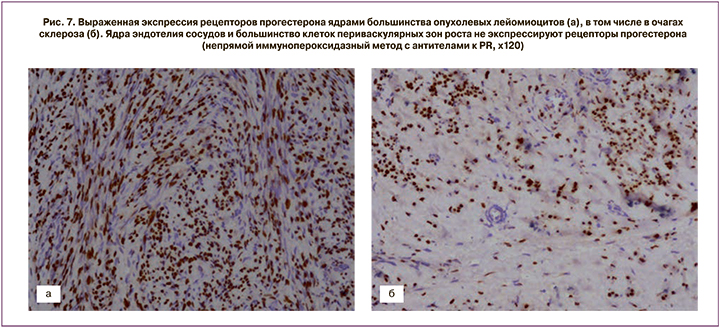
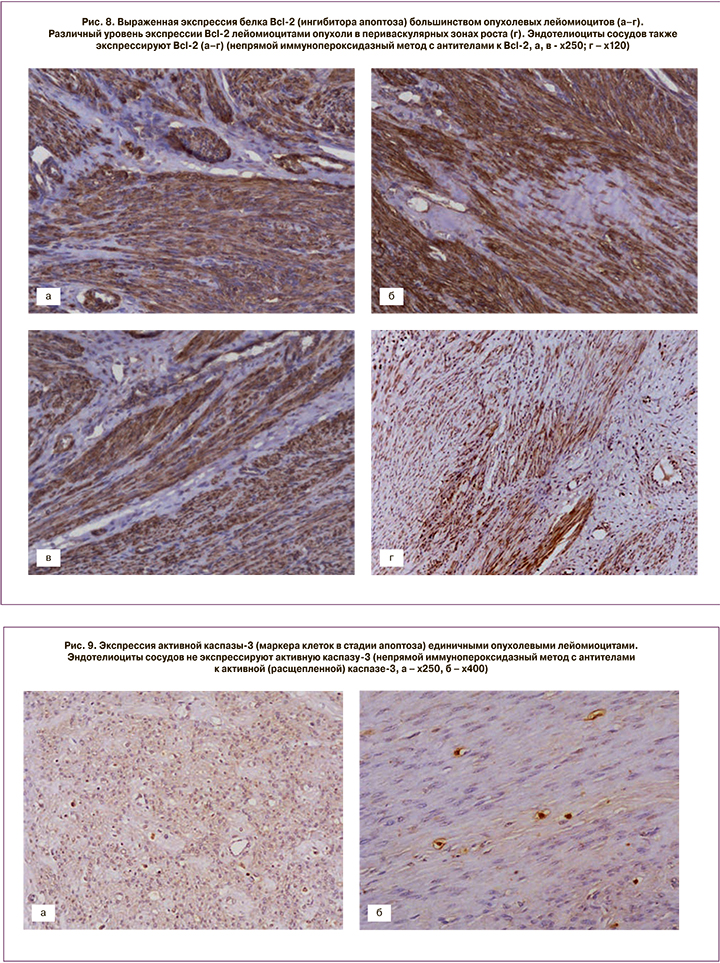
После проведения миомэктомии у пациенток с активным ростом миоматозных узлов и наличием кровотока в узле до начала лечения, кроме стандартного морфологического исследования, выполняли ИГХ-тесты (рис. 7, 8, 9).
Выраженная экспрессия рецепторов прогестерона отмечалась в ядрах большинства опухолевых лейомиоцитов. Ядра эндотелия сосудов не экспрессировали рецепторы прогестерона (см. рис. 7). Коэффициент экспрессии рецепторов прогестерона составил от 1,3 до 1,8 (в среднем М±σ – 1,7±0,12).
Экспрессия белка, ингибитора апоптоза, Bcl-2 выявлена во многих опухолевых лейомиоцитах и была высокой. Уровень экспрессии в периваскулярных зонах роста значительно варьировал в пределах одной опухоли: от выраженного (в участках со слабым склерозом и гиалинозом) до слабого (преимущественно в областях с выраженным склерозом и гиалинозом). Эндотелиоциты сосудов также экспрессировали Bcl-2 (см. рис. 8). Коэффициент экспрессии (КЭ) белка Bcl-2 составил от 1,24 до 1,84 (в среднем М±σ – 1,6±0,18).
Статистически значимой разницы в экспрессии Bcl-2 в 4 наблюдениях так называемых пролиферирующих лейомиом (по клинико-морфологической классификации И.С. Сидоровой и С.А. Левакова, 2002) [1] выявлено не было.
Процент опухолевых лейомиоцитов, цитоплазма которых экспрессировала активную (расщепленную) каспазу-3 (клеток, находящихся в стадии апоптоза), был крайне незначителен во всех полях зрения. Эндотелиоциты сосудов не экспрессировали активную каспазу-3 (см. рис. 9).
Разница в экспрессии активной (расщепленной) каспазы-3 при пролиферирующих лейомиомах отсутствовала
Процент опухолевых лейомиоцитов с экспрессией активной (расщепленной) каспазы-3 составил от 0,01 до 0,4% (в среднем М±σ – 0,1±0,12%).
Анализ результатов морфологического ИГХ-исследования свидетельствует о сохраняющейся повышенной экспрессии рецепторов прогестерона вне зависимости от клинико-морфологического варианта ММ. При этом медикаментозная терапия нередко приводит к снижению пролиферативной активности и трансформации активно растущей миомы в простую.
В пролиферирующих миоматозных узлах имеются признаки пролиферации опухолевых миоцитов, высокая клеточность, крупные размеры ядер. В миометрии, окружающем простые миоматозные узлы, нет каких-либо специфических изменений.
Простая миома является неактивной, медленно растущей опухолью с преобладанием соединительнотканных компонентов, фенотипической трансформацией миоцитов и снижением кровоснабжения в миоматозных узлах.
Пролиферирующие миомы следует отнести к активной множественной быстрорастущей опухоли матки с повышенной пролиферативной активностью и часто сопровождающейся пролиферативными (гиперпластическими) процессами в эндометрии, опухолевидными и доброкачественными опухолями яичников. Между этими крайними клинико-морфологическими вариантами развития существуют промежуточные варианты.
В настоящем исследовании аменорею и олигоменорею не оценивали как побочные эффекты.
При приеме Гинестрила к нежелательным явлениям отнесли повышение массы тела – 2% (1 пациентка – менее 3% от исходной массы), головную боль, слабость – 2 (4%) пациентки.
До назначения улипристала ацетата исключали пациенток с дисфункцией печени и почек. Прием Эсмии сопровождался обратимым после отмены утолщением эндометрия более 16 мм – 12%, приливами – 6%, психоэмоциональными расстройствами, головной болью и акне – по 12% соответственно.
На фоне введения Бусерелина-депо отмечали головную боль, психоэмоциональные расстройства – 14%, снижение либидо – 12% и бессонницу – 12%.
Побочные эффекты ни в одном наблюдении не потребовали отмены терапии, носили легкий характер. Невысокая частота нежелательных явлений, вероятно, обусловлена короткой продолжительностью курсов терапии и высокой мотивацией пациенток.
Заключение
Медикаментозная терапия, направленная на устранение симптомов ММ, является эффективным инструментом для органосбережения, подготовки к беременности и хирургическому лечению.
Агонист ГнРГ более выраженно уменьшает размеры матки при сочетании миомы с внутренним эндометриозом тела матки. Применение аГнРГ сопряжено с большей частотой побочных явлений по сравнению с улипристалом и мифепристоном.
Назначение улипристала требует обязательного дополнительного обследования, сопровождается большим количеством нежелательных реакций. При этом улипристал сопоставим с мифепристоном по степени и темпам уменьшения размеров миоматозных узлов.
Мифепристон наиболее активно переводит клинический вариант пролиферирующей миомы в простую. Важным аспектом выбора мифепристона является наиболее высокий профиль безопасности и возможность коррекции дозы. Мифепристон обладает наименьшим количеством побочных эффектов.



