Use of dinoprostone vaginal insert for cervical ripening and labor induction in routine obstetrical clinical practice: a multicenter study based on real-world evidence
Baev O.R., Tskhay V.B., Babich D.A., Bezhenar V.F., Semenov Yu.A., Serova O.F., Gabelova K.A., Zainulina M.S., Tsakhilova S.G., Omarova N.G., Sheldagayeva A.V., Mentsik M.M., Semenova Yu.E., Bryukhanova A.A., Tkacheva N.V., Peshkova N.V., Vasilkovskaya E.N., Skornyakova L.M., Muravina E.L., Samoylova Yu.A., Rasskazova T.V., Fatkullina I.B., Frolov A.L., Dalaeva T.Kh., Emasheva N.M., Guskova O.I., Klishina V.V., Kipriyanova I.I., Klimkina K.I., Afanasieva K.Yu., Gracheva M.S., Osmanov N.Sh., Magomedova L.I., Arslanbekova A.A., Pavlova T.Yu., Danilova S.N., Donskaya D.D.
Objective: To evaluate the obstetric and neonatal outcomes associated with the use of dinoprostone vaginal insert for cervical ripening and labor induction in routine obstetrical clinical practice.
Materials and methods: Multicenter clinical trial utilizing «real-world evidence» was conducted across 18 hospitals. The study included 503 primiparous and multiparous women with singleton pregnancies in cephalic presentation, gestational ages between 37 and 41+6 weeks, and a cervical Bishop score of 5 or less, who met all inclusion criteria, none with the exclusion criteria, and signed an informed consent form. Vaginal insert containing prostaglandin E2 (dinoprostone) was used for cervical ripening. After 24 h the insert was removed and the patients were re-evaluated. Based on the Bishop score, patients underwent either additional cervical ripening or labor induction.
Results: Labor began in 64.61% of patients before the end of the 24-hour interval while the dinoprostone vaginal insert was still in place. Failed cervical ripening and failed labor induction occurred in 2.78% and 2.78% of patients, respectively. The mean increase in the Bishop score was 2. A vaginal delivery was achieved for 77.14% of patients, while a cesarean was performed on 22.86% of patients. No infectious complications or significant side effects were reported.
Conclusion: The findings from our multicenter real-world evidence study indicate that the dinoprostone vaginal insert is an effective and safe method for cervical ripening and labor induction.
Authors’ contributions: Baev O.R., Tskhay V.B. – conception and design of the study, editing of the manuscript; Baev O.R., Tskhay V.B., Babich D.A., Bezhenar V.F., Semenov Yu.A., Gabelova K.A., Zainulina M.S., Tsakhilova S.G., Omarova N.G., Sheldagayeva A.V., Mentsik M.M., Semenova Yu.E., Bryukhanova A.A., Tkacheva N.V., Peshkova N.V., Vasilkovskaya E.N., Skornyakova L.M., Muravina E.L., Samoylova Yu.A., Rasskazova T.V., Fatkullina I.B., Frolov A.L., Dalaeva T.Kh., Emasheva N.M., Guskova O.I., Klishina V.V., Kipriyanova I.I., Klimkina K.I., Afanasieva K.Yu., Gracheva M.S.,
Osmanov N.Sh., Magomedova L.I., Arslanbekova A.A., Pavlova T.Yu., Danilova S.N., Donskaya D.D. – collection and processing of material; Babich D.A., Baev O.R. – statistical analysis, review of relevant publications, drafting of the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the V.I. Kulakov NMRC for OG&P (Ref. № 06 of 17.08.2023).
Patient Consent for Publication: All patients participating in the study signed an informed consent form. They were informed that the study data would be published without their names being used in written reports or publications.
Authors' Data Sharing Statement: The data supporting the findings of this study are available upon request from the corresponding author after approval from the principal investigator.
For citation: Baev O.R., Tskhay V.B., Babich D.A., Bezhenar V.F., Semenov Yu.A., Serova O.F., Gabelova K.A., Zainulina M.S., Tsakhilova S.G., Omarova N.G., Sheldagayeva A.V., Mentsik M.M., Semenova Yu.E., Bryukhanova A.A., Tkacheva N.V., Peshkova N.V., Vasilkovskaya E.N., Skornyakova L.M., Muravina E.L.,
Samoylova Yu.A., Rasskazova T.V., Fatkullina I.B., Frolov A.L., Dalaeva T.Kh., Emasheva N.M., Guskova O.I., Klishina V.V., Kipriyanova I.I., Klimkina K.I., Afanasieva K.Yu., Gracheva M.S., Osmanov N.Sh., Magomedova L.I., Arslanbekova A.A., Pavlova T.Yu., Danilova S.N., Donskaya D.D. Use of dinoprostone vaginal insert for cervical ripening and labor induction in routine obstetrical clinical practice: a multicenter study based on real-world evidence.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (6): 94-105 (in Russian)
https://dx.doi.org/10.18565/aig.2025.80
Keywords
In modern obstetric practice, cervical ripening and labor induction are common measures aimed at improving the outcomes for both mothers and newborns. Over the past 20 years, the frequency of induced labor has steadily increased, reaching 25–30% in developed countries [1, 2]. The effectiveness of labor induction and its outcomes are directly influenced by the degree of cervical "maturity," or readiness for labor [3, 4]. In this context, pre-induction of labor or cervical ripening is crucial for enhancing the effectiveness of labor induction. Currently, both pharmacological and mechanical methods are available for cervical ripening, differing in their mechanisms of action, conditions of use, and potential side effects. Mechanical methods, unlike pharmacological methods, have fewer contraindications, but are less comfortable for patients and require specific conditions for implementation [5]. Despite the relatively similar effectiveness of various pre-induction methods, professional communities tend to favor pharmacological agents for cervical ripening [6].
It is important to note that labor induction does not always result in successful outcomes. Available data indicate that the probability of an unsuccessful outcome can reach 27% [7]. Consequently, the question of selecting the optimal method for pre-induction and induction of labor remains a topic of ongoing discussion and relevance [5].
In the Russian Federation, dinoprostone, a synthetic prostaglandin E2, is approved for the preinduction and induction of labor and is available in two forms: an intracervical gel and a vaginal drug delivery system (VDDS). International studies suggest that the use of VDDS with dinoprostone offers several advantages over the gel form, including an increased probability of successful vaginal delivery within 24 h after cervical ripening begins and the ability to rapidly remove the system if necessary [8].
However, the final outcome of induced labor is not determined solely by the pre-induction method. The effectiveness of labor induction is also influenced by assessment practices, selection of additional cervical ripening methods, and overall labor management approaches adopted in obstetric institutions. Yet, domestic literature on the use of VDDS with dinoprostone for cervical ripening primarily features isolated single-center studies with small patient samples, which do not provide sufficient evidence to conclude its effectiveness in routine obstetric practice in Russia [9, 10].
Given these considerations, it is both important and urgent to investigate the effectiveness and safety of VDDS with dinoprostone for cervical ripening and labor induction in real clinical practice in Russia.
This multicenter study aimed to evaluate the effectiveness and safety of VDDS with dinoprostone for cervical ripening and labor induction in routine obstetric practice in the Russian Federation.
Materials and methods
A group of authors conducted a multicenter study based on real-world evidence to evaluate the efficacy and safety of VDDS for cervical ripening (pre-induction of labor) and labor induction from August 30, 2023, to February 28, 2024. The study was carried out in 18 hospitals (15 third-level and 3 second-level), with the participation of 615 pregnant women requiring cervical ripening and labor induction.
The study adhered to all the necessary ethical standards. Permission was obtained from the local ethics committees, and the study was reviewed and approved by the Research Ethics Committee of V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia (Ref. No. 06 of 17.08.2023). This study was conducted in accordance with the principles of the Declaration of Helsinki (1964; 2013). All patients included in the study provided informed consent.
Inclusion criteria for the study were as follows: age between 18 and 45 years; singleton pregnancy; cephalic presentation of the fetus; gestational age of 37 weeks (259 days) or more; unreadiness of the birth canal at the time of inclusion (Bishop score of 0–5 points); no contraindications to vaginal delivery or the use of other drugs for childbirth preparation (mifepristone, prostaglandin E2, oxytocin); and signed informed consent. The exclusion criteria included myoma or uterine malformations; a uterine scar from a previous cesarean section or other surgical intervention; severe heart disease; arterial hypertension of 160/100 mmHg or higher; severe dysfunction of the liver, kidneys, and adrenal glands; breech presentation of the fetus; estimated fetal weight less than 2000 g or more than 4500 g; acute or exacerbation of chronic infection; cardiotocography indicators indicating fetal distress; and indications for planned delivery by cesarean section.
A total of 503 patients met the inclusion criteria.
Patients received accessible explanations from the research physician regarding the indications for labor induction, procedure sequence, possible complications, side effects, and outcomes. After signing the informed consent and completing the anamnesis, general and obstetric examinations, and cardiotocographic assessment of the fetus, the patients underwent VDDS with dinoprostone inserted into the posterior vaginal fornix according to the manufacturer's instructions.
VDDS consists of a woven polyester sheath shaped like a long-looped tape designed for extraction, with one thin flat rectangular polymer plate with rounded corners placed inside. The active substance of the system is dinoprostone (10 mg), an analog of prostaglandin E2, while the auxiliary substance is a hydrogel of a cross-linked polymer and woven polyester tape. Unlike the gel form of dinoprostone, VDDS releases dinoprostone at a relatively constant rate (0.3 mg/h).
The onset of labor was defined as the appearance of regular uterine contractions (2-3 in 10 min or more) with progressive cervical effacement and dilation. The active phase of labor was considered to be achieved when complete cervical effacement and dilation of ≥ 5 cm occurred.
Indications for the removal of the VDDS with dinoprostone included: expiration of 24 hours after administration; complete "maturation" of the cervix (8 points or more on the Bishop scale); onset of labor; tachysystole (more than 5 contractions in 10 minutes) or uterine hypertonicity (contraction duration exceeding 90–120 seconds); signs of fetal distress; and indications of a pronounced systemic effect in the mother (hypotension, tachycardia).
The criterion for failure of VDDS use was the lack of a cervical maturation effect within 24 h, determined by the absence of changes in the Bishop score.
When cervical maturity reached ≥ 8 points, amniotomy was performed for labor induction. If contractions were absent within 4 h after amniotomy, labor induction with oxytocin was initiated. The lack of effective contractions with cervical dilation within 4 h after oxytocin administration was considered the criterion for ineffectiveness.
Clinical monitoring of the pregnant woman and fetus was performed. Before and after VDDS placement, and every 8 hours thereafter, including after VDDS removal and amniotomy, as well as continuously during oxytocin infusion and labor, the fetal condition was assessed using cardiotocography (FIGO classification 2015).
The primary outcome measures included changes in the Bishop score after VDDS placement, the number of women who went into labor before VDDS removal, the total number of vaginal deliveries, the number of vaginal deliveries within 24 hours of VDDS installation, and the failure rate of childbirth preparation.
Secondary outcome measures included the rate and severity of adverse reactions to VDDS, need for labor induction with oxytocin, duration of labor and anhydrous period, method of delivery, and newborn outcomes.
Statistical analysis
The data obtained during the study were subjected to statistical analyses using parametric and nonparametric methods. The collection, adjustment, systematization, and visualization of initial information were performed using Microsoft Office Excel 2016. Statistical analysis was conducted using IBM SPSS Statistics Trial Version (IBM Corporation, USA), Jamovi (JASP), and MedCalc Trial Version (MedCalc Software Ltd., Belgium). The normality of the distribution of continuous variables was tested using the Shapiro–Wilk test, along with the analysis of asymmetry and kurtosis. Continuous variables showing a normal distribution were expressed as mean (M) and standard deviation (SD), presented as M (SD), along with the 95% confidence interval (95% CI). For data deviating from a normal distribution, the median (Me) and interquartile range (Q1–Q3) were reported. Categorical data are presented as counts and percentages.
Results
The clinical, medical history and demographic data of the patients are presented in Table 1.
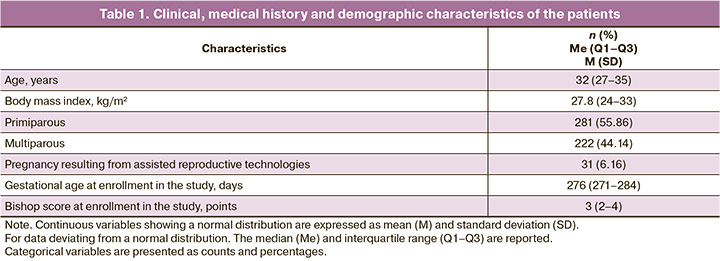
Among the study participants, there were slightly more primiparous women than multiparous ones. All the patients had full-term pregnancies. According to the Bishop scale, the degree of cervical maturity before inclusion in the study ranged from 0 to 5 points (median: 3 points). The most common indication for pre-induction or induction of labor was diabetes mellitus (Table 2). In 246 cases, VDDS placement was preceded by cervical ripening with mifepristone. The median Bishop score before mifepristone administration was 3 (2–3), and after cervical ripening with mifepristone, it was 4 (3–5).
Of the 503 cases, labor began against the background of the inserted VDDS in 325 cases (64.61%) before the end of the 24-hour period. Cervical "maturation" occurred in 164/503 (32.6%) cases. The median increase in Bishop score after cervical ripening with VDDS and dinoprostone was 2 points (1–3) (Table 3). In 14/503 cases (2.78%), there were no changes in the cervix; these cases were classified as having no effect on cervical ripening.
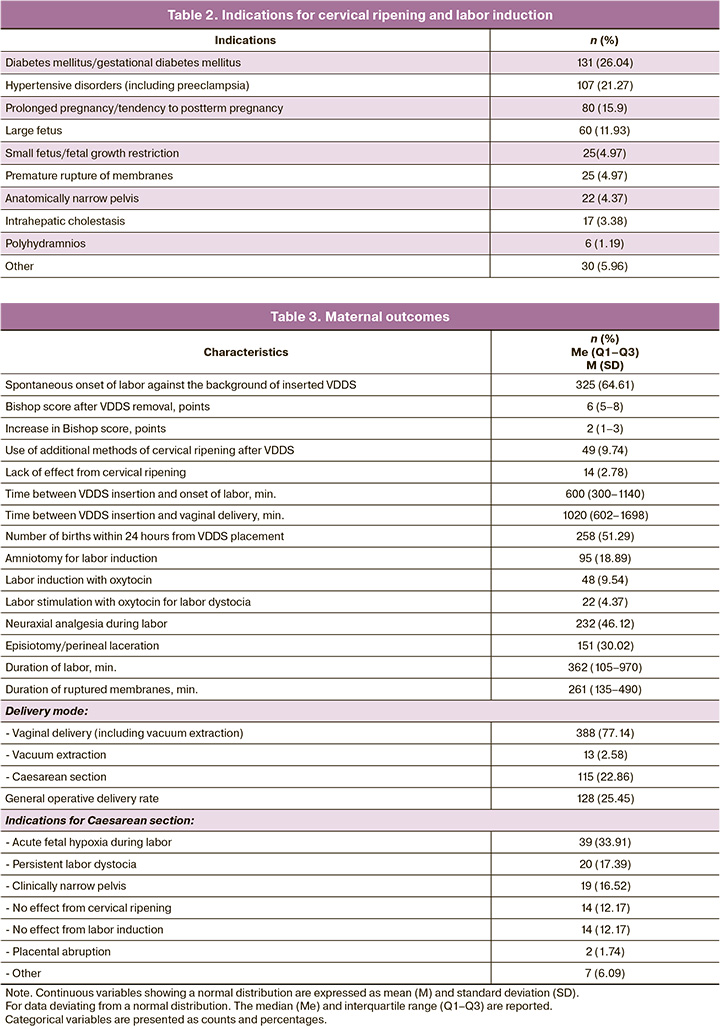
Among the 49 women in whom cervical maturity was less than 8 points according to the Bishop score after VDDS placement, additional cervical ripening methods (intracervical balloon or dinoprostone gel) were employed. Labor induction by amniotomy was performed in 18.89% of the cases, and labor induction by oxytocin was required twice less often.
A vaginal delivery was performed for 77.14% of patients. Labor dystocia was diagnosed in 4.37% of cases, and the average duration of labor stimulation was 242.3 (111.43) minutes. Moderate postpartum hemorrhage occurred in 13 patients (2.58%) and was successfully stopped by conservative measures. The mean labor duration was six hours, and the mean duration of ruptured membranes was four hours (Table 3). Within 24 h of VDDS placement, more than half of the women (51.29%) had vaginal delivery.
Operative vaginal delivery (vacuum extraction of the fetus) was performed in 13/503 (2.58%) cases due to acute fetal hypoxia or persistent weakness of labor. Surgical abdominal delivery was performed in 22.86% of the cases; the indications are presented in Table 3.
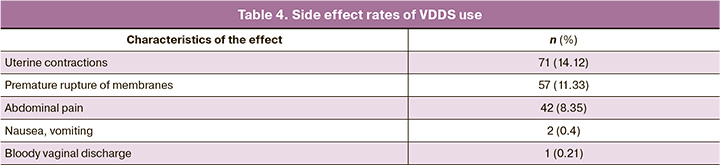
Postpartum hemorrhage occurred in 16/503 (3.18%) patients. The mean blood loss was 273.05 (132.96) ml during vaginal deliveries and 678.27 (215.64) ml during cesarean sections. Episiotomy or suturing of perineal lacerations was necessary in 151/503 (38.82%) vaginal deliveries, and manual examination of the postpartum uterine cavity was necessary in 35/503 (9.02%) vaginal deliveries. The most common effect of VDDS was precursor uterine contractions, which occurred in 14.12% of the cases (Table 4). Premature rupture of membranes occurred in 11.33% of the cases against the background of the inserted VDDS. In 8.35% of cases, women reported minor lower abdominal pain, which they described as "menstrual." There were no cases of diarrhea or placental abruption associated with VDDS. All observed effects were mild, and early removal of the VDDS was not necessary in any case.
Live babies were born in all cases. The average body weight of newborns was 3489 (451) g, and the frequency of large fetuses was 13.92%. The median Apgar score at 5 min after birth was 9 (8.5–9) points. In six infants (1.19%), the Apgar score at 5 min was below 7 points (from 4 to 6 points), and they were transferred to the neonatal intensive care unit for further examination and observation. All infants were discharged in satisfactory condition.
Discussion
Analysis of the study results demonstrated that the included pregnant women represented a cohort consistent with the general population. The most common indications for preinduction and induction of labor were diabetes mellitus, hypertensive disorders, and a tendency toward post-term pregnancy, which aligns with data typical of the general population and previous studies [10–14].
In our study, after the removal of VDDS, the mean increase in cervical maturity according to the Bishop score was 2 points, consistent with the results of earlier studies [10, 15]. In comparison, Gupta J.K. et al. (2022) reported that the use of mechanical dilators for cervical ripening led to a mean increase of three points on the Bishop scale [11].
When pre-inducing labor, it is crucial to consider that if the cervix is not “mature” enough after VDDS removal, additional cervical ripening methods may be necessary. Rankin K. et al. (2018) noted that the need for further cervical ripening when using a vaginal insert with dinoprostone occurs in 33% of cases [16]. In our study, the requirement for additional cervical ripening methods after using a vaginal insert with dinoprostone was recorded in only 9.87% of patients. This lower percentage may be attributed to the significant proportion of women who had previously received mifepristone for labor pre-induction. Notably, 64.61% of our patients entered labor following VDDS placement, a finding that aligns with the results from a multicenter study conducted by Beyer J. et al. (2022) [17]. The high percentage of deliveries within 24 h after VDDS placement underscores the system's effectiveness in both the pre-induction and induction of labor. This highlights the significant role of dinoprostone (a synthetic analog of prostaglandin E2) in the processes of cervical “maturation” and labor initiation, as it stimulates the production of endogenous prostaglandin F2α and enhances the myometrium's sensitivity to endogenous oxytocin [8]. Additionally, the low frequency of labor induction using oxytocin, required in only 9.54% of cases, further indicates the high efficacy of pre-induction labor using VDDS with dinoprostone. Overall, the frequency of oxytocin use in our study was 13.92%, which was significantly lower than that reported in other studies (26–57%) [18].
The interval from VDDS placement to the onset of labor was approximately 6 h, indicating a relatively rapid effect of the vaginal insert with dinoprostone. The mean time from VDDS placement to delivery was approximately 17 h, while the literature reports this figure to vary from 13 to 29 h. More than half of the women (51.29%) gave birth within 24 h of VDDS insertion. According to other researchers, the interval from the start of induction to delivery ranges from 12 to 36 h, with the percentage of vaginal deliveries within the first day after pre-induction ranging from 42.4% to 55.7% [11, 13, 18–20].
Vaginal deliveries after cervical ripening and labor induction occurred in 77.14% of the cases, while the cesarean section rate was 22.86%. The current literature indicates that when using the vaginal form of dinoprostone for cervical ripening, the rate of vaginal delivery ranges from 67.6% to 72.9%, whereas the cesarean section rate varies from 25.8% to 58.6% [8, 21–24]. Only one study reported a cesarean section rate after pre-induction/induction of VDDS that was lower than these values (14.3%). However, this study focused exclusively on low-risk pregnant women without gestational complications, with the sole indication being a tendency toward post-term pregnancy [25]. All children born to mothers in our multicenter study were alive and their birth weights corresponded to the average population values. The frequency of Apgar scores below 7 at the 5-minute mark was 1.19% in our study, which falls within the range reported in other studies (2.5–3.7%) [12, 26].
No infectious complications or significant side effects were observed. Moreover, the frequency and intensity of such manifestations were lower than those described in the available literature [26, 27]. It is important to note that the amount of blood loss after vaginal delivery and cesarean section, along with the need for episiotomy and manual examination of the postpartum uterine cavity, was within population values.
Currently, there is no consensus in the scientific literature regarding the criteria for success or failure of labor induction. The most commonly used indicators for assessing effectiveness include the frequency of vaginal deliveries, proportion of spontaneous vaginal deliveries, and percentage of deliveries within the first 24 hours from the start of the measures [7, 28–32].
Our results demonstrated a low rate of failure to respond to cervical ripening (2.78%), according to the Bishop score. When considering the rate of failure to respond to labor induction (2.78%), the overall failure rate for the preinduction/induction of labor was 5.57%. Literature data indicate that failure of labor induction when using vaginal forms of dinoprostone occurs in 7–18% of cases [29, 33].
Limitations of the study
Our study had several limitations. Owing to the extensive volume of primary information, we did not perform analyses based on birth parity, although the effectiveness of VDDS may differ across these groups. The effectiveness of VDDS relative to the initial maturity of the cervix has not been examined, which represents a potential area for a personalized approach. Furthermore, a differentiated analysis based on a preliminary preparation with mifepristone was not conducted. These limitations will be the subject of future research. However, important advantages of our work include its prospective design and participation from centers of varying levels, allowing us to consider this study as a "real-world evidence study."
Conclusion
The results of this multicenter "real-world evidence" study indicate that the use of VDDS with dinoprostone is an effective and safe method for pre-induction of labor and is suitable for routine obstetric practice.
References
- Declercq E., Belanoff C., Iverson R. Maternal perceptions of the experience of attempted labor induction and medically elective inductions: analysis of survey results from listening to mothers in California. BMC Pregnancy Childbirth. 2020; 20(1): 458. https://dx.doi.org/10.1186/s12884-020-03137-x
- Tsakiridis I., Mamopoulos A., Athanasiadis A., Dagklis T. Induction of labor: an overview of guidelines. Obstet. Gynecol. Surv. 2020; 75(1): 61-72. https://dx.doi.org/10.1097/OGX.0000000000000752
- Levine L.D. Cervical ripening: Why we do what we do. Semin. Perinatol. 2020; 44(2): 151216. https://dx.doi.org/10.1016/j.semperi.2019.151216
- Socha M.W., Flis W., Wartęga M., Kunicka A., Stankiewicz M. A review of the mechanism of action and clinical applications of osmotic dilators for cervical ripening in the induction of labor and in gynecology procedures. Med. Sci. Monit. 2023; 29: e940127. https://dx.doi.org/10.12659/MSM.940127
- Sanchez-Ramos L., Levine L.D., Sciscione A.C., Mozurkewich E.L., Ramsey P.S., Adair C.D. et al. Methods for the induction of labor: efficacy and safety. Am. J. Obstet. Gynecol. 2024; 230(3S): S669-S695. https://dx.doi.org/10.1016/j.ajog.2023.02.009
- Inducing labour. London: National Institute for Health and Care Excellence (NICE); 2021 Nov 4.
- Freret T.S., Woods G.T., James K.E., Kaimal A.J., Clapp M.A. Incidence of and risk factors for failed induction of labor using a contemporary definition. Obstet. Gynecol. 2021; 137(3): 497-504. https://dx.doi.org/10.1097/AOG.0000000000004257
- Shirley M. Dinoprostone vaginal insert: a review in cervical ripening. Drugs. 2018; 78(15): 1615-24. https://dx.doi.org/10.1007/s40265-018-0995-2
- Сакварелидзе Н.Ю., Цахилова С.Г, Паенди Ф.А. Прогностические предикторы эффективности и безопасности использования вагинальной вставки динопростона в подготовке шейки матки к родам. Эффективная фармакотерапия. 2022; 18(24): 12-4. [Sakvarelidze N.Yu., Czaxilova S.G, Paendi F.A. Predictive predictors of the efficacy and safety of using the dinoprostone vaginal insert in preparing the cervix for childbirth. Effective Pharmacotherapy. 2022; 18(24): 12-4. (in Russian)]. https://dx.doi.org/10.33978/2307-3586-2022-18-24-12-14
- Баев О.Р., Гусар В.А., Гайдарова А.Р., Эдильберг И.В. Применение вагинальной терапевтической системы с простагландином для индукции родов. Медицинский совет. 2022; 16(16): 84-91. [Baev O.R., Gusar V.A., Gaydarova A.R., Edilberg I.V. The use of a vaginal therapeutic system with prostaglandin for induction of labor. Medical Council. 2022; 16(16): 84-91. (in Russian)]. https://dx.doi.org/10.21518/2079-701X-2022-16-16-84-91
- Gupta J.K., Maher A., Stubbs C., Brocklehurst P., Daniels J.P., Hardy P.; Synthetic Osmotic Cervical Dilator for Induction of Labor in Comparison to Dinoprostone Vaginal insErt (SOLVE) collaborative group. A randomized trial of synthetic osmotic cervical dilator for induction of labor vs dinoprostone vaginal insert. Am. J. Obstet. Gynecol. MFM. 2022; 4(4): 100628. https://dx.doi.org/10.1016/j.ajogmf.2022.100628
- Devillard E., Petillon F., Rouzaire M., Pereira B., Accoceberry M., Houlle C. et al. Double balloon catheter (plus oxytocin) versus dinoprostone vaginal insert for term rupture of membranes: A randomized controlled trial (RUBAPRO). J. Clin. Med. 2022; 11(6): 1525. https://dx.doi.org/10.3390/jcm11061525
- De Bonrostro Torralba C., Tejero Cabrejas E.L., Envid Lázaro B.M., Franco Royo M.J., Roca Arquillué M., Campillos Maza J.M. Low-dose vaginal misoprostol vs vaginal dinoprostone insert for induction of labor beyond 41st week: A randomized trial. Acta Obstet. Gynecol. Scand. 2019; 98(7): 913-9. https://dx.doi.org/10.1111/aogs.13556
- Sarno L., Tesauro M., Carlea A., Quaglia F., Maruotti G.M., Pannella G. et al. Single versus double application of vaginal dinoprostone: maternal factors affecting responsiveness. J. Matern. Fetal Neonatal. Med. 2022; 35(24): 4763-7. https://dx.doi.org/10.1080/14767058.2020.1863367
- López-Jiménez N., García-Sánchez F., Pailos R.H., Rodrigo-Álvaro V., Pascual-Pedreño A., Moreno-Cid M. et al. Induction of labor with vaginal dinoprostone (PGE2) in patients with a previous cesarean section: obstetric and neonatal outcomes. J. Clin. Med. 2021; 10(22): 5221. https://dx.doi.org/10.3390/jcm10225221
- Rankin K., Chodankar R., Raymond K., Bhaskar S. Misoprostol vaginal insert versus dinoprostone vaginal insert: A comparison of labour and delivery outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019; 235: 93-6. https://dx.doi.org/10.1016/j.ejogrb.2018.07.025
- Beyer J., Jäger Y., Balci D., Kolb G., Weschenfelder F., Seeger S. et al. Induction of labor at term with oral misoprostol or as a vaginal insert and dinoprostone vaginal insert - a multicenter prospective cohort study. Geburtshilfe Frauenheilkd. 2022; 82(8): 868-73https://dx.doi.org/10.1055/a-1860-0419
- Zhao L., Lin Y., Jiang T.T., Wang L., Li M., Wang Y. et al. Vaginal delivery among women who underwent labor induction with vaginal dinoprostone (PGE2) insert: a retrospective study of 1656 women in China. J. Matern. Fetal Neonatal Med. 2019; 32(10): 1721-7. https://dx.doi.org/10.1080/14767058.2017.1416351
- Bhatia A., Teo P.L., Li M., Lee J.Y.B., Chan M.X.J., Yeo T.W. et al. Dinoprostone vaginal insert (DVI) versus adjunctive sweeping of membranes and DVI for term induction of labor. J. Obstet. Gynaecol. Res. 2021; 47(9): 3171-8. https://dx.doi.org/10.1111/jog.14907
- Maggi C., Mazzoni G., Gerosa V., Fratelli N., Prefumo F., Sartori E. et al. Labor induction with misoprostol vaginal insert compared with dinoprostone vaginal insert. Acta Obstet. Gynecol. Scand. 2019; 98(10): 1268-73. https://dx.doi.org/10.1111/aogs.13667
- Beckmann M., Gibbons K., Flenady V., Kumar S. Induction of labour using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicentre randomised controlled trial. BJOG. 2020; 127(5): 571-9. https://dx.doi.org/10.1111/1471-0528.16030
- Di Tommaso M., Pellegrini R., Ammar O., Lecis S., Huri M., Facchinetti F. Safety of the use of dinoprostone gel and vaginal insert for induction of labor: A multicenter retrospective cohort study. Int. J. Gynaecol. Obstet. 2025; 168(3): 1039-46. https://dx.doi.org/10.1002/ijgo.15952
- Shindo R., Aoki S., Nakanishi S., Obata S., Miyagi E. Impact of introducing a controlled-release dinoprostone vaginal insert for labor induction: a retrospective single-center study in Japan. Cureus. 2024; 16(1): e53180. https://dx.doi.org/10.7759/cureus.53180
- Taliento C., Manservigi M., Tormen M., Cappadona R., Piccolotti I., Salvioli S. et al. Safety of misoprostol vs dinoprostone for induction of labor: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023; 289: 108-28. https://dx.doi.org/10.1016/j.ejogrb.2023.08.382
- Itoh H., Ishii K., Shigeta N., Itakura A., Hamada H., Nagamatsu T. et al. Efficacy and safety of controlled-release dinoprostone vaginal delivery system (PROPESS) in Japanese pregnant women requiring cervical ripening: Results from a multicenter, randomized, double-blind, placebo-controlled phase III study. J. Obstet. Gynaecol. Res. 2021; 47(1): 216-25. https://dx.doi.org/10.1111/jog.14472
- Lauterbach R., Ben Zvi D., Dabaja H., Zidan R., Justman N., Vitner D. et al. Vaginal dinoprostone insert versus cervical ripening balloon for term induction of labor in obese nulliparas-a randomized controlled trial. J. Clin. Med. 2022; 11(8): 2138. https://dx.doi.org/10.3390/jcm11082138
- Anh N.D., Duc T.A., Ha N.T., Giang D.T., Dat D.T., Thuong P.H. et al. Dinoprostone vaginal insert for induction of labor in women with low-risk pregnancies: a prospective study. Med. Arch. 2022; 76(1): 39-44. https://dx.doi.org/10.5455/medarh.2022.76.39-44
- Grobman W.A., Bailit J., Lai Y., Reddy U.M., Wapner R.J., Varner M.W. et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Defining failed induction of labor. Am. J. Obstet. Gynecol. 2018; 218(1): 122.e1-122.e8. https://dx.doi.org/10.1016/j.ajog.2017.11.556
- Kamel R., Garcia F.S.M., Poon L.C., Youssef A. The usefulness of ultrasound before induction of labor. Am. J. Obstet. Gynecol. MFM. 2021; 3(6S): 100423. https://dx.doi.org/10.1016/j.ajogmf.2021.100423
- Bashirudin S.B., Omar S.Z., Gan F., Hamdan M., Tan P.C. Induction of labor after one previous cesarean: Predictors of vaginal birth. Eur. J. Obstet. Gynecol. Reprod. Biol. X. 2023; 20: 100249. https://dx.doi.org/10.1016/j.eurox.2023.100249
- Sørbye I.K., Oppegaard K.S., Weeks A., Marsdal K., Jacobsen A.F. Induction of labor and nulliparity: A nationwide clinical practice pilot evaluation. Acta Obstet. Gynecol. Scand. 2020; 99(12): 1700-9. https://dx.doi.org/10.1111/aogs.13948
- Kamel R.A., Negm S.M., Youssef A., Bianchini L., Brunelli E., Pilu G. et al. Predicting cesarean delivery for failure to progress as an outcome of labor induction in term singleton pregnancy. Am. J. Obstet. Gynecol. 2021; 224(6): 609.e1-609.e11. https://dx.doi.org/10.1016/j.ajog.2020.12.1212
- Tseng J.Y., Lin I.C., Chang W.H., Yeh C.C., Horng H.C., Wang P.H. Using dinoprostone vaginal insert for induction of labor: A single institute experience. Taiwan J. Obstet. Gynecol. 2020; 59(5): 723-7. https://dx.doi.org/10.1016/j.tjog.2020.07.017
Received 20.03.2025
Accepted 21.05.2025
About the Authors
Oleg R. Baev, Dr. Med. Sci., Professor, Head of the 1st Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4; Professor at the Department of Obstetrics, Gynecology, Perinatology and Reproductology, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia (Sechenov University), 119991, Russia, Moscow, Trubetskaya str., 8-2, metod_obsgyn@hotmail.com, https://orcid.org/0000-0001-8572-1971Vitaly B. Tskhay, Dr. Med. Sci., Professor, Head of the Department of Perinatology, Obstetrics and Gynecology of the Medical Faculty, Prof. V.F. Voino-Yasenetsky Krasnoyarsk State Medical University, Ministry of Health of Russia, 660022, Russia, Krasnoyarsk, P. Zheleznyak str., 1, +7(923)287-21-34, tchai@yandex.ru,
https://orcid.org/0000-0003-2228-3884
Dmitry A. Babich, PhD, Obstetrician-Gynecologist at the1st Maternity Department, Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Ac. Oparina str., 4, +7(903)295-05-05, d_babich@oparina4.ru, https://orcid.org/0000-0002-3264-2038
Vitaly F. Bezhenar, Dr. Med. Sci., Professor, Head of the Departments of Obstetrics, Gynecology and Neonatology/Reproductology, Head of the Clinic of Obstetrics and Gynecology, Pavlov First State Medical University, Ministry of Health of Russia, 197022, Russia, St. Petersburg, Leo Tolstoy str., 6-8; Main Supernumerary Specialist Obstetrician-Gynecologist of the Health Committee of St. Petersburg, +7(812)338-78-66, bez-vitaly@yandex.ru, https://orcid.org/0000-0002-7807-4929
Yuri A. Semenov, Dr. Med. Sci., Director, Urals Research Institute of Maternity and Child Care, Ministry of Health of Russia, 620028, Russia, Yekaterinburg, Repin str., 1, +7(343)371-87-68, u-sirius@mail.ru, https://orcid.org/0000-0002-3855-3650
Olga F. Serova, Dr. Med. Sci., Professor, Chief External Expert in Obstetrics and Gynaecology at the Moscow Region, Department of Health; Chief Physician, Moscow Regional Perinatal Center, 143900, Russia, Moscow region, Balashikha, Shosse Entuziastov, 12; Head of the Department of Obstetrics, Gynecology and Perinatology,
A.I. Burnazyan Federal Medical Biophysical Center of the Federal Medical and Biological Agency of Russia, +7(916)193-46-38, olga-serova@yandex.ru,
https://orcid.org/0000-0002-4088-4619
Karina A. Gabelova, PhD, Associate Professor at the Department of Obstetrics, Gynecology and Neonatology; Head of the Obstetrics Department of Pregnancy Pathology, Clinic for Obstetrics and Gynecology, Pavlov First State Medical University, Ministry of Health of Russia, 197022, Russia, St. Petersburg, Leo Tolstoy str., 6-8,
+7(812)338-78-66, kgabelova@mail.ru
Marina S. Zainulina, Dr. Med. Sci, Professor, Department of Obstetrics, Gynecology and Reproduction, Pavlov First State Medical University, Ministry of Health of Russia, 197022, Russia, St. Petersburg, Leo Tolstoy str., 6-8; Chief Physician, V.F. Snegirev Maternity Hospital No 6, 191014, Russia, St. Petersburg, Mayakovsky str., 5,
+7(812)338-78-66, zainulina@yandex.ru, https://orcid.org/0000-0002-2622-5000
Svetlana G. Tsakhilova, Dr. Med. Sci., Professor, Professor at the Department of Obstetrics, Gynecology and Reproductive Medicine at the N.A. Semashko Scientific and Educational Institute of Clinical Medicine, Russian University of Medicine, Ministry of Health of Russia, 127006, Russia, Moscow, Dolgorukovskaya str., 4, Tsakhilovas@mail.ru, https://orcid.org/0000-0002-4898-6919
Naira H. Omarova, Obstetrician-Gynecologist, V.F. Snegirev Maternity Hospital No 6, 191014, Russia, St. Petersburg, Mayakovsky str., 5, +7(812)73-54-93,
omarovanaira@mail.ru
Angelika V. Sheldagayeva, Obstetrician-Gynecologist, Senior Laboratory Assistant at the Department, Pavlov First State Medical University, Ministry of Health of Russia, 197022, Russia, St. Petersburg, Leo Tolstoy str., 6-8, +7(812)338-7866
Marina M. Menzik, Deputy Chief Physician for Obstetrics and Gynecology, Krasnoyarsk Interdistrict Clinical Hospital No. 20 named after I.S. Berzon, 660123, Russia, Krasnoyarsk, Instrumentalnaya str., 12, +7(391)262-18-96, gkb20@mail.ru
Yulia E. Semenova, Obstetrician-Gynecologist, Department of Pregnancy Pathology, Krasnoyarsk Interdistrict Clinical Hospital No. 20 named after I.S. Berzon,
660123, Russia, Krasnoyarsk, Instrumentalnaya str., 12, +7(391)262-18-96, gkb20@mail.ru
Anastasia A. Bryukhanova, Head of the Maternity Hospital Admission Department, Krasnoyarsk Interdistrict Clinical Hospital No. 20 named after I.S. Berzon,
660123, Russia, Krasnoyarsk, Instrumentalnaya str., 12, +7(391)262-18-96, gkb20@mail.ru
Nadezhda V. Tkacheva, Obstetrician-Gynecologist, Department of Pregnancy Pathology, Krasnoyarsk Interdistrict Clinical Hospital No. 20 named after I.S. Berzon,
660123, Russia, Krasnoyarsk, Instrumentalnaya str., 12, +7(391)262-18-96, gkb20@mail.ru
Natalia V. Peshkova, Head of the Maternity Department, District Clinical Hospital, 628012, Russia, Khanty-Mansiysk Autonomous Okrug – Yugra, Khanty-Mansiysk,
Kalinina str., 40, +7(3467)33-35-71, roddomokb@mail.ru
Elena N. Vasilkovskaya, Deputy Head for Obstetric and Gynecological Care, District Clinical Hospital, 628012, Russia, Khanty-Mansiysk Autonomous Okrug –Yugra,
Khanty-Mansiysk, Kalinina str., 40, +7(3467)33-35-71, roddomokb@mail.ru
Lyudmila M. Skornyakova, Deputy Chief Physician for Obstetric and Gynecological Care, Stavropol Regional Clinical Perinatal Center No. 1, Russia,
355029, Russia, Stavropol Territory, Stavropol, Semashko str., 3/1 in block 486, +7(8652)25-71-59, Skornyakova_lm@mail.ru
Elena L. Muravina, PhD, Deputy Chief Physician for Obstetrics and Gynecology, S.S. Yudin City Clinical Hospital of the Department of Health of the City of Moscow,
115446, Russia, Moscow, Kolomenskiy passage, 4, build. 2, gkb-yudina@zdrav.mos.ru
Yulia A. Samoylova, PhD, Head of the First Obstetric Department of Pregnancy Pathology, Maternity Hospital, S.S. Yudin City Clinical Hospital of the Department of Health of the City of Moscow, 115446, Russia, Moscow, Kolomenskiy passage, 4, build. 2; Teaching Assistant at the Department of Obstetrics, Gynecology and Perinatology of the Faculty of Medicine, I.M. Sechenov First Moscow State Medical University, Ministry of Health of Russia, samoylova2005@yandex.ru
Tatiana V. Rasskazova, Obstetrician-Gynecologist, Senior Resident at the First Obstetric Department of Pregnancy Pathology, Maternity Hospital, S.S. Yudin City Clinical Hospital of the Department of Health of the City of Moscow, 115446, Russia, Moscow, Kolomenskiy passage, 4, build. 2, gkb-yudina@zdrav.mos.ru
Irina B. Fatkullina, Dr. Med. Sci, Deputy Chief Physician, Republican Clinical Perinatal Center, Ministry of Health of the Republic of Bashkortostan,
450106, Russia, Ufa, Batyrskaya str., 41; Associate Professor at the Department of Obstetrics and Gynecology No. 1, Bashkir State Medical University,
Ministry of Health of Russia, fib1971@mail.ru, https://orcid.org/0009-0004-9453-6718
Alexey L. Frolov, PhD, Head of the Operating Department No. 2, Republican Clinical Perinatal Center, Ministry of Health of the Republic of Bashkortostan,
450106, Russia, Ufa, Batyrskaya str., 41, frolrpc@yandex.ru, https://orcid.org/0009-0004-0678-1503
Tanzila К. Dalaeva, Obstetrician-Gynecologist, Republican Clinical Perinatal Center, Ministry of Health of the Republic of Bashkortostan,
450106, Russia, Ufa, Batyrskaya str., 41, tanzila_dalaeva@mail.ru, https://orcid.org/0009-0009-3523-7722
Narkes M. Emasheva, Head of the Department of Obstetric Pathology of Pregnancy, Republican Clinical Hospital named after G.G. Kuvatov,
450005, Russia, Republic of Bashkortostan, Ufa, Dostoevsky str., 132; +7(347)279-03-97, ufa.rkbkuv@doctorrb.ru
Olga I. Guskova, Chief of the Perinatal Center, Saratov City Clinical Hospital No. 8, 410052, Russia, Saratov, Odesskaya str., 46A, build. 2, +7(8452)39-31-10, sargkb8@mail.ru
Victoria V. Klishina, Deputy Chief Physician for Obstetrics and Gynecology, V.Y. Mishekurin Novgorod Regional Clinical Perinatal Center,
173017, Russia, Veliky Novgorod, Derzhavina str., 1, +7(8162)63-73-60, priemokrd@mail.ru
Irina I. Kipriyanova, PhD, Deputy Chief Physician in Obstetrics, Regional Perinatal Center, 454141, Russia, Chelyabinsk, Vorovskiy str., 70, build. 12, +7(351)225-24-27, kipriyanovaii@list.ru
Kseniya I. Klimkina, Head of the Obstetric Department of Pregnancy Pathology, Regional Perinatal Center,
454141, Russia, Chelyabinsk, Vorovskiy str., 70, build. 12, +7(351)225-24-27
Ksenia Yu. Afanasieva, Head of the Department of Pregnancy Pathology, Moscow Regional Perinatal Center,
143900, Russia, Moscow region, Balashikha, Shosse Entuziastov, 12, +7(495)529-50-13, KseniyaTarkhova@mail.ru
Marina S. Gracheva, Head of the Department of Pregnancy Pathology at the 1st Obstetric Hospital, Yekaterinburg Clinical Perinatal Center,
620137, Russia, Yekaterinburg, Komsomolskaya str., 9, +7(343) 374-51-08, terris808061@gmail.com
Nizam S. Osmanov, Chief Physician, Republican Perinatal Center named after Omarov S.-M.A., 367027, Russia, Republic of Dagestan, Makhachkala, Akhmed Magomedov str., 2, +7(8722)64-01-28, gbu_rpc@e-dag.ru
Lyudmila I. Magomedova, Deputy Chief Physician for Obstetric and Gynecological Care, Republican Perinatal Center named after Omarov S.-M.A.,
367027, Russia, Republic of Dagestan, Makhachkala, Akhmed Magomedov str., 2, +7(8722)64-01-26, gbu_rpc@e-dag.ru
Amina A. Arslanbekova, PhD, Deputy Chief Physician for Clinical and Expert Work, Republican Perinatal Center named after Omarov S.-M.A.,
367027, Russia, Republic of Dagestan, Makhachkala, Akhmed Magomedov str., 2, +7(8722)64-01-28, gbu_rpc@e-dag.ru
Tatyana Yu. Pavlova, PhD, Deputy Minister of Health of the Republic of Sakha (Yakutia), 677010, Russia, Republic of Sakha (Yakutia), Yakutsk, Lermontov str., 126, tatyanaupavl@mail.ru
Sargylana I. Danilova, Director, Republican Hospital No. 1 – National Center of Medicine named after M.E. Nikolaev,
677010, Russia, Republic of Sakha (Yakutia), Yakutsk, Sergelyakhskoe shosse str., 4, rb1ncm@gov14.ru
Danara D. Donskaya, Deputy Chief Physician for Obstetrics and Gynecology, Yakut Republican Clinical Hospital,
677005, Russia, Republic of Sakha (Yakutia), Yakutsk, Stadukhina str., 81, +7(4112)43-20-41, gorclinbol@mail.ru
Corresponding authors: Oleg R. Baev, o_baev@oparina4.ru; Dmitry A. Babich, d_babich@oparina4.ru



