Clinical and diagnostic criteria for postpartum endometritis according to delivery mode
Aim. To investigate risk factors, clinical and diagnostic criteria for the development of postpartum endometritis according to delivery mode.Barinov S.V., Lazareva O.V., Shkabarnya L.L., Savculich V.E., Sycheva A.S., Tirskaya Yu.I., Khoroshkin E.A., Kadtsyna T.V., Medyannikova I.V., Chulovsky Yu.I., Beznoshchenko G.B.
Materials and methods. The present study retrospectively analyzed 97 cases of postpartum endometritis, including 55 (group A) and 42 (group B) patients who underwent cesarean and vaginal delivery, respectively.
Results. The mean age of patients was 27.7 (5.7) years. In group A, 12/55 (21.8%) women had repeat emergency caesarean delivery and 74.5% (41/55) required an emergency caesarean section. In group A, patients were significantly more likely to have gynecological diseases (χ2=3.562; p=0.056), preeclampsia (χ2=0.743; p=0.003), leukocytosis (χ2=3.762; p=0.052), stab neutrophils (χ2=6.169; p=0.013), anemia (χ2=4.767; p=0.029), thrombocytosis (χ2=11.526; p=0.000), hypoproteinemia (χ2=6.047; p=0.014), hypercoagulability (χ2=4.342; p=0.037). The symptoms of endometritis developed statistically significantly more often on days 2–4 after cesarean section (χ2=7.590; p=0.006). Uterine subinvolution and hematometra were detected by ultrasound in 50/55 (90.9%) women in group A and in 41/42 (97.6%) in group B. Placenta accreta spectrum was observed in 3/55 (5.5%) and 4/42 (9.5%) cases in groups A and B, respectively. The main identified microflora in puerperal women included Staphilococcus epidermidis, Esherichia coli, and enterococci in 28/97 (28.9%), 20/97 (20.0%), and 25/97 (25.8%) cases, respectively.
Conclusion. Cesarean section is associated with more severe postpartum endometritis and septic complications than vaginal delivery.
Keywords
Postpartum endometritis accounts for 39% of postpartum infections, one of the leading causes of maternal mortality [1–3]. Endometritis is the leading cause of postpartum sepsis [4, 5]. Factors that may increase the risk of postpartum endometritis are obesity, diabetes mellitus, chronic infectious diseases, vaginal infections, preeclampsia, prolonged duration of ruptured membranes, multiple vaginal examinations, intrauterine infection, manual removal of the placenta, excessive blood loss during childbirth, anemia, and cesarean section [3, 6, 7]. Surgical delivery is associated with a 20-fold and 25-fold higher likelihood of endometritis and infection-related mortality, respectively. Endometritis rates were estimated at 1–3% after vaginal delivery and 5–10 times higher (13–90%) following cesarean section. This increase is associated with maternal factors, type of surgery (elective or emergency), comorbidities, antibiotic prophylaxis, operation characteristics, country socio-economic level, and perioperative complications [7, 8]. Each subsequent cesarean section increases the risk of developing surgical complications and postpartum endometritis [7, 8]. Post-cesarean endometritis has a severe course, characterized by higher rates of septic complications, leading to reoperation, hysterectomy and adverse long-term consequences, uterine scar dehiscence, and reduced health-related quality of life [9–11].
This study aimed was to investigate risk factors, clinical and diagnostic criteria for the development of post-cesarean endometritis compared with endometritis after vaginal delivery.
Materials and methods
This retrospective controlled study included 97 puerperal women with postpartum endometritis who were managed at the Omsk Perinatal Center of the Regional Clinical Hospital from 2016 to 2020. The study participants were divided according to delivery mode into two groups, comprising patients after abdominal cesarean section (group A, n=55) and women who had a vaginal delivery (group B, n=42).
History taking included past obstetrics and gynecological history, course of pregnancy, childbirth, postpartum period. Clinical evaluation included somatic status, gynecological examination, biochemical and coagulation tests, urinalysis, bacteriology of the uterine cavity, pelvic transvaginal and transabdominal ultrasound (US), and hysteroscopy.
Statistical analysis
Statistical analysis was performed using the Statistica 10 software (StatSoft Inc., USA) and Microsoft Excel. Quantitative variables showing normal distribution were expressed as means (M) and standard deviation (SD). Qualitative variables were summarized as counts and percentages. Categorical variables were compared by the Chi-square test (χ2). Differences were considered statistically significant at p<0.05. The odds ratio (OR) and 95% confidence interval (CI) were calculated, and the corresponding p-test for postpartum hysterectomy versus vaginal delivery cases.
Results
The study findings included data obtained on the anamnesis, characteristics of the course of pregnancy, childbirth, and the postpartum period. Data on the time of disease manifestation, laboratory, and clinical parameters in each group were collected.
The patients’ age ranged from 16 to 39 years, with a mean of 27.7 (5.7) years, including 27.8 (4.1) and 27.3 (3.4) years in groups A and B, respectively. The body mass index was 25.6 (7.3) kg/m2, 25.3 (6.3), and 25.5 (5.8) kg/m2 in groups A and B, respectively. Index birth as the first in 34/97 (35.1%) women. Data of obstetric and gynecological history and pregnancy complications are presented in Table 1.
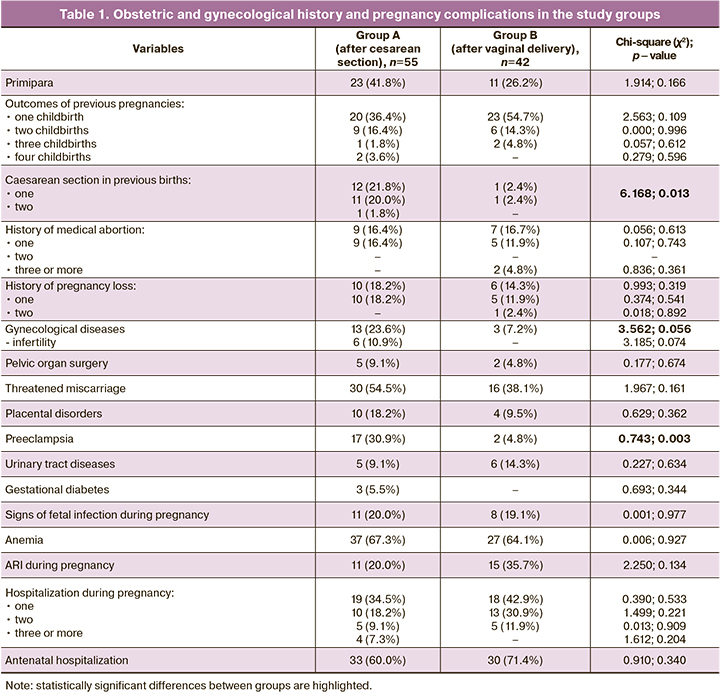
The average length of antenatal hospital stay in group A was 5.4 (3.7) days (min. – 1 day, max. – 17 days) and 3.2 (1.4) days (min. – 1 day, max. – 19 days) in group B. Elective and emergency cesarean delivery was performed in 14/55 (25.5%) and 41/55 (74.5%) women, respectively. In most puerperal women (12/14 – 85.7%), the indication for elective surgery was a post-cesarean uterine scar, and 2/14 (14.3%) had a combination of indications. The indications for emergency caesarean section were labor abnormality [22/41 (53.6%)], fetal hypoxia during labor [10/41 (24.4%)], severe preeclampsia [2/41 (4.9%)], and a combination of factors [8/41 (19.54)].
Clinical manifestations of endometritis symptoms were diagnosed in group A on day 11.4 (4.1), in group B – on day 10.6 (5.2) and developed in subjects in groups A and B, respectively: on 2–4th day 12/55 (21.8%) and 2/42 (4.8%) women, χ2=4.314; p=0.038; on the 5–8th day – in 10/55 (18.2%) and 21/42 (50.0%), χ2=9.117; p=0.003; on the 9–13th day – in 21/55 (38.2%) and 10/42 (23.7%), χ2=1.650; p=0.199; on the 14–20th day – in 8/55 (14.5%) and 7/42 (16.7%), χ2=0.000; p=0.996; on days 21–40th – in 4/55 (7.3%) and 2/42 (4.8%), χ2=0.007; p=0.934.
Of the clinical manifestations, the most common symptom of endometritis was fever (88/97 – 90.7%), which was observed in 50/55 (90.9%) women in group A and in 38/42 (90.5%) patients in group B (χ2=0.079; p=0.779). Complaints of purulent vaginal discharge were reported by 20/97 (20.6%) puerperal women, including 13/55 (23.6%) in group A and 7/42 (16.7%) in group B (χ2=0.345; p=0.557). Excessive bloody vaginal discharge was reported by 19/97 (19.6%) women, including 9/55 (16.4%) in group A and 10/42 (23.8%) in group B (χ2=0.432; p=0.511). Lower abdomen pain was reported by 26/97 (26.8%) patients, including (18/55 (32.7%) in group A and 8/42 (19.1%) in group B, χ2=1.972; p=0.160). In a bimanual vaginal examination, 79/97 (81.4%) of the subjects had subinvolution and softening of the uterus, of whom 43/55 (78.2%) and 36/42 (85.7%) in groups A and B, respectively (χ2=0.465; p=0.495).
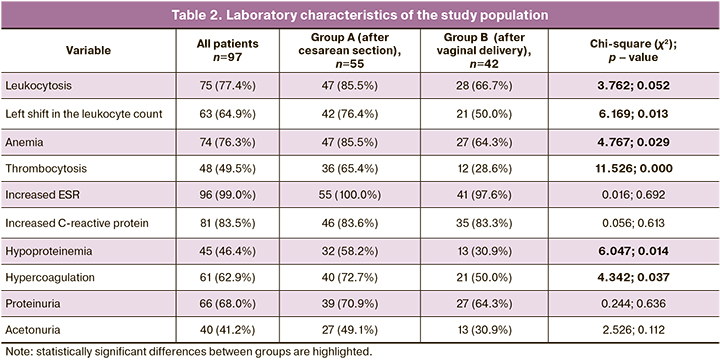
Laboratory characteristics of the study population are presented in Table 2.
All patients underwent ultrasound examination. The main ultrasound signs were uterine subinvolution, distension of the uterine cavity, and signs of hematometra. These changes were observed in 50/55 (90.9%) and 41/42 (97.6%) in groups A and B, respectively (χ2=0.672; p=0.350). In the group after cesarean section, no changes in ultrasound were observed in 5/55 (9.1%) of puerperal women, four of whom developed peritonitis symptoms on days 2–3 postoperatively. These patients had a fever and transient symptoms of ileus 4–6 hours after cesarean section. Despite antibacterial therapy and fluid resuscitation, they developed peritonitis requiring relaparotomy and hysterectomy. In 7/55 (12.7%) women of group A, an ultrasound showed signs of hematoma due to postoperative wound dehiscence, and 3/55 (5.5%) patients had signs of pathologic adherence of placental villi, which was confirmed by subsequent relaparotomy and histological examination. In the group of patients after vaginal delivery, only 1/42 (2.4%) of the puerperal women had no ultrasound changes. She was diagnosed with endometritis on the 19th day of the postpartum period. In 4/42 (9.5%) women, in addition to subinvolution and hematometra, signs of pathologic adherence of placental villi were found. They underwent relaparotomy, excision of the pathological site, and metroplasty.
All parturient women underwent hysteroscopy. Hysteroscopic findings included distension of the uterine cavity, and signs of hematometra, fibrin deposits, and areas of necrotic decidual tissue. In group A, 4/55 (7.3%) parturient women had pathologic adherence of placental villi. In group B, 2/42 (4.8%) women had residual parts of the placenta (onset of endometritis on 10 and 18 days postpartum), and 4/42 (9.5%) were found to have pathologic adherence of placental villi requiring relaparotomy and metroplasty.
Microbiological characteristics of samples obtained from the uterine cavity of the parturient women are presented in Figure 1.
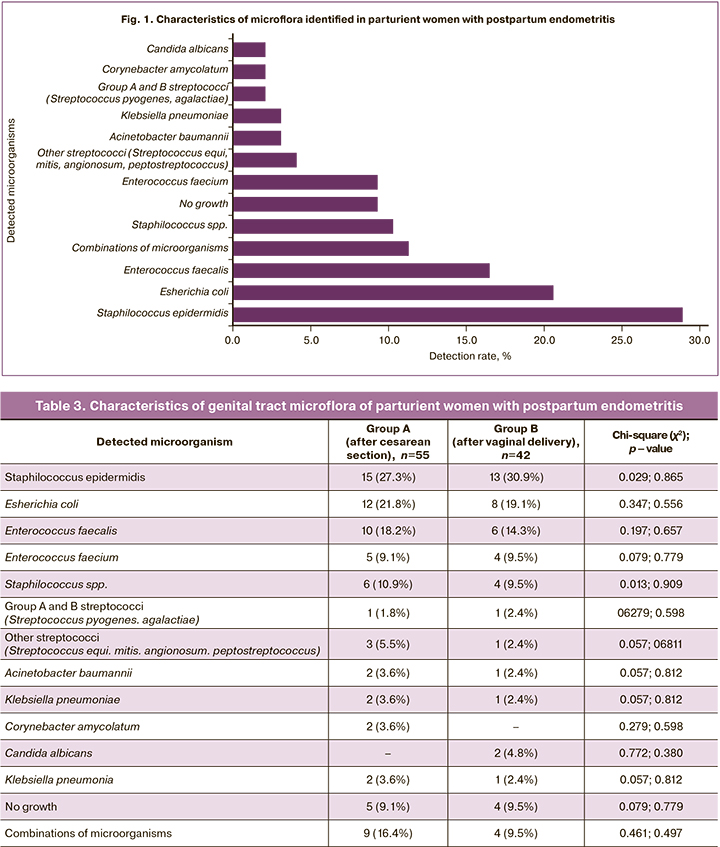
Detection rates of microorganisms in the study groups are shown in Table 3.
All puerperal women were administered etiologic treatment with broad-spectrum antibacterial drugs in consultation with a clinical pharmacologist. The choice of the drug combination was determined by the disease severity and the spectrum of pathogens. The therapy included fluid resuscitation, detoxification, venous thromboembolism prevention, local treatment with intrauterine irrigation with a cooled antiseptic solution, and low-frequency ultrasound therapy of uterine cavity with an antiseptic solution, intrauterine carbon sorbent injection with a sorption effect on microorganisms and their toxins [12].
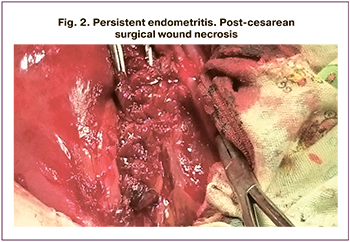 In group A, a second surgery was performed in 31/55 (56.4%) postpartum women; 15/55 (27.3%) of them underwent metroplasty and excision of the inflamed uterine scar (Fig. 2) with adjacent myometrium down to healthy tissue and re-suturing of the uterus with singlerow separate sutures. Postpartum radical hysterectomy was performed in 15/55 (27.3%) patients. One patient had relaparotomy with metroplasty for endometritis with pathologic adherence of placental villi, but three days later underwent a hysterectomy without appendages due to persistent endometritis and lack of treatment effect. Indications for hysterectomy were pelvic peritonitis [2/16 (12.5%)], diffuse peritonitis [5/16 (31.3%)], persistent endometritis, parametritis [5/16 (31.3%)], endometritis with multiple organ failure and preeclampsia [2/16 (12.5%)], and endometritis co-occurring with severe community-acquired pneumonia [1/16 (6.3%)].
In group A, a second surgery was performed in 31/55 (56.4%) postpartum women; 15/55 (27.3%) of them underwent metroplasty and excision of the inflamed uterine scar (Fig. 2) with adjacent myometrium down to healthy tissue and re-suturing of the uterus with singlerow separate sutures. Postpartum radical hysterectomy was performed in 15/55 (27.3%) patients. One patient had relaparotomy with metroplasty for endometritis with pathologic adherence of placental villi, but three days later underwent a hysterectomy without appendages due to persistent endometritis and lack of treatment effect. Indications for hysterectomy were pelvic peritonitis [2/16 (12.5%)], diffuse peritonitis [5/16 (31.3%)], persistent endometritis, parametritis [5/16 (31.3%)], endometritis with multiple organ failure and preeclampsia [2/16 (12.5%)], and endometritis co-occurring with severe community-acquired pneumonia [1/16 (6.3%)].
Most women in group B were treated conservatively [36/42 (85.7%)]. Six of 42 (14.3%) patients underwent surgery, including hysterectomy [3/42 (7.1%)] (two patients due to persistent endometritis and sepsis and one due to the panmetritis with concomitant pathologic adherence of placental villi). Three of 42 (7.1%) women underwent metroplasty for endometritis with concomitant pathologic adherence of placental villi.
In groups A and B, hysterectomy was performed in 16/55 (29.1%) and 3/42 (7.1%) patients, respectively; χ2=5.956; p=0.015. The odds ratio (OR) for post-cesarean hysterectomy was 5.33 (95% CI: 1.43–19.78) compared to vaginal delivery.
Discussion
The literature discusses various possible factors predisposing to postpartum endometritis, including maternal age, first birth, smoking during pregnancy, overweight, which are controversial and can only indirectly increase the risks of postpartum infection [4, 7]. The average age of the patients in our study was 27.7 (5.7) years. Most of the women were not overweight. Most of them were multiparous (64.9%).
Patients in group A were statistically significantly more likely to have gynecological diseases (p=0.056), and almost half of them had a history of infertility. These diseases are associated with infectious factors, which, combined with operative delivery, increase the risk of postpartum infection [4]. However, statistically significant differences in the detected gynecological pathology in our study can be regarded as another reason that determined the indication for operative delivery.
Among patients with post-cesarean endometritis, 21.8% of women underwent second and one the third surgery. The first cesarean section usually predisposes to a second operation and increases the risk of endometritis and perioperative complications [6, 13, 14].
Chronic diseases weaken the body's defense mechanisms and promote the activation of infectious factors. More than half of patients in both groups had anemia, which is a significant factor for the development of postpartum endometritis. And additional postoperative blood weakens the body's defense mechanisms, increasing the risk of infectious complications [14].
Respiratory viral infection during pregnancy increases the likelihood of infectious agent persistence, infection of the amniotic fluid, and, as a consequence, postpartum endometritis [15].
Patients in the study group were significantly more likely to have preeclampsia (p=0.003), an additional risk factor for postpartum endometritis [4]. Symptoms of threatened termination of pregnancy were observed in almost half of the subjects (47.4%). An infectious factor is one of the leading causes of threatened miscarriage, which increases the risk of postpartum septic complications and placental disorders [16].
38.1% of the study participants were hospitalized during pregnancy. Each hospitalization increases the risk of nosocomial infection and contributes to antibiotic resistance. Currently, the reduction of postpartum length of hospital stay after cesarean delivery is considered an essential strategy for puerperal women [3, 17]. More than half of the study participants had an indication of prenatal hospitalization. Prolonged antenatal hospitalization may increase the risk of endometritis and postoperative infectious complications [17].
The main indication for elective cesarean delivery in patients of group A was a uterine scar after cesarean delivery (85.7%). A global trend is an increase in the cesarean delivery rates, which implies repeat operative delivery increasing the risk of postpartum infectious complications [8, 18]. Repeat cesarean section can be a risk factor for complications associated with technical issues, increased operative time, more significant blood loss, and postoperative complications [18, 19]. Zejnullahu V.A. (2019) reported that women with a history of a previous cesarean section were 7.4 times more likely to develop a surgical wound infection than those with no past cesarean section [18].
Our study participants were three times more likely to undergo emergency surgery. Many authors note an increase in the number of postpartum endometritis when performing a cesarean section for emergency indications, especially after the onset of labor [8, 9]. Although cesarean delivery can, in some cases, reduce maternal and perinatal mortality, the indications for the operation must be evaluated very cautiously. In this connection, the World Health Organization (WHO) recommends reducing cesarean section rates to 10–15%, taking into account the characteristics of the health care facility and local clinical guidelines [3, 20].
The onset of the first endometritis symptoms depends on many factors, including the presence of intrauterine infection, the specificity of etiotropic microbes, the characteristics of pregnancy and childbirth, concomitant pathology, and the physical and psychological state of the woman. In most cases, postpartum endometritis develops in the first week after delivery [4, 19], consistent with our study result. The symptoms of endometritis developed statistically significantly more often on days 2–4 after cesarean section compared with the group after vaginal delivery (p=0.038), which confirms the data on the early development of endometritis symptoms after surgical delivery. This is facilitated by operational stress, blood loss, abdominal cavity infection, and early generalization of infection from the surgical wound [4]. On days 5–8, endometritis symptoms developed more often after vaginal delivery (p=0.003), which was statistically significant.
In our study, only 9.3% of patients had no fever. Other symptoms (pelvic pain, purulent discharge, bleeding) were less common. Symptoms of uterine subinvolution and softening were observed in most patients in the study groups (78.2% and 85.7%, respectively). Many authors have reported fever as the most common symptom of endometritis. Uterine bleeding is a consequence of the soft and subinvoluted uterus and placental tissue remnants [21, 22]. There were no significant differences between the study groups in terms of clinical symptoms, which indicates the uniformity of endometritis clinical manifestation regardless of delivery mode.
Patients after cesarean section were statistically significantly more likely to have leukocytosis, left shift to band neutrophils, anemia, thrombocytosis, hypoproteinemia, and hypercoagulability, a more severe course of the disease aggravated by postoperative trauma, and a higher risk of developing complications. Some authors have established a high diagnostic value of the group of neutrophils for the diagnosis and prognosis of postpartum endometritis [4, 23]. It should be noted that postpartum endometritis may manifest no peripheral blood inflammatory reaction. In our study, a third of the patients in the group after vaginal delivery had no leukocytosis.
There are conflicting data regarding the reliability of ultrasound for endometritis diagnosis, which creates difficulties in interpreting ultrasonography findings [24]. Ultrasound signs of endometritis are hematometra and uterine subinvolution [4]. Ultrasound signs of subinvolution were recorded in 90.9% of women in group A, and 97.6% of patients in group B. Ultrasound assessment of uterine subinvolution is ambiguous. On the one hand, this symptom is more common in multiparous women after giving birth to a large baby and resolves spontaneously without any consequences. On the other hand, subinvolution may be one of the signs of endometritis [25]. The time of the onset of this symptom is of clinical importance. The ultrasound presentation of post-cesarean endometritis may not differ significantly from that after uncomplicated delivery [25]. The ultrasound presentation of endometritis is not always informative. Faure K. et al. (2019) showed that an ultrasound scan could determine a physiological hematometra, the presence of echogenic inclusions or even air bubbles, which disappear in about 50% of cases one week after delivery and in 6% of cases by three weeks after delivery without additional surgical interventions. There is no characteristic ultrasound presentation of postpartum endometritis, but ultrasonography helps to detect retention of parts of the placenta and pathologic adherence of placental villi [4].
Detection rates of microorganisms in the groups were comparable, which indicates a low probability of infection during operative delivery according to established aseptic techniques. In the last 5–7 years, the leading microorganisms detected in puerperal women were Staphilococcus epidermidis, Esherichia coli, Enterococcus faecalis [18, 26–29], which is consistent with our study. According to our data, Staphilococcus epidermidis (28.9%), Esherichia coli (20.6%), enterococci (25.8%) were detected among puerperal women; other staphylococci occurred in 10.3% of patients; group A and B streptococci were detected in 2.1% of the subjects. Microorganisms such as Acinetobacter baumannii, Klebsiella pneumoniae, Corynebacter amycolatum, Candida albicans, Klebsiella pneumonia were detected in 2–4% of puerperal women. In 9.3% of the patients, no microorganism growth was observed; in 13.4% of women, a combination of microbiota was noted.
Several microorganisms were detected less often, but their presence is often accompanied by severe complications. Acinetobacter baumannii (infection associated with death from infectious, toxic shock, and multiple organ failure) [26] was detected in two groups A patients who were treated in the intensive care unit for severe postpartum endometritis. One of them also had bilateral polysegmental community-acquired pneumonia. Subsequently, she underwent a radical hysterectomy for persistent endometritis. Corynebacterium amycolatum causes postoperative wound infection. This microorganism was detected in two group A patients, one of whom underwent reoperation and radical hysterectomy for persistent endometritis and parametritis combined with subaponeurotic hematoma [30].
In our study, 56.4% of patients after cesarean delivery had complicated postpartum septic infection forms requiring reoperation and hysterectomy in half of the cases. After operative delivery, the risk of radical hysterectomy was 5.3-fold higher than after vaginal delivery in the case of postpartum endometritis, which is consistent with many studies investigating complications after cesarean section [13, 15, 18].
Conclusion
Post-cesarean endometritis has an early onset, more severe clinical course, and more pronounced laboratory changes than endometritis after a vaginal delivery; it is associated with complications and a 5-time higher rate of organ-removing surgery.
References
- Bebell L.M., Ngonzi J., Bazira J., Fajardo Y., Boatin A.A., Siedner M.J. et al. Antimicrobial-resistant infections among postpartum women at a Ugandan referral hospital. PloS One. 2017; 12(4): e0175456. https://dx.doi.org/10.1371/journal.pone.0175456.
- Ngonzi J., Tornes Y.F., Mukasa P.K., Salongo W., Kabakyenga J., Sezalio M. et al. Puerperal sepsis, the leading cause of maternal deaths at a Tertiary University Teaching Hospital in Uganda. BMC Pregnancy Childbirth. 2016; 16(1): 207. https://dx.doi.org/10.1186/s12884-016-0986-9.
- Радзинский В.Е. Акушерская агрессия. М.: Редакция журнала StatusPraesens; 2017. 872 с. [Radzinsky V.E. Obstetric aggression. M .: StatusPraesens Editorial Office. 2017. 872 p. (in Russian)].
- Faure K., Dessein R., Vanderstichele S., Subtil D. Postpartum endometritis: CNGOF and SPILF pelvic inflammatory diseases guidelines. Gynecol. Obstet. Fertil. Senol. 2019; 47(5): 442-50. https://dx.doi.org/10.1016/j.gofs.2019.03.013.
- Meaney-Delman D., Bartlett L.A., Gravett M.G., Jamieson D.J. Oral and intramuscular treatment options for early postpartum endometritis in low-resource settings: a systematic review. Obstet. Gynecol. 2015; 125(4): 789-800. https://dx.doi.org/10.1097/AOG.0000000000000732.
- Tuuli M.G., Liu L., Longman R.E., Odibo A.O., Macones G.A., Cahill A.G. Infectious morbidity is higher after second-stage compared with first-stage cesareans. Am. J. Obstet. Gynecol. 2014, 211(4): 410.e1-6. https://dx.doi.org/ 10.1016/j.ajog.2014.03.040.
- Racicot K., Mor G. Risks associated with viral infections during pregnancy. J. Clin. Invest. 2017; 127(5): 1591-9. https://dx.doi.org/10.1172/JCI87490.
- Краснопольский В.И., Логутова Л.С., Буянова С.Н., Чечнева М.А., Ахвледиани К.Н. Результаты оперативной активности в современном акушерстве. Журнал акушерства и женских болезней. 2015; 64(2): 53-8. [Krasnopol’skii V.I., Logutova L.S., Buyanova S.N., Chechneva M.A., Ahvlediani K.N. The results of surgical activity in modern obstetrics. Journal of obstetrics and female diseases. 2015; 64(2): 53-8. (in Russian)].
- Smaill F.M., Grivell R.M. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst. Rev. 2014; (10): CD007482. https://dx.doi.org/10.1002/14651858.CD007482.pub3.
- Манухин И.Б., Гогсадзе И.Г., Гогсадзе Л.Г., Пономарева Ю.Н. Дифференцированная лечебная тактика у пациенток с эндометритом после кесарева сечения. Хирург. 2014; 2: 35-40. [Manuchin I.B. Differential treatment tactics in patients with postcaesarean endometritis. Surgeon. 2014; 2: 35-40. (in Russian)].
- Доброхотова Ю.Э., Михалева Л.М., Насырова Н.И., Апонович И.А., Залесская С.А. Состояние репродуктивной системы пациенток, перенесших реконструктивно-пластические операции на матке. Акушерство и гинекология. 2017; 8: 42-8. [Dobrokhotova Yu.E., Mikhaleva L.M., Nasyrova N.I., Aponovich I.A., Zalesskaya S.A. The reproductive system of patients who have undergone reconstructive plastic surgery of the uterus. Obstetrics and Gynecology. 2017; 8: 42-8. (in Russian)]. https://dx.doi.org/10.18565/aig.2017.8.42-8.
- Баринов С.В., Блауман Е.С., Лазарева О.В., Долгих В.Т., Попова Л.Д., Новиков Д.Г., Пьянова Л.Г. Новый метод лечения больных с послеродовым эндометритом. Российский вестник акушера-гинеколога. 2019; 19(4): 65-71. [Barinov S.V., Blauman E.S., Lazareva O.V., Dolgih V.T., Popova L.D., Novikov D.G., Pyanova L.G. A new method of treating patients with postpartum endometritis. Russian Bullet of the Obstetrican-Gynecologist, 2019; 19(4): 65-71. (in Russian)].
- Tuuli M.G., Liu J., Stout M.J., Martin S., Cahill A.G., Odibo A.O. et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N. Engl. J. Med. 2016; 374(7): 647-55. https://dx.doi.org10.1056/NEJMoa1511048.
- Axelsson D., Brynhildsen J., Blomberg M. Postpartum infection in relation to maternal characteristics, obstetric interventions and complications. J. Perinat. Med. 2018; 46(3): 271-8. https://dx.doi.org/10.1515/jpm-2016-0389.
- Иванников Н.Ю., Митичкин А.Е., Димитрова В.И., Слюсарева О.А., Хлынова С.А., Доброхотова Ю.Э. Современные подходы в лечении послеродовых гнойно-септических заболеваний. Медицинский совет. 2019; 7: 58-69. [Ivannikov N. Y., Mitichkin A. E., Dimitrova V. I., Slyusareva O. A., Khlynova S. A., Dobrokhotova Yu. E. Modern approaches in the treatment of postpartum purulent-septic diseases. Medical Council. 2019; 7: 58-69. (in Russian)]. https://dx.doi.org/10.21518/2079-701X.
- Woodd S.L., Montoya A., Barreix M., Pi L., Calvert C., Rehman A.M. et al. Incidence of maternal peripartum infection: a systematic review and meta-analysis. PLoS Med. 2019; 16(12): e1002984. https://dx.doi.org/10.1371/journal.pmed.1002984.
- Blumenfeld Y.J., El-Sayed Y.Y., Lyell D.J., Nekson L.M., Butwick A.J. Risk factors for prolonged postpartum length of stay following cesarean delivery. Am. J. Perinatol. 2015; 32(9): 825-32. https://dx.doi.org/10.1055/s-0034-1543953.
- Zejnullahu V.A., Isjanovska R., Sejfija Z., Zejnullahu V.A. Surgical site infections after cesarean sections at the University Clinical Center of Kosovo: rates, microbiological profile and risk factors. BMC Infect. Dis. 2019; 19(1): 752. https://dx.doi.org/10.1055/s-0034-1543953.
- Callaghan W.M., Creanga A.A, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet. Gynecol. 2012; 120(5): 1029-36. https://dx.doi.org/10.1097/aog.0b013e31826d60c5.
- Betran A.P., Torloni M.R., Zhang J.J., Gulmezoglu A.M. WHO Working Group on Caesarean Section. WHO statement on caesarean section rates. BJOG. 2016; 123(5): 667-70. https://dx.doi.org/10.1111/1471-0528.13526.
- Kawakita T., Landy H.J. Surgical site infections after cesarean delivery: epidemiology, prevention and treatment. Matern. Health Neonatol. Perinatol. 2017; 3: 12. https://dx.doi.org/10.1186/s40748-017-0051-3.
- Akladios C.Y., Sananes N., Gaudineau A., Boudier E., Langer B. Secondary postpartum hemorrhage. J. Gynecol. Obstet. Biol. Reprod. (Paris). 2014; 43(10): 1161-9. https://dx.doi.org/10.1016/j.jgyn.2014.10.008.
- Agarkov N.M., Golovchenko O.V., Blinkov Y.A., Kulabukhov A.S., Yakovlev S.A., Budnik I.V. et al. Diagnosis of acute endometritis on hematologic indicators, and given discriminant models. Klin. Lab. Diagn. 2018; 63(6): 361-4. https://dx.doi.org/10.18821/0869-2084-2018-63-6-361-364.
- Братчикова О.А., Чехонацкая М.Л., Яннаева Н.Е. Ультразвуковая диагностика послеродового эндометрита. Саратовский научно-медицинский журнал. 2014; 10(1): 65-9. [Bratchikova O.A., Chekhonatskaya M.L., Yannaeva N.E. Ultrasound diagnostics of postpartum endometritis (review). SaratovJournal of Medical Scientific Research. 2014;10(1):65-9. (in Russian)].
- Laifer-Narin S.L., Kwak E., Kim H., Hecht E.M., Newhouse J.H. Multimodality imaging of the postpartum or posttermination uterus: evaluation using ultrasound, computed tomography, and magnetic resonance imaging. Curr. Probl. Diagn. Radiol. 2014; 43(6): 374-85. https://dx.doi.org/10.1067/j.cpradiol.2014.06.001.
- Самойлова Т.Е., Кохно Н.И., Докудаева Ш.А. Микробные ассоциации при послеродовом эндометрите. РМЖ. Медицинское обозрение. 2018; 1: 6-13. [Samoylova T.E., Kohno N.I., Dokuchaeva S.A. Microbial association with postpartum endometritis. RMG. Medical review. 2018; 1: 6-13. (in Russian)].
- Ahmed S., Kawaguchiya M., Ghosh S., Paul S.K., Urushibara N., Mahmud C. et al. Drug resistance and molecular epidemiology of aerobic bacteria isolated from puerperal infections in Bangladesh. Microb. Drug Resist. 2015; 21(3): 297-306. https://dx.doi.org/10.1089/mdr.2014.0219.
- Faiz S.A. Bacteriology of gynaecological surgical site infection in a Medical University Hospital. JSOGP. 2017; 7(3): 2017.
- Sáez-López E., Guiral E., Fernández-Orth D., Villanueva S., Gonce A., Lopez M. et al. Vaginal versus obstetric infection escherichia coli isolates among pregnant women: antimicrobial resistance and genetic virulence profile. PLoS One. 2016; 11(1): e0146531. https://dx.doi.org/10.1371/journal.pone.0146531.
- Rizvi M., Rizvi M.W., Shaheen, Sultan A., Khan F., Shukla I. et al. Emergence of coryneform bacteria as pathogens in nosocomial surgical site infections in a tertiary care hospital of North India. J. Infect. Public Health. 2013; 6(4): 283-8. https://dx.doi.org/10.1016/j.jiph.2013.01.005.
Received 08.07.2020
Accepted 14.10.2020
About the Authors
Sergey V. Barinov, Dr.Med.Sci., Professor, Head of the Department of Obstetrics and Gynecology №2, Omsk State Medical University. Tel.: +7(3812)24-06-58.E-mail: barinov_omsk@mail.ru. ORCID: 0000-0002-0357-7097. 644043, Russia, Omsk, Lenina str., 12.
Oksana V. Lazareva, Ph.D., Associate Professor, Department of Obstetrics and Gynecology №2, Omsk State Medical University. Tel.: +7(904)584-66-99.
E-mail: lazow@mail.ru. ORCID: 0000-0002-0895-4066. 644043, Russia, Omsk, Lenina str., 12.
Lyudmila L. Shkabarnya, Head of the Department of Gynecology, Perinatal Centre of Omsk Regional Clinical Hospital. Tel.: +7(3812)25-15-78. E-mail: l_shka@mail.ru. ORCID: 0000-0001-6080-1828. 644011, Russia, Omsk, Beresovaya str., 3.
Vladimir E. Savculich, Physician at the Department of Gynecology, Perinatal Centre of Omsk Regional Clinical Hospital. Tel.: +7(3812)23-22-37. E-mail: erutar_sava@mail.ru. ORCID: 0000-0002-1191-7645. 644011, Russia, Omsk, Beresovaya str., 3.
Anastasia S. Sycheva, Resident at the Department of Obstetrics and Gynecology №2, Omsk State Medical University. Tel.: +7(904)524-06-58. E-mail: defenceless123@mail.ru.
ORCID: 0000-0002-6453-7216. 644043, Russia, Omsk, Lenina str., 12.
Yuliya I. Tirskaya, Dr.Med.Sci., Associate Professor, Department of Obstetrics and Gynecology №2, Omsk State Medical University. Tel.: +7(3812)24-06-58.
E-mail: yulia.tirskaya@yandex.ru. ORCID: 0000-0001-5365-7119. 644043, Russia, Omsk, Lenina str., 12.
Yegor A. Khoroshkin, Medical Student, Omsk State Medical University. Tel.: +7(960)985-51-25. E-mail: drrussian@mail.ru. ORCID: 0000-0001-5365-7119.
644043, Russia, Omsk, Lenina str., 12.
Tat’yana V. Kadtsyna, Ph.D., Associate Professor, Department of Obstetrics and Gynecology №2, Omsk State Medical University. Tel.: +7(3812)24-06-58.
E-mail: tatianavlad@list.ru. ORCID: 0000-0002-0348-5985. 644043, Russia, Omsk, Lenina str., 12.
Irina V. Medyannikova, Dr.Med.Sci., Associate Professor, Department of Obstetrics and Gynecology №2, Omsk State Medical University. Tel.: +7(3812)24-06-58.
E-mail: mediren@gmail.com. ORCID: 0000-0002-0348-5985. 644043, Russia, Omsk, Lenina str., 12.
Yurij I. Chulovsky, Ph.D., Associate Professor, Department of Obstetrics and Gynecology №2, Omsk State Medical University. Tel.: +7(3812)24-06-58.
E-mail: akusheromsk@rambler.ru. 644043, Russia, Omsk, Lenina str., 12.
Galina B. Beznoshchenko, Dr.Med.Sci., Professor, Department of Obstetrics and Gynecology №2, Omsk State Medical University. Tel.: +7(3812)24-06-58.
E-mail: akusheromsk@rambler.ru. 644043, Russia, Omsk, Lenina str., 12.
For citation: Barinov S.V., Lazareva O.V., Shkabarnya L.L., Savkulich V.E., Sycheva A.S., Tirskaya Yu.I., Khoroshkin E.A., Kadtsyna T.V., Medyannikova I.V., Chulovsky Yu.I., Beznoshchenko G.B. Clinical and diagnostic criteria for postpartum endometritis according to delivery mode.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2020; 12: 108-116 (in Russian)
https://dx.doi.org/10.18565/aig.2020.12.108-116



