Changes in the composition of intestinal microbiota and their association with cortisol, melatonin, tumor necrosis factor-α, and interleukin-17 levels in women with idiopathic recurrent miscarriage
Gumenyuk L.N., Gavrilov M.V., Puchkina G.A., Asanova F.S., Medzhitova D.R., Bordyugov M.D., Tishchenko V.A., Rudeva K.A.
Objective: This study aimed to evaluate changes in the taxonomic composition of the intestinal microbiota and examine their relationship with serum levels of cortisol, melatonin, tumor necrosis factor-α (TNF-α), and interleukin-17 (IL-17) in women with idiopathic recurrent miscarriage (IRM).
Materials and methods: This study included 55 women with IRM and 60 women with healthy pregnancies. The taxonomic composition of the gut microbiota and the serum concentrations of cortisol, melatonin, TNF-α, and IL-17 were analyzed.
Results: The study found that women with IRM exhibited changes in the taxonomic composition of intestinal microbiota, characterized by a significant decrease in α-diversity of the bacterial community (Chao1 index p=0.014) and a decrease in the abundance of Bifidobacterium (p<0.001), Lachnospira (p=0.032), Roseburia (p=0.003), and Coprococcus (p=0.012). Additionally, there was an increase in the abundance of Ruminococcus (p<0.001) and Klebsiella (p=0.002). Significant correlations were observed between cortisol levels and the presence of Lachnospira bacteria (r=-0.51; p=0.002) and between melatonin and the presence of Coprococcus bacteria (r=-0.49; p=0.012). Correlations were also identified between TNF-α and IL-17 concentrations and the Chao1 index (r=-0.51, p=0.002; r=-0.54, p=0.001, respectively), TNF-α and the abundance of Ruminococcus bacteria (r=0.51; p=0.002), and IL-17 and the abundance of Bifidobacterium (r=-0.52; p=0.001).
Conclusion: Potential modifications to the gut microbiota may have preventive and therapeutic implications in women with IRM.
Authors' contributions: Gumenyuk L.N. – idea and design of the study, drafting of the manuscript; Gavrilov M.V., Puchkina G.A. – statistical analysis; Asanova F.S., Rudeva K.A. – analysis and interpretation of the obtained data; Medzhitova D.R., Bordyugov M.D. – data collection; Tishchenko V.A. – participation in the research design.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: There was no funding for this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the S.I. Georgievsky Medical Institute, V.I. Vernadsky Crimean Federal University.
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Gumenyuk L.N., Gavrilov M.V., Puchkina G.A., Asanova F.S., Medzhitova D.R., Bordyugov M.D., Tishchenko V.A., Rudeva K.A. Changes in the composition of intestinal microbiota and their association with
cortisol, melatonin, tumor necrosis factor-α, and interleukin-17 levels in
women with idiopathic recurrent miscarriage.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2024; (6): 37-45 (in Russian)
https://dx.doi.org/10.18565/aig.2024.34
Keywords
Recurrent miscarriage (RM) is a heterogeneous condition defined as two or more clinical pregnancy losses before 22 weeks of gestation, occurring in 2–5% of pregnant women. After two previous miscarriages, the risk of miscarriage more than doubles, reaching 36–38% [1].
The etiological factors of RM include chromosomal abnormalities (2–6%), anatomical changes in the uterus (10–15%), infectious and inflammatory conditions (2–6%), endocrine disorders (17–20%), autoimmune diseases (20%), and thrombophilic conditions (10%) [2]. However, approximately 50% of RM cases are unexplained (idiopathic). Idiopathic RM (IRM) remains a pressing issue in reproductive medicine.
Current research suggests that aberrant expression of proinflammatory cytokines, leading to an impaired immune response, is a critical factor in the pathogenesis of IRM [3]. In this context, tumor necrosis factor alpha (TNF-α) and interleukin (IL)-17, which have embryotoxic and antitrophoblastic activities, are of particular interest [4]. There is evidence that women with IRM typically have significantly higher levels of TNF-α in their peripheral blood (by 40–70% compared to controls) [5], including the initiation of apoptosis and inhibition of trophoblast invasion, activation of thrombokinase, a decrease in the expression of nucleotide-binding domain oligomerization, and increased cytotoxicity of dNK cells, which, in turn, leads to insufficient remodeling of the spiral artery, thrombosis, trophoblast infarction and detachment [6], dysfunction of decidual cells, immunological rejection of the fetus, and, as a consequence, miscarriage. Most researchers believe that serum IL-17 levels are elevated in women with IRM [4] and serve as independent prognostic factors for IRM. The increased level of IL-17 inversely correlates with the content of Treg cells in the peripheral blood and decidua, a decrease in the level of which potentiates the processes of embryo rejection and is independently associated with activation of the expression of the nuclear transcription factor NF-kB, a reduction in the expression level of progesterone receptors, and a weakening of its functional activity. This leads to decidua dysplasia, insufficient nutritional support of the embryo, stimulation of myometrial contractility, and ultimately miscarriage [7].
At the same time, the immune system is closely associated with the neuroendocrine system. Dysfunction of the epiphyseal-pituitary-adrenal axis has been implicated in increasing the risk of IRM, with a particular emphasis on changes in cortisol and melatonin secretion [8]. Analysis of hormonal status in women with IRM revealed excessive cortisol and insufficient melatonin levels [9]. In addition, changes in cortisol levels are associated with important etiological factors of IRM, such as decreased fibrinolytic activity of the vascular wall, disruption of full trophoblast invasion and function, induction of apoptosis, and inhibition of progesterone secretion. Conversely, a decrease in melatonin levels is associated with the immunological rejection of trophoblasts (due to inhibited progesterone secretion) and stimulation of myometrial contractility (by increasing prostaglandin synthesis) [10].
Several studies have demonstrated the role of gut microbiota in the pathophysiology of IRM due to its key role in forming and modulating neuro-immune-endocrine reactions. Convincing data show qualitative changes in the microbial landscape of women with IRM [11]. Generally, these women show a trend toward decreased bacterial diversity and reduced beneficial commensals, accompanied by an increase in pathobionts [11–14]. However, information regarding specific microbial composition is fragmentary and contradictory. Moreover, there are only isolated reports on the association between the gut microbiota and inflammatory biochemical markers in women with IRM. No studies have assessed the relationship between gut microbiota members and cortisol and melatonin concentrations in IRM.
Therefore, the relationship between gut microbiota and IRM remains unclear.
This study aimed to evaluate changes in the taxonomic composition of the gut microbiota and examine their relationship with the serum levels of cortisol, melatonin, TNF-α, and IL-17 in women with IRM.
Materials and methods
This study was conducted at the Gynecological Department of the Perinatal Center of the Semashko Republican Clinical Hospital (Simferopol). The prospective comparative study included 55 women with primary IRM (mean age: 31.6 [26.9; 33.9] years) who formed the study group, and 60 women with healthy pregnancy (HP) (mean age, 30.3 [25.9; 33.2] years) who were self-referred for abortion (control group).
The inclusion criteria for study group were confirmed diagnosis of IRM, age of women up to 35 years, normal karyotype of the couple, and informed consent to participate in the study.
The study group did not include underweight or overweight women; patients with genetic and anatomic causes of RM; chronic infectious, inflammatory, endocrine, autoimmune, thrombophilic, oncologic diseases; chronic pathology of the digestive and hepatobiliary system; irritable bowel syndrome; bacterial, viral, and fungal infectious diseases; smokers; and patients taking medications affecting the stool; mental pathology; smokers; with changes in stool (diarrhea/constipation) and taking medications that affect stool for 30 days; vaccinated within 60 days before inclusion in the study; and taking antibiotics, antiviral, probiotic, and prebiotic medications within 90 days before inclusion in the study.
The control group included women under 35 years of age; pregnancy up to 22 weeks (confirmed by ultrasound); physiologically occurring pregnancy; uncomplicated gynecological and obstetric history; history of at least one successful pregnancy; no chronic extragenital pathology and no history of allergic reactions; no history of mental pathology; the frequency of respiratory infections not more than three times a year; without infectious and acute diseases, changes in stool (diarrhea/constipation), and not taking drugs that affect the stool within 60 days before inclusion in the study; not taking antibiotics, antiviral, probiotic, or prebiotic drugs within 90 days before inclusion in the study; and agreed to participate in the study.
The control group did not include women with preconceptional underweight or obesity, pregnancy induced by assisted reproductive technologies, high risk of miscarriage, elevated body temperature above 36.9°C; and refusal to participate in the study.
The diagnosis of RM was established according to the criteria of the European Society of Human Reproduction and Embryology (ESHRE) [15].
The taxonomic composition of the gut microbiota was analyzed in all women with IRM and control group. Stool samples were collected from all participants according to the study protocol. Samples were transported and stored at -80°C until metagenomic analysis. Total DNA was isolated from the stool samples of study participants using the phenol extraction method. The nucleotide sequence of the isolated DNA was determined using the high-performance sequencer SOLiD5500 Wildfire, Applied Biosystems (USA), using the shotgun sequencing method [16].
Read quality filtering and taxonomic classification were assessed using the QIIME software version 1.9.1 [17]. The taxonomic identity of the reads was determined as follows: first, reference values of bacterial operational taxonomic units (OTUs) were selected based on a comparison of the 16S rRNA gene reads obtained with the GreenGenes database (version 13.5) [18], and the taxonomic identity of these OTUs was then determined according to the HITdb human gut microbiota database using the RDP protocol [19].
The qualitative and quantitative composition of the gut microbiota was analyzed by identifying the species, genera, and phyla of microorganisms. To evaluate the α-diversity of the community, the Chao1 index was calculated; to calculate the indicator of the number of taxa recorded (Sobs) and the indicator reflecting the actual number of taxa (ACE), the program Mothur (version 1.22.0) (http://www. mothur. org) were used.
Blood serum was used to assess the concentrations of cortisol, melatonin, TNF-α, and IL-17 by enzyme-linked immunosorbent assay using standard kits from JSC Vector-Best (Russia) and Immuno Biological Laboratories (Germany), according to the manufacturer's instructions.
Statistical analysis
Statistical analysis was performed using STATISTICA 8.0 (StatSoft Inc., USA). Continuous variables showing a normal distribution were expressed as mean (M) and standard deviation (SD); otherwise, they were reported as median (Me) and interquartile interval (Q1; Q3). Categorical variables were expressed as counts and percentages. Continuous variables between the groups were compared using the parametric Student's t-test and Mann–Whitney U test. Categorical variables were compared using the χ2 (chi-square) test. The direction and strength of the relationships between the variables were assessed using Spearman's rank correlation coefficient. Differences were considered statistically significant at p<0.05.
Results
The characteristics of the women with IRM and control group are shown in Table 1. The groups were similar in terms of age (p=0.122) and body mass index (p=0.087).
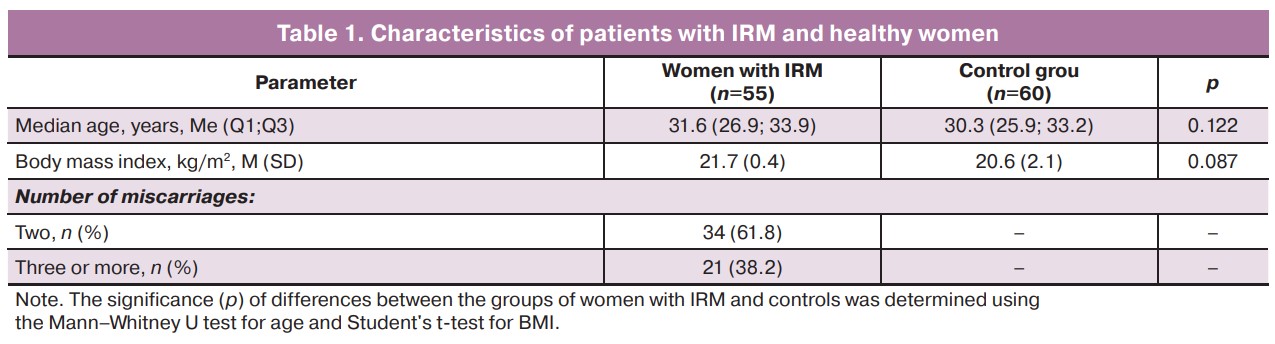
Analysis of the taxonomic composition of the gut microbiota revealed a statistically significant reduction in the α-diversity of the bacterial community (Chao1 index, p=0.014) and a trend towards a decrease in the ACE and Sobs indices (p=0.053 and p=0.051, respectively) in the group of women with IRM compared with women in the control group (Fig. 1).
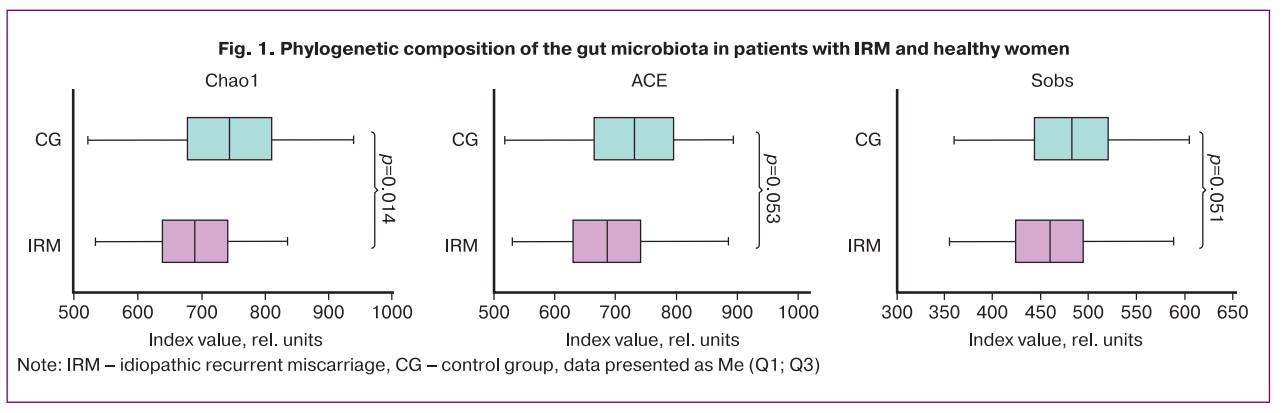
Compared to patients in control group, changes in the generic composition of the gut microbiota in the group of women with IRM were characterized by a statistically significant decrease in the abundance of Bifidobacterium (p<0.001), Lachnospira (p=0.032), Roseburia (p=0.003), Coprococcus (p=0.012) and an increase abundance of Ruminococcus (p<0.001) and Klebsiella (p=0.002) (Fig. 2).
Women with IRM had significantly higher serum levels of cortisol, TNF-α, and IL-17, and lower melatonin levels than women in the control group (Table 2).
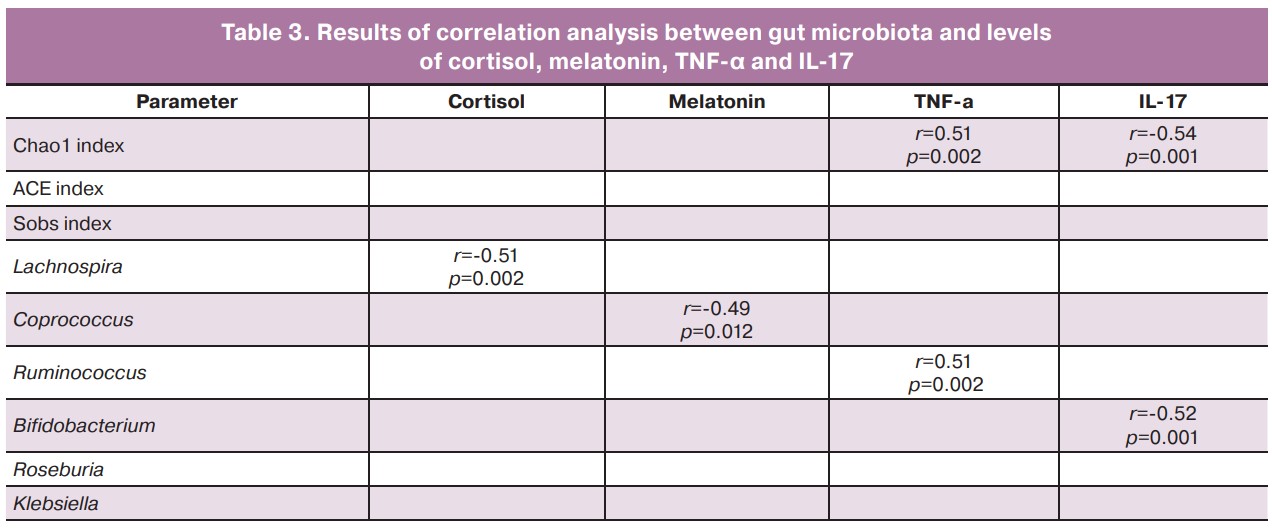
Statistically significant correlations were observed between cortisol levels and the abundance of Lachnospira bacteria (r=-0.51; p=0.002) and between melatonin levels and the presence of Coprococcus bacteria (r=-0.49; p=0.012). Relationships between the concentrations of TNF-α and IL-17 and the Chao1 index were revealed (r=-0.51, p=0.002; r=-0.54, p=0.001, respectively). The concentration of TNF-α was positively correlated with the abundance of Ruminococcus bacteria (r=0.51, p=0.002), and the concentration of IL-17 was inversely correlated with the abundance of Bifidobacterium bacteria (r=-0.52, p=0.001) (Table 3).
Discussion
This study specifies changes in the taxonomic composition of the gut microbiota and evaluates their relationship with the serum concentrations of cortisol, melatonin, TNF-α, and IL-17 in women with IRM.
Previous studies have demonstrated changes in the composition of the gut microbiota in women with IRM [11–14]. We also found that the composition of the gut microbial community in women with IRM was fundamentally different from that in women with physiological pregnancy. In particular, a reduction in bacterial α-diversity was observed in women with IRM compared to that in women with physiological pregnancy, which was confirmed by a statistically lower Chao1 index. Similar results were reported by Liu Y. et al. [13] and Guang Y. et al. Women with IRM also have a reduced abundance of bacteria with immunomodulatory potential – representatives of the genera Bifidobacterium, Lachnospira, Roseburia, Coprococcus, and Prevotella, which are known to produce short-chain fatty acids (SCFA), especially butyrate and propionate. A decrease in the levels of the latter is accompanied by an increase in histone deacetylase and blockade of the receptors coupled to the G proteins GPR41, GPR43, and GPR109A, and consequently, the development of chronic inflammation [21]. In contrast, we found an increased presence of potential pathobionts, namely bacteria of the genera Ruminococcus and Klebsiella. Our results are in partial agreement with those of other studies. Cui Y. et al. [12] reported a decrease in the presence of Prevotella, Roseburia, and Lachnospira bacteria, and an increase in the presence of Ruminococcus and Klebsiella bacteria was typical for women with IRM. In a study by Liu Y. et al. [13], a decrease in the abundance of Lachnospira, Roseburia, and Prevotella, and Jin M. et al. [11] showed that a decrease in the presence of Prevotella was typical for women with IRM. The inconsistency of the data presented may be largely due to the fact that the study was conducted in different geographical areas and there were differences in the methodology for including patients in the study. We did not include women with complicated gynecological history and concurrent extragenital pathology, as well as those who took probiotics, prebiotics, and symbiotic agents in the 3 months prior to the study, to exclude their influence on the study results. It is also important to note the small sample size in previous studies [12, 13].
As already indicated, women with IRM have increased blood concentrations of cortisol, TNF-α, IL-17, and a decrease in the concentration of melatonin, the role of which in the pathogenesis of IRM has been confirmed [4, 5, 8]. Our results were comparable to those reported in the literature. In women with IRM, in comparison with women with a healthy pregnancy, significant differences were observed in the plasma concentrations of cortisol, melatonin, TNF-α, and IL-17. It is important that individual members of the gut microbial community in women with IRM be associated with the plasma concentrations of the studied biomarkers. This may indicate the existence of a relationship between the composition and abundance of the gut microbiota and IRM. A negative correlation with plasma cortisol concentration was shown for the genus Lachnospira, indicating a possible significant role of these bacteria in the dysregulation of the functioning of the hypothalamic-pituitary-adrenal axis in IRM. During the analysis of the research data, we found no studies that examined the relationship between gut microbiota and cortisol production in women with IRM. At the same time, Zhang Q. et al. [22] showed that in patients with Cushing's syndrome, Lachnospira was negatively correlated with cortisol expression. A similar pattern was reported in a study by Michels N. et al. [23], where a reduction in the abundance of Lachnospira bacteria was strongly associated with higher cortisol concentrations in healthy children aged 8–16 years. We found a plausible interpretation of this correlation in the literature. Lachnospira are one of the main butyrate-producing bacteria. Because SCFAs can cross the blood-brain barrier, their participation in the regulation of hypothalamic-pituitary activity through a direct effect on the secretory function of pituitary neurons of the medial paraventricular nucleus can be discussed. Evidence that SCFAs can influence cortisol expression has been reported in experimental and clinical studies. Thus, an experimental study by van de Wouw M. et al. [24] showed that the 7-day use of functional doses of SCFAs by direct administration into the colon increased their concentration in the blood and reduced the intensity of the cortisol response to psychosocial stress in healthy individuals.
A statistically significant correlation was found between melatonin levels and the presence of Coprococcus bacteria. It can be assumed that one of the reasons for the correlation we established is the ability of the latter to block signals in the p-CREB-binding protein complex arylalkylamine-N-acetyltransferase due to the inhibition of tryptophan, which is a precursor of serotonin, from which melatonin is subsequently produced. The studies by domestic and foreign authors describe similar close associations of Coprococcus abundance with the concentrations of tryptophan metabolites and serum melatonin in patients with juvenile idiopathic arthritis and type 2 diabetes mellitus [25, 26].
When discussing our results regarding the relationship between gut microbiota and blood levels of TNF-α and IL-17 in a group of women with IRM, it is important to note that only some of them are consistent with the literature data. The relationship between the gut microbiota and proinflammatory cytokines was previously investigated by Liu Y. et al. [13].
In patients with IRM, a decrease in bacterial diversity was associated with an increase in the blood concentrations of TNF-α and IL-17 [13]. Our data do not contradict those of the aforementioned studies. This finding may indicate that, in patients with IRM, the proinflammatory effects of the microbiome are likely caused by holistic gut dysbiosis. In addition, we found a direct connection between the level of TNF-α in the blood and the presence of Ruminococcus. Ruminococcus bacteria synthesize lipopolysaccharide glucoramnan, which, by activating TLR4-mediated reactions, initiates the production of pro-inflammatory cytokines, including TNF-α, by dendritic cells of the bone marrow [27]. To date, a sufficient pool of studies has accumulated indicating that Bifidobacterium is capable of influencing severity of inflammatory reactions [28–30]. Thus, a decrease in the number of Bifidobacterium is associated with the development of preeclampsia [28], shortened lifespan of the heart allograft, and an increased risk of developing autoimmune conditions [29] and inflammatory bowel diseases [30]. It is known that a decrease in the abundance of bacteria of the genus Bifidobacterium is associated with activation of histone acetylation and suppression of DNA methylation, which, in turn, leads to increased activation of transcription mediated by NF-kB and intensified expression of IL-17. The negative correlation between the blood concentrations of IL-17 and Bifidobacterium abundance found in our study provides further evidence for this association. At the same time, our results do not agree with those of the study by Liu Y. et al. [13], in which a negative relationship was established between the blood concentration of IL-17 and the presence of Prevotella in women with IRM. The inconsistency of our results with the data of the compared study, as mentioned above, is most likely a consequence of differences in the study design, namely, the inclusion of women with IRM without gynecological pathology and metabolic disorders. In contrast, Liu Y. et al. [13] did not exclude the presence of polycystic ovary syndrome and insulin resistance. This might have influenced the differences in the associations of TNF-α and IL-17 with gut microbiota in women with IRM compared to those available in the literature. However, the data presented by Liu Y. et al. [13] and our results suggest a significant role for the gut microbial community in the immunogenesis of IRM. Therefore, the cause-effect relationship between the gut microbiota and the systemic profile of proinflammatory cytokines in IRM requires more in-depth investigation and further research in this direction.
Conclusion
The study results showed that women with IRM have a significant decrease in microbial diversity and changes in the taxonomic composition of the gut microbiota. In addition, statistical results showed significant correlations between specific members of the gut microbial community and hormonal and inflammatory markers, indirectly supporting the notion that the abundance and composition of gut microbiota are associated with IRM. These findings suggest that modifying the gut microbiota may be a preventive and therapeutic approach for women with IRM.
References
- Lund M., Kamper-Jørgensen M., Nielsen H.S., Lidegaard Ø., Andersen A.-M.N., Christiansen O.B. Prognosis for live birth in women with recurrent miscarriage: what is the best measure of success? Obstet. Gynecol. 2012; 119(1): 37-43. https://dx.doi.org/10.1097/AOG.0b013e31823c0413.
- Abu-Heija A. Thrombophilia and recurrent pregnancy loss: Is heparin still the drug of choice? Sultan Qaboos Univ. Med. J. 2014; 14(1): e26-36. https://dx.doi.org/10.12816/0003333.
- Mor G., Aldo P., Alvero A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017; 17(8): 469-82. https://dx.doi.org/10.1038/nri.2017.64.
- Saifi B., Rezaee S.A., Tajik N., Ahmadpour M.E., Ashrafi M., Vakili R. et al. Th17 cells and related cytokines in unexplained recurrent spontaneous miscarriage at the implantation window. Reprod. Biomed. Online. 2014; 29(4): 481-9. https://dx.doi.org/10.1016/j.rbmo.2014.06.008.
- Shaarawy M., Nagui A.R. Enhanced expression of cytokines may play a fundamental role in the mechanisms of immunologically mediated recurrent spontaneous abortion. Acta Obstet. Gynecol. Scand. 1997; 76(3): 205-11. https://dx.doi.org/10.3109/00016349709048142.
- Azizieh F.Y., Raghupathy R.G. Tumor necrosis factor-α and pregnancy complications: a prospective study. Med. Princ. Pract. 2015; 24(2): 165-70. https://dx.doi.org/10.1159/000369363.
- Sha J., Liu F., Zhai J., Liu X., Zhang Q., Zhang B. Alteration of Th17 and Foxp3+ regulatory T cells in patients with unexplained recurrent spontaneous abortion before and after the therapy of hCG combined with immunoglobulin. Exp. Ther. Med. 2017; 14(2): 1114-8. https://dx.doi.org/10.3892/etm.2017.4574.
- McCarthy R., Jungheim E.S., Fay J.C., Bates K., Herzog E.D., England S.K. Riding the rhythm of melatonin through pregnancy to deliver on t.ime. Front. Endocrinol. (Lausanne). 2019; 13(10): 616. https://dx.doi.org/10.3389/fendo.2019.00616.
- Palmer K.T., Bonzini M., Harris E.C., Linaker C., Bonde J.P. Work activities and risk of prematurity, low birth weight and pre-eclampsia: an updated review with meta-analysis. Occup. Environ. Med. 2013; 70(4): 213-2. https://dx.doi.org/10.1136/oemed-2012-101032.
- Sandyk R., Anastasiadis P.G., Anninos P.A., Tsagas N. The pineal gland and spontaneous abortions: Implications for therapy with melatonin and magnetic field. Int. J. Neurosci. 1992; 62(3-4): 243-50. https://dx.doi.org/10.3109/00207459108999775.
- Jin M, Li D., Ji .R., Liu W., Xu X., Feng X. Changes in gut microorganism in patients with positive immune antibody-associated recurrent abortion. Biomed. Res. Int. 2020; 2020: 4673250. https://dx.doi.org/10.1155/2020/4673250.
- Cui Y., Zou L., Ye Q., Li D., Wu H., He L. Gut microbiota composition and functional prediction in recurrent spontaneous abortion. Research Square; 2021. https://dx.doi.org/10.21203/rs.3.rs-906730/v1.
- Liu Y., Chen H., Feng L., Zhang J. Interactions between gut microbiota and metabolites modulate cytokine network imbalances in women with unexplained miscarriage. NPJ Biofilms Microbiomes. 2021; 7(1): 24. https://dx.doi.org/10.1038/s41522-021-00199-3.
- Быкова С.В., Сабельникова Е.А., Парфенов А.И., Гудкова Р.Б., Крумс Л.М., Чикунова Б.З. Репродуктивные расстройства у женщин с целиакией. Влияние этиотропной терапии. Экспериментальная и клиническая гастроэнтерология. 2011; 3: 12-8. [Bykova S.V., Sabelnikova E.A., Parfenov A.I., Gudkova R.B., Krums L.M., Chikunova B.Z. Reproductive disorders in women with celiac disease. Impact of etiotropic therapy. Experimental and Clinical Gastroenterology. 2011; (3): 12-8. (in Russian)].
- Bender Atik R., Christiansen O.B., Elson J., Kolte A.M., Lewis S., Middeldorp S. et al.; ESHRE Guideline Group on RPL. ESHRE guideline: recurrent pregnancy loss.:an update in 2022. Hum. Reprod. Open. 2023; 2023(1): hoad002. https://dx.doi.org/10.1093/hropen/hoad002.
- Mitra S., Förster-Fromme K., Damms-Machado A., Scheurenbrand T., Biskup S., Huson D.H., Bischoff S.C. Analysis of the intestinal microbiota using SOLiD 16S rRNA gene sequencing and SOLiD shotgun sequencing. BMC Genomics. 2013;14(Suppl. 5): S16. https://dx.doi.org/10.1186/1471-2164-14-S5-S16.
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010; 7(5): 335-6. https://dx.doi.org/10.1038/nmeth.f.303.
- DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K. et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006; 72(7):5069-72. https://dx.doi.org/10.1128/AEM.03006-05.
- Ritari J., Salojärvi J., Lahti L., de Vos W.M. Improved taxonomic assignment of human intestinal 16S rRNA sequences by a dedicated reference database. BMC Genomics. 2015; 16: 1056. https://dx.doi.org/10.1186/s12864-015-2265-y.
- Guang Y., Shen X., Tan Y., Tang S., Chen J., Zhang L. et al. Systematic analysis of microbiota in pregnant Chinese women and its association with miscarriage. Ann. Transl. Med. 2022; 10(20): 1099. https://dx.doi.org/10.21037/atm-22-4115.
- Vinolo M.A.R., Rodrigues H.G., Nachbar R.T., Curi R. Regulation of Inflammation by short chain fatty acids. Nutrients. 2011; 3(10): 858-76. https://dx.doi.org/10.3390/nu3100858.
- Zhang Q., Hu W.M., Deng Y.L., Wan J.J., Wang Y.J, Jin P. Dysbiosis of gut microbiota and decreased propionic acid associated with metabolic abnormality in Cushing's syndrome. Front. Endocrinol. (Lausanne). 2023; 13: 1095438. https://dx.doi.org/10.3389/fendo.2022.1095438.
- Michels N., Van de Wiele T., Fouhy F., O'Mahony S., Clarke G., Keane J. Gut microbiome patterns depending on children's psychosocial stress: Reports versus biomarkers. Brain Behav. Immun. 2019; 80: 751-762. https://dx.doi.org/10.1016/j.bbi.2019.05.024.
- van de Wouw M., Boehme M., Lyte J.M., Wiley N., Strain C., O'Sullivan O. et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018; 596(20): 4923-44. https://dx.doi.org/10.1113/JP276431.
- Поросюк М.В., Клементьев Д.Д., Ходов Н.А., Гуменюк Л.Н., Эсатова Э.С., Середа Е.В. и др. Изменения микробиоты кишечника у больных ювенильным идиопатическим артритом. Вестник РГМУ. 2022; 6: 13-9. [Porosyuk M.V., Klementiev D.D., Hodov N.A., Gumenyuk L.N., Esatova E.S., Sereda E.V. et al. Gut microbiota alterations in patients with juvenile idiopathic arthritis. Bulletin of RSMU. 2022; (6): 13-9. (in Russian)]. https://dx.doi.org/10.24075/brsmu.2022.060.
- Huang X., Qiu Y., Gao Y., Zhou R., Hu Q., He Z. et al. Gut microbiota mediate melatonin signalling in association with type 2 diabetes. Diabetologia. 2022; 65(10): 1627-41. https://dx.doi.org/10.1007/s00125-022-05747-w.
- Henke M.T., Kenny D.J., Cassilly C.D., Vlamakis H., Xavier R.J., Clardy J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn's disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA. 2019; 116(26): 12672-7. https://dx.doi.org/10.1073/pnas.1904099116.
- Miao T., Yu Y., Sun J., Ma A., Yu J., Cui M. et al. Decrease in abundance of bacteria of the genus Bifidobacterium in gut microbiota may be related to pre-eclampsia progression in women from East China. Food Nutr. Res. 2021; 65. https://dx.doi.org/10.29219/fnr.v65.5781.
- Vatanen T., Kostic A.D., d'Hennezel E., Siljander H., Franzosa E.A., Yassour M. et al.; DIABIMMUNE Study Group. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016; 165(4): 842-53. https://dx.doi.org/10.1016/j.cell.2016.04.007.
- Henrick B.M., Chew S., Casaburi G., Brown H.K., Frese S.A., Zhou Y. et al. Colonization by B. infantis EVC001 modulates enteric inflammation in exclusively breastfed infants. Pediatr. Res. 2019; 86(6): 749-57. https://dx.doi.org/10.1038/s41390-019-0533-2.
Received 13.02.2024
Accepted 23.05.2024
About the Authors
Lesya N. Gumenyuk, Dr. Med. Sci., Professor at the Department of Psychiatry, Narcology, Psychotherapy with a course of general and medical psychology,S.I. Georgievsky Medical Institute of V.I. Vernadsky Crimean Federal University, 295051, Russia, Republic of Crimea, Simferopol, Lenin Blvd., 5/7, lesya_gymenyuk@mail.ru, https://orcid.org/0000-0003-2785-388
Mikhail V. Gavrilov, Head of Operational Gynecology, GUTA Clinic, 119454, Russia, Moscow, Fadeev str., 4A-1, centr.gyn@yandex.ru, https://orcid.org/0000-0002-3957-2087
Galina A. Puchkina, Associate Professor at the Department of Obstetrics, Gynecology and Perinatology No. 1, S.I. Georgievsky Medical Institute of V.I. Vernadsky Crimean Federal University, 295051, Russia, Republic of Crimea, Simferopol, Lenin Blvd, 5/7, puchkina.g.a@mail.ru, https://orcid.org/0000-0002-8882-8317
Feride S. Asanova, Student at the S.I. Georgievsky Medical Institute of V.I. Vernadsky Crimean Federal University, 295051, Russia, Republic of Crimea, Simferopol, Lenin Blvd., 5/7, feride.asanova.01.01@mail.ru, https://orcid.org/0009-0003-3919-6485
Diana R. Medzhitova, Student at the S.I. Georgievsky Medical Institute of V.I. Vernadsky Crimean Federal University, 295051, Russia, Republic of Crimea, Simferopol,
Lenin Blvd., 5/7, medzhitova9032001@mail.ru, https://orcid.org/0009-0008-8642-3851
Maxim D. Bordyugov, Student at the S.I. Georgievsky Medical Institute of V.I. Vernadsky Crimean Federal University, 295051, Russia, Republic of Crimea, Simferopol,
Lenin Blvd., 5/7, maksjing4@mail.ru, https://orcid.org/0009-0002-4031-8649
Vladislav A. Tishchenko, Student at the S.I. Georgievsky Medical Institute of V.I. Vernadsky Crimean Federal University, 295051, Russia, Republic of Crimea, Simferopol,
Lenin Blvd., 5/7, lacebrra2016@gmail.com, https://orcid.org/0009-0003-3344-0766
Ksenia A. Rudeva, Student at the S.I. Georgievsky Medical Institute of V.I. Vernadsky Crimean Federal University, 295051, Russia, Republic of Crimea, Simferopol, Lenin Blvd., 5/7, rudeva00@mail.ru, https://orcid.org/0009-0001-1857-1157
Corresponding author: Lesya N. Gumenyuk, lesya_gymenyuk@mail.ru



