Changes in the serum concentrations of CCL chemokines (MCP-1, MIP-1α, MIP-1β, RANTES, and EOTAXIN) in women with uterine myoma
Objective. To investigate the serum concentrations of the chemokines MCP-1, MIP-1α, MIP-1β, RANTES and Eotaxin in women with uterine myoma (UM).Konenkov V.I., Koroleva E.G., Orlov N.B., Prokofyev V.F., Shevchenko A.V., Novikov A.M., Dergacheva T.I.
Subjects and methods. Thirty-six Caucasian patients with UM who had undergone surgical treatment as laparoscopic myomectomy were followed up. Serum chemokine concentrations were simultaneously analyzed using the standardized Bio-Plex® multiplex immunoassay methods based on xMAP technology.
Results. In the patients with UM, the increase in the serum concentration of RANTES (p < 0.01) was shown to be accompanied with the decrease in that of chemokines, such as Eotaxin, MIP-1α, and MIP-1β (p < 0.001, р < 0.05, and р < 0.001, respectively). At the same time, the reduced concentrations of the chemokines CCL2 (MCPТ1), CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL11 (Eotaxin) directly correlated with each other.
Conclusion. An analysis of the findings may lead to the conclusion that there is chemokine network dysfunction in UM, which is manifested by differently directional and correlated changes in the serum concentration of CC chemokines in UM.
Keywords
Uterine myoma (UM), or leiomyoma, is a benign monoclonal tumor of the smooth muscle compartment (myometrium) of the uterus. According to some reports, up to 77% of all women will develop UM in their lifetime, and some of them will suffer from substantial symptoms, including pelvic discomfort, dysmenorrhea, menorrhagia, anemia, urinary incontinence, recurrent pregnancy loss, preterm labor, and in some cases infertility [1, 2]. Among factors promoting UM growth, the key role is played by the loss of control over cell migration, proliferation, differentiation, disorganization of the extracellular matrix, and associated inflammatory changes. One of the major contributing factors in these interrelated processes is impairment of local angiogenesis, which is directly influenced by individual and combined actions of growth factors, cytokines, and chemokines. Some of the identified UM-related growth factors are transforming growth factor (TGF-β), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF). Many of these growth factors including EGF, PDGF, and VEGF-A are overexpressed in UM compared to myometrial smooth muscle, although the EGF receptor (EGFR) is expressed at the same levels in both tissues [3].
Many cytokines, including tumor necrosis factor-alpha (TNF-α), erythropoietin, interleukin (IL) -1 and IL-6, are involved in the development of uterine leiomyoma. Compared with myometrial tissue, in leiomyomas expression of eotaxin mRNA, inflammatory macrophage proteins (MIP-lα, MIP-Iβ) and beta-chemokine receptors belonging to the class of integral membrane proteins (CCR3) were found to be changed. In simple leiomyoma, the levels of MIP-1, CCR5, and eotaxin mRNA are statistically significantly lower than in myometrial tissue. The expression level of eotaxin mRNA in myometrial tissue is significantly higher in solitary than in multiple leiomyomas and in simple than proliferating ones. [4]. It has been shown that chemokines and their receptors (MIP-1α, MIP-1β, RANTES, eotaxin, eotaxin-2, IL-8, CCR1, CCR3, CCR5, CXCR1, and CXCR2) can be mediators of the tumor grows in UM [5, 6]. MCP-1 mRNA levels are higher in myometrium compared to leiomyoma tissue, and estrogens and progestins decrease the production of this chemokine protein, which indicates that MCP-1 may have antitumor activity in UM [7]. The increased mast cell content in UM positively correlates with the concentration of RANTES and eotaxin [8].
Chemokines are cytokines with chemoattractant properties (chemotactic cytokines) and a molecular weight of 7 to 12 kDa. Chemokines are characterized by several features, including the presence of four conserved cysteine residues. Their receptors often contribute to rapid and reversible changes in cellular metabolism or migration, unlike other cytokine receptors, which usually cause slower and irreversible processes of proliferation and apoptosis.
Members of the chemokine family include CC chemokines, which have two adjacent cysteines, CXC chemokines, with two cysteines separated by one amino acid, C-chemokines characterized by the presence of two cysteines, and CX3C chemokines with three amino acids between the two cysteines [9]. The most significant representatives of this group of proteins that attract inflammatory cells to the pathological foci are proteins produced by macrophages and other activation cells: MCP-1 - CCL2 (macrophage chemotactic protein-1), MIP-1α - CCL3 and MIP-1β - CCL4 (macrophage inflammatory protein α and β), RANTES - CCL5 (Regulated upon Activation Normal T-cell Expressed and Secreted) and Eotaxin (CCL11) [10, 11].
Analysis of their serum concentrations in patients with UM was the main objective of this study. Its distinctive feature is the simultaneous analysis of serum chemokine concentrations using standardized modern methods of multiplex immunoassay based on xMAP technology, licensed by Luminex, created using flow fluorimetry, polystyrene microspheres, two fluorescent dyes at different ratios, laser detection, and digital signal processing for providing results of up to 100 various analyzes from one sample.
Material and methods
Patients’ description. The study comprised 36 patients of Caucasian origin, who underwent laparoscopic myomectomy for uterine myoma. The median age of patients was 42.0 (38.5; 45.8) years and ranged from 23 to 54 years. Among them, 35.9% were overweight or obese (body mass index (BMI)> 24.9). Twenty (55.6%) patients had menstrual irregularities in the form of polymenorrhea. A history of previous births, induced termination of pregnancy, spontaneous miscarriages and no pregnancies was reported by 28, 25, 8, and 5 patients, respectively. Twenty eight and 23 patients complained of pulling lower abdominal pain and bleeding, respectively. Fibroid size, as measured by pelvic ultrasound ranged from 5 to 180 mm with the median fibroid size 97.5 (80.5; 110.0) mm. In 19 and 7 patients fibroids were detected in the anterior and posterior uterine wall, respectively, and 2 patients had a combined fibroid localization. Histologic studies of preoperative hysteroscopic biopsy specimens reported endometrial polyp and simple endometrial hyperplasia in 7 and 6 patients, respectively.
Methods for cytokine determination. Before cytokine analysis, once-frozen at -80 ˚С serum samples were completely thawed at room temperature and centrifuged at 10 000 rpm for 10 min at 4 C to remove the sediment. Concentrations of 27 cytokines were determined using the Bio-Plex Pro™ Human Cytokine 27-Plex Immunoassay by flow-through fluorometry on a Bio-Plex 200 two-beam laser analyzer, manufactured by Bio-Rad (USA). After creating a calibration curve according to validated standards, serum concentrations of cytokines and chemokines, including CCL chemokines MCP-1, MIP-1α, MIP-1β, RANTES, and Eotaxin, were simultaneously determined. The data were processed using the Bio-Plex Manager Software version 4.1. The concentration of cytokines was expressed in pg/ml.
Statistical analysis. Statistical analysis was performed using Statistica 10.0 (StatSoft) and IBM SPSS Statistics 23 (USA) statistical software. The distribution of continuous variables was tested for normality using the Shapiro – Wilk and Kolmogorov-Smirnov test with Lilliefors correction. The data were analyzed using parametric tests including the Student’s one-sample test (approximate method) and the Student’s t-test to compare the means with the on-line calculator (http://medstatistic.ru/calculators/averagestudent.html), as well as non-parametric (Spearman’s rank correlation) statistical methods. Quantitative variables showing normal distribution (RANTES and Eotaxin) were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise (MCP-1, MIP-1α, MIP-1β) the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format were reported. The critical level of significance when testing statistical hypotheses was considered at p <0.05.
Results and discussion
A single-step analysis of serum levels of 5 chemokines in one sample showed that they had multidirectional changes. Particularly noteworthy is a significant dispersion of serum concentration of these regulatory factors. This observation is confirmed by both minimum and maximum cytokine concentrations presented in Table 1, and significant differences in their mean and median levels.
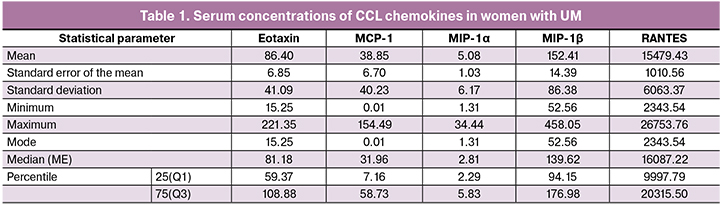
Especially noteworthy are significant differences in the absolute serum concentrations of chemokines with pro-inflammatory activity in patients with UM expressed in picograms per 1 ml. For instance, the median level of MIP-1α is 2.81 (2.29; 5.83) pg/ml, while the median level of RANTES in the same sample reaches 15479 (6063) pg/ml, i.e., is 5,000 times higher. This, of course, refers to the absolute protein concentrations, and not to the comparative activity of these regulatory factors, which can only be compared using cell-based test systems.
To compare our findings with studies of healthy women, we turned to the normative values from a sample of 94 healthy women of Caucasian origin obtained by the Center for Human Immunology, Autoimmunity and Inflammation, National Institutes of Health, Bethesda, Maryland, collaborating with the developer of the 27-plex kit from Bio-Rad test system [12]. These studies investigated serum levels of cytokines taking into account gender, age, the ethnicity of the study subjects, the time of the study, and even the use of various plastic plates for serum samples.
Besides, the authors of this study showed that serum chemokine concentrations were not significantly associated with age in women aged 40 years, similar to the age of our study participants. The reference concentrations [M (SD)] of 5 chemokines were as follows: MCP-1 - 55.40 (45.43), MIP-1α - 15.10 (21.52), MIP-1β - 173.50 (105.23), RANTES – 11848.90 (7298.92), and Eotaxin - 257.40 (209.01). The findings of these studies were obtained using Bio-Plex Manager 6.2. Statistical software and were recognized as reference values for healthy women of Caucasian origin. In the article, the term “level in healthy women” will refer to the concentrations of 5 chemokines taken from the above source of literature and reported as arithmetic mean (M) and standard deviation (SD).
As shown in table 1, the most stable were serum concentrations of MIP-1β both in healthy women and leiomyoma patients with almost identical levels (p = 0.24).
Serum levels of Eotaxin, MCP-1, and MIP-1α in women with UM were statistically significantly lower than in healthy women (p < 0,001, р = 0,04, and р<0,001, respectively). The greatest differences were observed in the concentrations of MIP-1α and eotaxin, which were almost three times lower in UM patients. These data probably indicate low activity of inflammatory or eosinophilic allergic processes occurring in the myometrium during the development of UM, since the chemokine CCL11 and members of its subfamily CC24 and CCL26 are chemoattractants for eosinophilic cells [13]. It can also be assumed that a decrease in the level of MIP-1α leads to insufficient activation of T-lymphocytes, which in turn can serve as one of the factors of insufficient control over the myocyte proliferation, and an insufficient level of such a strong chemotactic factor as MCP-1 leads to a reduced intensity of migration of cells with antigen-presenting function into the foci of a poorly controlled foci of proliferative activity of myocytes. The concomitant decrease in the activity of these most important regulatory processes can contribute to disrupting the normal course of myometrial remodeling in UM.
Contrary findings were observed in serum RANTES concentrations, which were significantly higher (p = 0.01) in women with UM. Given the important role of this protein in the recruitment and differentiation of monocytes in the arterial wall, it can be assumed that an increase in RANTES serum level may reflect the activation of neoangiogenesis in growing leiomyoma foci [14]. Besides, the complex of CCL5 and its receptor CCR5 is involved in the recruitment of regulatory immune cells in the tumor, inducing local immunosuppression, and contributing to tumor progression [15].
The multidirectional nature of changes in the studied cytokines in UM was also confirmed by the results of the correlation analysis presented in Table 2.
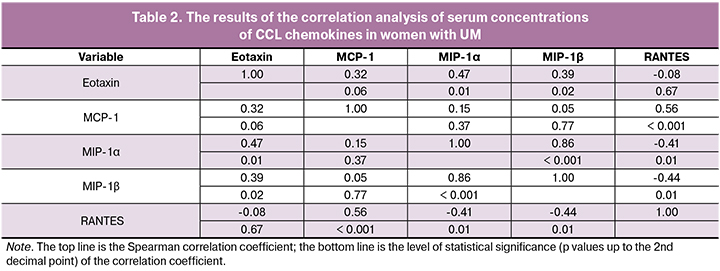
These findings show that in patients with UM, an increase in serum concentration of RANTES is accompanied by a decrease in the serum levels of Eotaxin, MIP-1α, and MIP-1β (negative correlations). At the same time, the decrease in the concentrations of chemokines CCL2, CCL3, CCL4, and CCL11 directly correlated with each other.
Conclusion
Summing up the results of our study, it can be concluded that the development of leiomyoma is associated with impaired functioning of the chemokine network manifested by multidirectional and correlated changes in serum concentrations of CCL chemokines.
Uterine myoma (UM), or leiomyoma, is a benign monoclonal tumor of the smooth muscle compartment (myometrium) of the uterus. According to some reports, up to 77% of all women will develop UM in their lifetime, and some of them will suffer from substantial symptoms, including pelvic discomfort, dysmenorrhea, menorrhagia, anemia, urinary incontinence, recurrent pregnancy loss, preterm labor, and in some cases infertility [1, 2]. Among factors promoting UM growth, the key role is played by the loss of control over cell migration, proliferation, differentiation, disorganization of the extracellular matrix, and associated inflammatory changes. One of the major contributing factors in these interrelated processes is impairment of local angiogenesis, which is directly influenced by individual and combined actions of growth factors, cytokines, and chemokines. Some of the identified UM-related growth factors are transforming growth factor (TGF-β), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF). Many of these growth factors including EGF, PDGF, and VEGF-A are overexpressed in UM compared to myometrial smooth muscle, although the EGF receptor (EGFR) is expressed at the same levels in both tissues [3].
Many cytokines, including tumor necrosis factor-alpha (TNF-α), erythropoietin, interleukin (IL) -1 and IL-6, are involved in the development of uterine leiomyoma. Compared with myometrial tissue, in leiomyomas expression of eotaxin mRNA, inflammatory macrophage proteins (MIP-lα, MIP-Iβ) and beta-chemokine receptors belonging to the class of integral membrane proteins (CCR3) were found to be changed. In simple leiomyoma, the levels of MIP-1, CCR5, and eotaxin mRNA are statistically significantly lower than in myometrial tissue. The expression level of eotaxin mRNA in myometrial tissue is significantly higher in solitary than in multiple leiomyomas and in simple than proliferating ones. [4]. It has been shown that chemokines and their receptors (MIP-1α, MIP-1β, RANTES, eotaxin, eotaxin-2, IL-8, CCR1, CCR3, CCR5, CXCR1, and CXCR2) can be mediators of the tumor grows in UM [5, 6]. MCP-1 mRNA levels are higher in myometrium compared to leiomyoma tissue, and estrogens and progestins decrease the production of this chemokine protein, which indicates that MCP-1 may have antitumor activity in UM [7]. The increased mast cell content in UM positively correlates with the concentration of RANTES and eotaxin [8].
Chemokines are cytokines with chemoattractant properties (chemotactic cytokines) and a molecular weight of 7 to 12 kDa. Chemokines are characterized by several features, including the presence of four conserved cysteine residues. Their receptors often contribute to rapid and reversible changes in cellular metabolism or migration, unlike other cytokine receptors, which usually cause slower and irreversible processes of proliferation and apoptosis.
Members of the chemokine family include CC chemokines, which have two adjacent cysteines, CXC chemokines, with two cysteines separated by one amino acid, C-chemokines characterized by the presence of two cysteines, and CX3C chemokines with three amino acids between the two cysteines [9]. The most significant representatives of this group of proteins that attract inflammatory cells to the pathological foci are proteins produced by macrophages and other activation cells: MCP-1 - CCL2 (macrophage chemotactic protein-1), MIP-1α - CCL3 and MIP-1β - CCL4 (macrophage inflammatory protein α and β), RANTES - CCL5 (Regulated upon Activation Normal T-cell Expressed and Secreted) and Eotaxin (CCL11) [10, 11].
Analysis of their serum concentrations in patients with UM was the main objective of this study. Its distinctive feature is the simultaneous analysis of serum chemokine concentrations using standardized modern methods of multiplex immunoassay based on xMAP technology, licensed by Luminex, created using flow fluorimetry, polystyrene microspheres, two fluorescent dyes at different ratios, laser detection, and digital signal processing for providing results of up to 100 various analyzes from one sample.
Material and methods
Patients’ description. The study comprised 36 patients of Caucasian origin, who underwent laparoscopic myomectomy for uterine myoma. The median age of patients was 42.0 (38.5; 45.8) years and ranged from 23 to 54 years. Among them, 35.9% were overweight or obese (body mass index (BMI)> 24.9). Twenty (55.6%) patients had menstrual irregularities in the form of polymenorrhea. A history of previous births, induced termination of pregnancy, spontaneous miscarriages and no pregnancies was reported by 28, 25, 8, and 5 patients, respectively. Twenty eight and 23 patients complained of pulling lower abdominal pain and bleeding, respectively. Fibroid size, as measured by pelvic ultrasound ranged from 5 to 180 mm with the median fibroid size 97.5 (80.5; 110.0) mm. In 19 and 7 patients fibroids were detected in the anterior and posterior uterine wall, respectively, and 2 patients had a combined fibroid localization. Histologic studies of preoperative hysteroscopic biopsy specimens reported endometrial polyp and simple endometrial hyperplasia in 7 and 6 patients, respectively.
Methods for cytokine determination. Before cytokine analysis, once-frozen at -80 ˚С serum samples were completely thawed at room temperature and centrifuged at 10 000 rpm for 10 min at 4 C to remove the sediment. Concentrations of 27 cytokines were determined using the Bio-Plex Pro™ Human Cytokine 27-Plex Immunoassay by flow-through fluorometry on a Bio-Plex 200 two-beam laser analyzer, manufactured by Bio-Rad (USA). After creating a calibration curve according to validated standards, serum concentrations of cytokines and chemokines, including CCL chemokines MCP-1, MIP-1α, MIP-1β, RANTES, and Eotaxin, were simultaneously determined. The data were processed using the Bio-Plex Manager Software version 4.1. The concentration of cytokines was expressed in pg/ml.
Statistical analysis. Statistical analysis was performed using Statistica 10.0 (StatSoft) and IBM SPSS Statistics 23 (USA) statistical software. The distribution of continuous variables was tested for normality using the Shapiro – Wilk and Kolmogorov-Smirnov test with Lilliefors correction. The data were analyzed using parametric tests including the Student’s one-sample test (approximate method) and the Student’s t-test to compare the means with the on-line calculator (http://medstatistic.ru/calculators/averagestudent.html), as well as non-parametric (Spearman’s rank correlation) statistical methods. Quantitative variables showing normal distribution (RANTES and Eotaxin) were expressed as means (M) and standard deviation (SD) and presented as M (SD); otherwise (MCP-1, MIP-1α, MIP-1β) the median (Me) and the quartiles Q1 and Q3 in the Me (Q1; Q3) format were reported. The critical level of significance when testing statistical hypotheses was considered at p <0.05.
Results and discussion
A single-step analysis of serum levels of 5 chemokines in one sample showed that they had multidirectional changes. Particularly noteworthy is a significant dispersion of serum concentration of these regulatory factors. This observation is confirmed by both minimum and maximum cytokine concentrations presented in Table 1, and significant differences in their mean and median levels.
Especially noteworthy are significant differences in the absolute serum concentrations of chemokines with pro-inflammatory activity in patients with UM expressed in picograms per 1 ml. For instance, the median level of MIP-1α is 2.81 (2.29; 5.83) pg/ml, while the median level of RANTES in the same sample reaches 15479 (6063) pg/ml, i.e., is 5,000 times higher. This, of course, refers to the absolute protein concentrations, and not to the comparative activity of these regulatory factors, which can only be compared using cell-based test systems.
To compare our findings with studies of healthy women, we turned to the normative values from a sample of 94 healthy women of Caucasian origin obtained by the Center for Human Immunology, Autoimmunity and Inflammation, National Institutes of Health, Bethesda, Maryland, collaborating with the developer of the 27-plex kit from Bio-Rad test system [12]. These studies investigated serum levels of cytokines taking into account gender, age, the ethnicity of the study subjects, the time of the study, and even the use of various plastic plates for serum samples.
Besides, the authors of this study showed that serum chemokine concentrations were not significantly associated with age in women aged 40 years, similar to the age of our study participants. The reference concentrations [M (SD)] of 5 chemokines were as follows: MCP-1 - 55.40 (45.43), MIP-1α - 15.10 (21.52), MIP-1β - 173.50 (105.23), RANTES – 11848.90 (7298.92), and Eotaxin - 257.40 (209.01). The findings of these studies were obtained using Bio-Plex Manager 6.2. Statistical software and were recognized as reference values for healthy women of Caucasian origin. In the article, the term “level in healthy women” will refer to the concentrations of 5 chemokines taken from the above source of literature and reported as arithmetic mean (M) and standard deviation (SD).
As shown in table 1, the most stable were serum concentrations of MIP-1β both in healthy women and leiomyoma patients with almost identical levels (p = 0.24).
Serum levels of Eotaxin, MCP-1, and MIP-1α in women with UM were statistically significantly lower than in healthy women (p < 0,001, р = 0,04, and р<0,001, respectively). The greatest differences were observed in the concentrations of MIP-1α and eotaxin, which were almost three times lower in UM patients. These data probably indicate low activity of inflammatory or eosinophilic allergic processes occurring in the myometrium during the development of UM, since the chemokine CCL11 and members of its subfamily CC24 and CCL26 are chemoattractants for eosinophilic cells [13]. It can also be assumed that a decrease in the level of MIP-1α leads to insufficient activation of T-lymphocytes, which in turn can serve as one of the factors of insufficient control over the myocyte proliferation, and an insufficient level of such a strong chemotactic factor as MCP-1 leads to a reduced intensity of migration of cells with antigen-presenting function into the foci of a poorly controlled foci of proliferative activity of myocytes. The concomitant decrease in the activity of these most important regulatory processes can contribute to disrupting the normal course of myometrial remodeling in UM.
Contrary findings were observed in serum RANTES concentrations, which were significantly higher (p = 0.01) in women with UM. Given the important role of this protein in the recruitment and differentiation of monocytes in the arterial wall, it can be assumed that an increase in RANTES serum level may reflect the activation of neoangiogenesis in growing leiomyoma foci [14]. Besides, the complex of CCL5 and its receptor CCR5 is involved in the recruitment of regulatory immune cells in the tumor, inducing local immunosuppression, and contributing to tumor progression [15].
The multidirectional nature of changes in the studied cytokines in UM was also confirmed by the results of the correlation analysis presented in Table 2.
These findings show that in patients with UM, an increase in serum concentration of RANTES is accompanied by a decrease in the serum levels of Eotaxin, MIP-1α, and MIP-1β (negative correlations). At the same time, the decrease in the concentrations of chemokines CCL2, CCL3, CCL4, and CCL11 directly correlated with each other.
Conclusion
Summing up the results of our study, it can be concluded that the development of leiomyoma is associated with impaired functioning of the chemokine network manifested by multidirectional and correlated changes in serum concentrations of CCL chemokines.
References
- McWilliams M.M., Chennathukuzhi V.M. Recent advances in uterine fibroid etiology. Semin. Reprod. Med. 2017; 35(2): 181-9. https://doi.org/10.1055/s-0037-1599090.
- Ordulu Z. Fibroids: genotype and phenotype. Clin. Obstet. Gynecol. 2016; 59(1): 25-9. https://doi.org/10.1097/GRF.0000000000000177.
- Parker W.H. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil. Steril. 2007; 87(4): 725-36. https://doi.org/10.1016/j.fertnstert.2007.01.093.
- Сысоев К.А., Кулагина Н.В., Чухловин А.Б., Морозова Е.Б., Тотолян А.А. Экспрессия мРНК хемокинов и хемокиновых рецепторов в тканях миометрия и лейомиомы матки. Бюллетень экспериментальной биологии и медицины. 2008; 145(1): 91-7. [Syssoev K.A., Kulagina N.V., Chukhlovin A.B., Morozova E.B., Totolian A.A. Expression of mRNA for chemokines and chemokine receptors in tissues of the myometrium and uterine leiomyoma. Byulleten’ eksperimental’noy biologii i meditsiny/Bulletin of Experimental Biology and Medicine. 2008; 145(1): 91-97. (in Russian)]
- Bonfield T.L., Panuska J.R., Konstan M.W., Hilliard K.A., Hilliard J.B., Ghnaim H. et al. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 1995; 152(6): 2111-8. https://doi.org/10.1164/ajrccm. 152.6.8520783.
- Mehrad B., Keane M.P., Strieter R.M. Chemokines as mediators of angiogenesis. Thromb. Haemost. 2007; 97(5): 755-62.
- Sozen I., Olive D.L., Arici A. Expression and hormonal regulation of monocyte chemotactic protein-1 in myometrium and leiomyomata. Fertil. Steril. 1998; 69(6): 1095-102.
- Lü J.Q., Zhu X.Q., Dong K., Xiang M., Lin Y., Hu Y. Study of the mechanism of mast cell increase in cellular leiomyoma of uterus. Zhonghua Fu Chan Ke Za Zhi. 2007; 42(6): 390-3.
- Toda E., Terashima Y., Sato T., Hirose K., Kanegasaki S., Matsushima K. FROUNT is a common regulator of CCR2 and CCR5 signaling to control directional migration. J. Immunol. 2009; 183(10): 6387-94. https://doi.org/10.4049/jimmunol.0803469.
- Aldinucci D., Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014; 2014: 292376. https://doi.org/10.1155/2014/292376.
- Conti P., DiGioacchino M. MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc. 2001; 22(3): 133-7.
- Biancotto A., Wank A., Perl S., Cook W., Olnes M.J., Dagur P.K. et al. Correction: Baseline Levels and Temporal Stability of 27 Multiplexed Serum Cytokine Concentrations in Healthy Subjects. PLoS One. 2015; 10(7): e0132870. https://doi.org/10.1371/journal.pone.0132870.
- Ahmadi Z., Hassanshahi G., Khorramdelazad H., Zainodini N., Koochakzadeh L. An overlook to the characteristics and roles played by eotaxin network in the pathophysiology of food allergies: allergic asthma and atopic dermatitis. Inflammation. 2016; 39(3): 1253-67. https://doi.org/10.1007/s10753-016-0303-9.
- Spinas E., Kritas S.K., Saggini A., Mobili A., Caraffa A., Antinolfi P. et al. Role of mast cells in atherosclerosis: a classical inflammatory disease. Int. J. Immunopathol. Pharmacol. 2014; 27(4): 517-21. https://doi.org/10.1177/039463201402700407.
- Velasco-Velázquez M., Xolalpa W., Pestell R.G. The potential to target CCL5/CCR5 in breast cancer. Expert Opin. Ther. Targets. 2014; 18(11): 1265-75. https://doi.org/10.1517/14728222.2014.949238.
Received 04.03.2019
Accepted 19.04.2019
About the Authors
Konenkov Vladimir I., Ph.D., Professor, Academician of Russian Sciences Academy, Honored Worker of Science of the Russian Federation, Scientific Director of RICEL - Branch of ICG SB RAS, Head of clinical immunogenetics laboratory. Phone: +7(383)333-64-09. E-mail: vikonenkov@gmail.com. ORCID.org/0000-0001-7385-6270630060 Russia, Novosibirsk, Timakova Street, 2.
Koroleva Elena G., MD, Researcher of Cell Technologies Laboratory, Research Institute of Clinical and Experimental Lymphology - Branch of the FRC Institute of Cytology and Genetics SB RAS. Phone: +7(383)335-93-32. E-mail: lymphology@niikel.ru
630060 Russia, Novosibirsk, Timakova Street, 2.
Orlov Nikolay B., Ph.D., major researcher of Clinical Immunogenetics Laboratory RICEL - Branch of the FRC Institute of Cytology and Genetics SB RAS.
Phone: +7 (383) 311-05-40. E-mail: nbo700@mail.ru. ORCID.org/0000-0002-3437-7151. 630060 Russia, Novosibirsk, Timakova Street, 2.
Prokof’ev Viktor F., PhD, Leading Researcher of Clinical Immunogenetics Laboratory, RICEL - Branch of the FRC Institute of Cytology and Genetics SB RAS.
Phone: +7 (383) 311-05-40. E-mail: vf_prok@mail.ru. ORCID.org/0000-0001-7290-1631. 630060 Russia, Novosibirsk, Timakova Street, 2.
Shevchenko Alla V., Ph.D., Leading Researcher, Clinical Immunogenetics Laboratory, RICEL - Branch of the FRC Institute of Cytology and Genetics SB RAS.
Phone: +7 (383) 311-05-40. E-mail: shalla64@mail.ru. ORCID.org/0000-0001-5898-950X. 630060 Russia, Novosibirsk, Timakova Street, 2.
Novikov Alexey M., MD, Junior Researcher, Laboratory of Cell Technologies, RICEL - Branch of the FRC Institute of Cytology and Genetics SB RAS.
Phone: +7 (383) 335-93-32. E-mail: novis.ngmu@gmail.com. 630060 Russia, Novosibirsk, Timakova Street, 2.
Dergacheva Tatyana I., PhD, professor, leading researcher of functional morphology of lymphatic system laboratory RICEL - Branch of the FRC Institute of Cytology
and Genetics SB RAS. Phone: +7 (383) 333-54-24. E-mail: dr-tanja@yandex.ru.
630060 Russia, Novosibirsk, Timakova Street, 2.
For citation: Konenkov V.I., Koroleva E.G., Orlov N.B., Prokofyev V.F., Shevchenko A.V., Novikov A.M., Dergacheva T.I. Changes in the serum concentrations of CCL chemokines (MCP-1, MIP-1α, MIP-1β, RANTES, and EOTAXIN) in women with uterine myoma.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2019; (8): 107-111(in Russian).
http://dx.doi.org/10.18565/aig.2019.8.107-111



