Assessment of hemostatic function in women with a history of recurrent pregnancy loss using thrombodynamics and blood clot contraction tests
Aim. To identify hemostatic abnormalities in non-pregnant patients with a history of recurrent pregnancy loss (RPL).Peshkova A.D., Safiullina S.I., Asarova D.G., Khafizova A.F., Ataullakhanov F.I., Litvinov R.I.
Materials and methods. Thirty-five patients with RPL and 25 parous women without a complicated obstetric history underwent clinical evaluation including thrombodynamics test and the measurement of blood clot contraction kinetics.
Results. Patients with a history of RPL had a statistically significantly higher hypercoagulability and a significant decrease in the ability of the clot to contract. Patients with three or more pregnancy losses have a statistically significantly higher hypercoagulability and greater changes in clot contraction, compared with patients who lost one or two pregnancies. Besides, miscarriages after 10 weeks’ gestation were associated with a greater decrease in clot contraction than early miscarriages.
Conclusion. Chronic hypercoagulable state and decreased blood clot contraction form a premorbid background in women with RPL. These findings suggest the possibility of using thrombodynamics and blood clot contraction as diagnostic tests to identify women at high risk of pregnancy loss.
Keywords
Recurrent pregnancy loss (RPL) is one of the major medical and social problems affecting 10–25% of all clinical pregnancies and up to 50% of pregnancies in women aged 40 and older [1, 2]. In Russia, the incidence of spontaneous miscarriages is 15–23%; about 80% of reproductive losses occur in the first trimester [3].
One of the pathogenetic factors of RPL is inherited and acquired hemostatic disorders. Physiological activation of the blood coagulation system begins at the earliest pregnancy stages, promotes the blastocyst implantation, and prevents peri-implantation hemorrhage during endovascular invasion of trophoblast [4]. On the other hand, excessive deposition of fibrin at the implantation site and micro-thrombosis of the spiral arteries and arterioles impairs the invasion of the fertilized egg and can lead to both pregnancy loss and primary placental insufficiency resulting in obstetric complications [5]. Inherited thrombophilia, including factor V Leiden mutation, prothrombin gene mutation, protein C, protein S, and antithrombin III deficiency, can cause RPL both in early and late pregnancy [6–8]. Therefore, RPL may be associated with both acquired and inherited hemostatic disorders, which are based on a predisposition to intravascular fibrin formation. It is important to identify maternal prothrombotic state during antenatal care for early initiation of anticoagulant therapy to prevent miscarriage.
Despite the crucial importance of detecting pre-thrombotic conditions, traditional coagulation tests fail to assess the thrombotic potential adequately and, as a rule, have low sensitivity to detect intravascular activation of the hemostatic system, including during pregnancy [9]. In this regard, of interest is thrombodynamics, a new global hemostasis assay that was shown to be sensitive to both hypercoagulability and hypocoagulability and can detect early changes in the hemostasis system underlying pre-thrombotic conditions [10, 11]. This method is based on registering spatial fibrin clot growth after activation of clotting in a thin layer of plasma after contact with an immobilized tissue factor bearing surface.
In-vitro coagulation is accompanied by spontaneous contraction of the blood clot. This process is termed as clot contraction or retraction. Since platelets are the driving force of the blood clot contraction, this process can be used as a test to characterize their quantity and functional state. Apart from platelets, the contraction process is caused by pathological changes in the molecular and cell composition in the peripheral blood [12]. Therefore, the study of blood clot contraction can provide additional information about the integral changes in the coagulation potential. Clinical studies have shown that in the blood of patients with prothrombotic conditions, such as ischemic stroke, venous thrombosis, systemic lupus erythematosus, clot contraction is significantly inhibited due to platelet dysfunction caused by their chronic hyperactivity and energy depletion [13-16]. It can, therefore, be assumed that similar processes can develop in women with a chronic hypercoagulable state associated with RPL, and the study of contraction of blood clots will provide additional information on the pathogenesis of RPL and may have clinical significance.
In light of the above, this study was aimed to investigate the hemostatic system in women with a history of RPL using two new laboratory tests - thrombodynamics and kinetics of blood clots contraction. Along with studying the pathogenetic role of hemostatic disorders, the aim of the work was to assess the diagnostic value of thrombodynamics tests and blood clot contraction in women with a history of RPL in comparison with other clinical and laboratory parameters at the stage of pregnancy planning.
Materials and methods
The study group comprised 35 women with a history of recurrent pregnancy loss. Their obstetric history included two or more miscarriages before 10 weeks of gestation, one or more miscarriages at 10–22 weeks of gestation, or one or more preterm births at 22–34 weeks. The clinical characteristics of the patients are presented in the table. The control group included 25 parous women without a complicated obstetric history and having no personal and family history of thrombosis. Patients with a history of RPL and the control group subjects were comparable in age [mean age 32 (5) years and 30 (4) years, respectively]. The study was conducted at the stage of pregnancy planning. Exclusion criteria from this study were anticoagulant, thrombolytic, or antiplatelet therapy, regardless of the reason within two weeks before the examination, as well as having antiphospholipid syndrome (APS). All participants gave written informed consent for the baseline clinical examination.
Blood samples for the study were obtained according to the requirements of the Research Ethics Committee of the Kazan Federal University (extract from protocol No. 3 of 03.23.17). Venous blood samples were collected in vacuum tubes with 3.8% sodium citrate (9:1 v/v) and analyzed within 4 hours after collection. One whole blood sample was used to analyze clot contraction. The second blood sample was centrifuged at 2000 g for 10 min at room temperature, and then the top three-quarters of platelet-poor plasma was collected for coagulation. For thrombodynamics test, platelet-poor plasma was used, which was obtained by additional centrifugation of platelet-poor plasma for 5 min at 10,000 g at room temperature. For analysis, the upper part (9/10 volume) of platelet-free plasma was selected. A third blood sample was stabilized with EDTA (final concentration of 1.6 mg/ml) for hematological analysis.
Kinetics of the directional fibrin clot formation (thrombodynamics). The principle of thrombodynamics is based on optical registration of the fibrin clot formation and growth after contact with an activator insert, on the surface of which a tissue factor is immobilized. After that, coagulation is activated in the adjacent plasma layer. The process of the fibrin clot formation and growth is recorded by a camera in scattered light using the Thrombodynamics Recording device (GemaKor Ltd., Russia).
To analyze thrombodynamics parameters, platelet-free plasma was transferred to a test tube with reagent I (contact phase enzyme inhibitor), then into a test tube with reagent II (calcium acetate). The resulting sample was transferred to a measuring well (120 μl), followed by the immersion of an activator insert with tissue factor, and then registration and recording of fibrin formation were started. The cuvette was registered optically every 6 s for 30 minutes. The obtained images were automatically processed by the program with the calculation of the following parameters: 1) lag-time — the time needed to start fibrin formation from the moment the plasma contacts with the activating surface; 2) the initial clot growth rate - the mean clot growth rate, calculated within 2–6 min after the start of clot growth; 3) stationary clot growth rate - the mean clot growth rate calculated between 15 and 25 min after the start of clot growth; 4) fibrin clot size 30 minutes after the plasma contacts the activator insert; 5) clot density - an optical indicator equal to the intensity of light scattering by a fibrin clot, which is proportional to fibrin network density.
Kinetics of clot contraction. The kinetics and degree of clot contraction were recorded in-vitro using Thrombodynamics Recorder device. The method is based on optical recording of decreasing size of the clot over time. To determine the contraction of a blood clot, a cuvette measuring 12´7´1mm was rinsed with a 4% Triton X-100 solution in 0.15 M NaCl to prevent the clot from sticking to the inner cuvette walls. Then, 2 mM calcium chloride and 1 U/ml thrombin were added to citrate to initiate clotting and activate platelets. Then, 80 μl of the sample was transferred into each of the channels of the transparent cuvette, and the cuvette itself was placed in a thermostatically controlled chamber of the Thrombodynamics Recorder at 37°C. Clot size was tracked from digitized images every 15 seconds for 20 minutes and then the images were analyzed using a special software to obtain the following parameters of the clot contraction kinetics: 1) the final degree of contraction is the percentage of the clot contraction relative to its initial size after 20 min of registration; 2) lag time - the time of reaching 95% of the clot original size; 3) the mean contraction rate is the percentage of the clot contraction per time unit; 4) the area under the kinetic curve, reflecting the mechanical work of platelets in the clot contraction.
Laboratory studies of hemostasis and cellular composition of blood. The hemostatic system was assessed using a Sysmex CA-1500 Automated Blood Coagulation Analyzer (Sysmex, Canada). The blood coagulation tests included activated partial thromboplastin time (APTT), prothrombin time (PT), thrombin time (TT), Clauss fibrinogen concentration, antithrombin III (AT III), plasminogen, C and S proteins, D-dimer and were measured using Immulite 2000 Immunoassay System (Siemens Healthcare Diagnostics Inc., USA). The content of soluble fibrin-monomer complexes (SFMCs) was determined using the ortho-phenanthroline test (reagents from Technologiya Standard, Russia).
A complete blood count test was performed in whole blood stabilized with EDTA using a hematology analyzer (Siemens Inc., Japan). Hematological parameters included the red blood cell count, mean corpuscular volume, hematocrit, hemoglobin, mean corpuscular hemoglobin concentration, color index, white blood cell count, the content of monocytes, neutrophils, lymphocytes, eosinophils, basophils, platelets, and mean platelet volume.
Statistical analyses were performed according to SAMPL guidelines [17] using the primary data without transformations and outliers. The analysis aimed at identifying differences in hemostatic parameters between the study and control groups as well as between subgroups within the study group. The distribution of continuous variables was tested for normality using the Shapiro-Wilk and D’Agostino tests. Continuous variables showing normal distribution were compared using the Student’s t-test. Normally distributed variables were expressed as means (M) and standard deviation (SD) and presented as M (SD).
To examine the relationships between various clinical and laboratory parameters and taking into account symmetric data distribution, the Pearson correlation coefficients test was conducted. The results are presented as r (a / b), where r is the Pearson linear correlation coefficient; a and b are the lower and upper boundaries of the 95% confidence interval (CI) for the correlation coefficient calculated using the online calculator [18]. The critical level of significance when testing statistical hypotheses was considered at p <0.05. Statistical analyses were performed using GraphPad Prism 7 statistical software.
Results
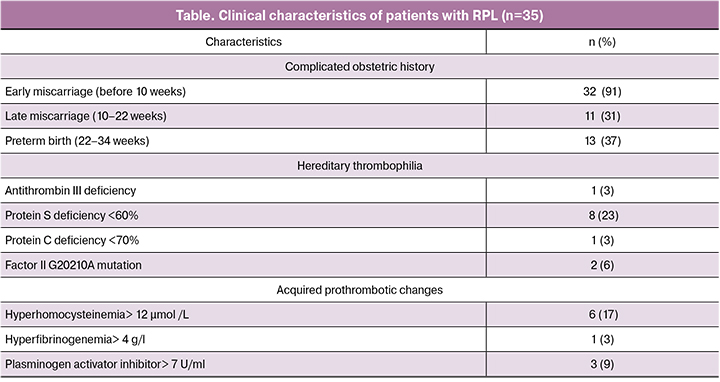
Compared with the control group, patients in the study group had significantly different thrombodynamics parameters, including higher mean initial and stationary clot growth rate and clot size, although they were within the reference range, which together indicates relatively hypercoagulative state already at the stage of pregnancy planning (Figure 1). It should be noted that thrombodynamics is an integral hemostasis assay that provides information about global coagulation potential without deciphering the mechanisms impairing this potential.
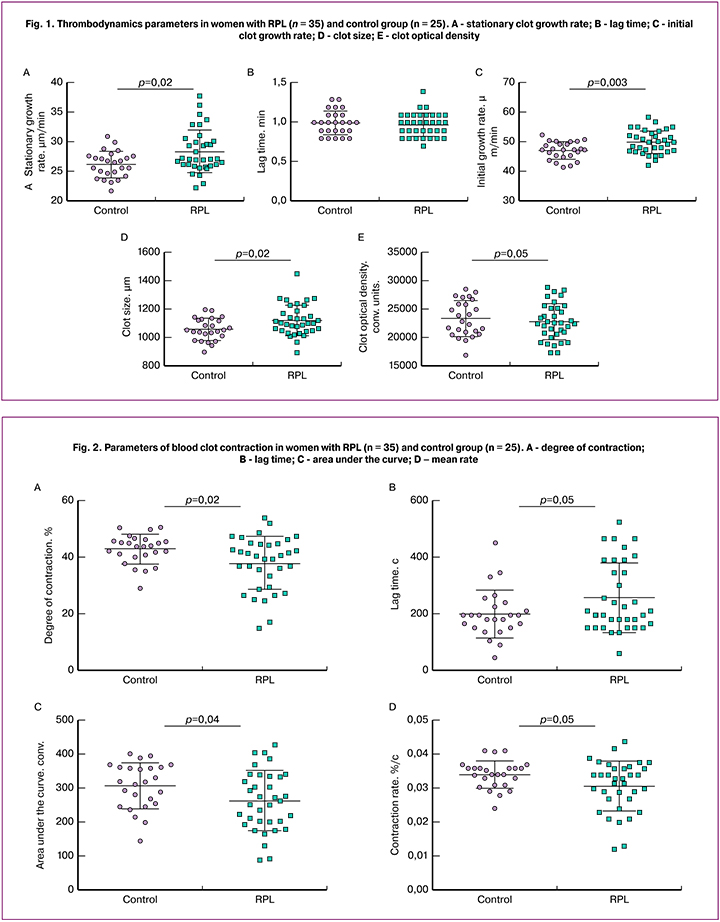
Patients in the study group were found to have a statistically significant decrease in the parameters of clot contraction, compared with control subjects (Fig. 2). This was evidenced by the inhibition of all stages of contraction including a decrease in the mean degree and rate of contraction, area under the kinetic curve, and longer lag time. To identify possible mechanisms of reducing blood clot contraction in women with RPL, the parameters of contraction were compared with the results of thrombodynamics and other laboratory tests.
A correlation analysis of thrombodynamics parameters and routine laboratory tests in both groups revealed the following statistically significant associations. There was a positive correlation between clot density and fibrinogen concentration [r = 0.72 (0.61/0.84); p = 0.001], D-dimer level [r = 0.32 (0.07/0.53); p = 0.04], plasminogen [r = 0.56 (0.36 / 0.71); p <0.001], and negative correlation with thrombin time [r = -0.39 (-0.59 -0.15); p = 0.01]. The initial clot growth rate was negatively correlated with APTT [r = -0.38 (-0.58/-0.14); p = 0.01], protein C concentration [r = -0.28 (-0.5/-0.03); p = 0.02], prothrombin time [r = -0.33 (-0.54/-0.08); p = 0.01], and hematocrit [r = -0.50 (-0.67 / -0.28); p = 0.01]. The stationary clot growth rate was negatively correlated with APTT [r = -0.32 (-0.53/-0.07); p = 0.02], protein C level [r = -0.35 (-0.55 / -0.11); p = 0.01], and hematocrit [r = -0.44 (-0.62/-0.21); p = 0.01]. The size of the clot had negative correlation with protein C concentration [r = -0.30 (-0.51/-0.05); p = 0.05], APTT [r = -0.36 (-0.56/-0.12); p = 0.02], prothrombin time [r = -0.32 (-0.53/-0.07); p = 0.02], and hematocrit [r = -0.40 (-0.59/-0.16); p = 0.04]. The lag time was negatively correlated with the prothrombin index [r = -0.27 (-0.49/-0.02); p = 0.05] and hematocrit [r = -0.44 (-0.62 / -0.21); p = 0.01]. These findings validate thrombodynamics as an integral test for studying the hemostatic system, which is sensitive to hypercoagulable conditions, including in patients with RPL.
There was a statistically significant positive correlation between APTT and a degree of [r = 0.26 (0.01 / 0.48); p = 0.02] and mean rate [r = 0.26 (-0.01 / 0.48); p = 0.02] of clot contraction, as well as a positive correlation between plasminogen activator inhibitor level and the degree of [r = 0.43 (0.2 / 0.62); p = 0.05] and speed [r = 0.43 (0.2 / 0.62); p = 0.05] of clot contraction. Platelet count had a direct correlation with the degree [r = 0.31 (0.04 / 0.54); p = 0.03] and speed [r = 0.31 (0.05 / 0.52); p = 0.02] of clot contraction, as well as with the area under the kinetic curve [r = 0.30 (0.05 / 0.51); p = 0.04]. Besides, a positive correlation was found between mean hemoglobin concentration and the degree [r = 0.36 (0.12 / 0.56); p <0.001] and speed [r = 0.35 (0.11 / 0.55); p <0.001] of clot contraction, as well as the area under the kinetic curve of contraction [r = 0.38 (0.14 / 0.58); p <0.001]. The lag time was negatively correlated with platelet distribution width [r = -0.43 (-0.62 / -0.2); p <0.001], mean corpuscular volume [r = -0.35 (-0.55 / -0.11); p = 0.01], and mean corpuscular hemoglobin concentration [r = -0.43 (-0.62 / -0.2); p = 0.01] and positively correlated with red cell distribution width [r = 0.39 (0.15 / 0.59); p <0.001]. These results suggest the diagnostic accuracy of the test of blood clot contraction kinetics as an indicator of the hemostatic system, the value of which goes beyond the indicator of platelet functional state.
Women with a history of three or more pregnancy losses had higher hypercoagulability compared with patients who lost one or two pregnancies (mean stationary clot growth rate of 30.3 (3.6) and 27.6 (3.4) μm/min., p = 0.05; clot size 1174 (99) and 1100 (107) μm, p = 0.05, respectively). Moreover, in patients of the first subgroup, the clot growth rate was above the reference range shifting to pathological hypercoagulability. Clot contraction rates also underwent statistically significant changes in the subgroup of patients with three or more pregnancy losses. The mean degree of contraction are 32 (9)% versus 39 (11)%; p = 0.05, contraction rates - 0.026 (0.007)% /s versus 0.032 (0.008)%/s .; p = 0.04, area under the kinetic curve - 215 (65) conventional units against 273 (92) conventional units; p = 0.04 were statistically significantly lower compared with patients who lost one or two pregnancies. On the contrary, the mean contraction lag time was statistically significantly longer in women with a history of three or more miscarriages - 283 (110) s versus 252 (120) s.; p = 0.05. Thus, the increase in the number of miscarriages was directly related to the severity of hypercoagulable state in terms of thrombodynamics, as well as to impaired blood clot contraction. These results confirm the important pathogenetic role of plasma and platelet hemostatic disorders in the pathogenesis of recurrent pregnancy loss.
Analysis of the relationship between thrombodynamics parameters and gestational age in patients of the study group showed a statistically significant decrease in the mean degree of clot contraction of 34 (9)% versus 40 (10)%; p = 0.05 and longer contraction lag time of 305 (131) s versus 213 (98) s; p = 0.03] in patients who lost pregnancy after 10 weeks compared with patients who had miscarriages at earlier gestational age. No statistically significant differences were observed in thrombodynamics parameters depending on gestational age.
Discussion
RPL may be caused by a variety of etiological and pathogenetic factors and have different mechanisms, such as chromosomal abnormalities, anatomical, endocrine or immune defects, and infections, although in many cases, the causes of RPL remain unclear [19–22]. Normal pregnancy is characterized by a hemostatic balance, which prevents fibrin accumulation in placental vessels and intervillous space [23]. However, patients with an imbalance in the hemostatic equilibrium resulting in hypercoagulability and thrombophilia have a high risk of miscarriage [24]. Among the causes of prothrombotic conditions during pregnancy are an increase in the factor VII activity, low level of PAI-1, genetic polymorphisms, APS, low levels of physiological anticoagulants, a change in fibrinolytic activity, and others [7, 8, 20, 25, 26].
The current study investigated the prothrombotic potential in women with RPL by analyzing parameters of thrombodynamics and blood clot contraction. There is a reason to believe that, along with other causes, RPL is the result of the pathological hypercoagulable state of pregnancy, which leads to placental or chorionic microthrombosis ultimately resulting in preterm birth [27], or, more often, early miscarriage [22] ]. This may be due to fewer placental and uterine vessels, which leads to a greater propensity to undergo partial or total occlusion as a result of microthrombosis without being compensated by collateral circulation [28, 29].
To our knowledge, no clinical studies have been previously conducted to characterize thrombodynamics and blood clot contraction in RPL. This study’s findings provide evidence that compared with healthy women, patients with a history of RPL tend to have prolonged, persistent hypercoagulable state as measured by thrombodynamics and a significant decrease in the ability of the clot to contract. Hypercoagulability and decreased clot contraction in RPL are consistent with increased levels of fibrinogen and D-dimer, as well as with the increased formation of SFMCs [30, 31]. Besides, according to published data, patients with RPL have been found to have vascular endothelium damage and increased expression of tissue factor, which may exacerbate pathological hypercoagulability [32].
Reduced blood clot contraction indicates platelet dysfunction in patients with RPL due to prolonged cell hyperactivation against the background of hypercoagulability and thrombinemia [13, 33]. This assumption is consistent with the evidence on a high level of spontaneous platelet activation in patients with a history of two or more miscarriages [34]. It is important to emphasize that the decrease in the total contractile function of platelets in patients with RPL occurs despite a statistically significant increase in platelet count, compared with the control group [264 (81) × 109/L versus 225 (32) × 109/L; p = 0.02].
Patients with RPL and a history of more than two miscarriages, the impairment of clot contractions and thrombodynamics were more pronounced than in patients with a history of one or two miscarriages, which is consistent with the literature [28, 29]. Along with the number of miscarriages, the timing of pregnancy loss matters. Our findings suggest that late miscarriages are associated with a greater decrease in clot contraction than early miscarriages. It is possible that the pregnancy loss at later gestational ages (more than 10 weeks) is largely due to impaired hemostatic function, while early fetal loss (before 10 weeks), as a rule, is associated with other pathogenetic factors (genetic, endocrine and gynecological) not associated with prothrombotic status [35, 36].
These findings, in combination with the literature data, support a pathogenetic role of inherited and acquired hemostatic disorders, which may be detected by testing thrombodynamics and kinetics of blood clot contraction. These changes are the result of changes in blood composition, causing prolonged hypercoagulable state and platelet dysfunction, including contractile function, which leads to impaired contraction, i.e., mechanical remodeling of blood clots and microthrombi. These disorders can lead to placental microcirculatory disturbances and, as a result, to termination of pregnancy. These results indicate that the analysis of thrombodynamics and blood clot contraction may be used for laboratory testing to evaluate the hemostatic system before and during pregnancy to identify high-risk patients and timely detect obstetric complications.
Conclusion
Our findings suggest that compared with healthy women, patients with a history of RPL have a statistically significant increase in the blood clot growth rate and size as measured by thrombodynamics and a decrease in its contraction parameters. These observations indicate the presence of hypercoagulability and a predisposition to thrombosis. Patients with a history of three or more pregnancy losses have a statistically significantly higher hypercoagulability and greater changes in clot contraction, compared with patients who have lost one or two pregnancies. These findings imply the possibility of using thrombodynamics and blood clot contraction in women with a history of miscarriage as diagnostic tests to determine the risk of pregnancy loss.
References
- Brezina P.R., Kutteh W.H. Classic and cutting-edge strategies for the management of early pregnancy loss. Obstet Gynecol Clin North Am. 2014; 41(1): 1–18. doi: 10.1016/j.ogc.2013.10.011
- Larsen E.C. Christiansen O.B., Kolte A.M., Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013; 11: 154. doi: 10.1186/1741-7015-11-154.
- Ведищев С.И., Прокопов А.Ю., Жабина У.В., Османов Э.М.. Современные представления о причинах невынашивания беременности. Вестник ТГУ. 2013; 18(4): 1309–12. [Vedischev S.I., Prokopov A.Yu., Zhabina V.V., Osmanov E.M. Modern ideas about the causes of miscarriage. Bulletin of TSU. 2013; 18 (4): 1309–12. (In Russian)]
- Cui C., Yang S., Zhang J., Wang G., Huang S., Zhang Y., Qiao R. Trimester-specific coagulation and anticoagulation reference intervals for healthy pregnancy. Thromb Res. 2017; 156: 82–6. doi: 10.1016/j.thromres.2017.05.021.
- De Jong P.G., Goddijn M., Middeldorp S. Testing for inherited thrombophilia in recurrent miscarriage. Semin Reprod Med. 2011; 29(6): 540–7. doi: 10.1055/s-0031-1293207.
- Shahina L., Lathi R. Recurrent pregnancy loss: evaluation and treatment. Obstet Gynecol Clin North Am. 2015; 42(1): 117–34. doi: 10.1016/j.ogc.2014.10.002.
- Dossenbach-Glaninger A., van Trotsenburg M., Dossenbach M., Oberkanins C., Moritz A., Krugluger W., Huber J., Hopmeier P. Plasminogen activator inhibitor 1 4G/5G polymorphism and coagulation factor XIII Val34Leu polymorphism: impaired fibrinolysis and early pregnancy loss. Clin Chem. 2003; 49(7): 1081–6.
- Kim J.J., Choi Y.M., Lee S.K., Yang K.M., Paik E.C., Jeong H.J., Jun J.K., Han A.R., Hong M.A. The PAI-1 4G/5G and ACE I/D polymorphisms and risk of recurrent pregnancy loss: a case-control study. Am J Reprod Immunol. 2014; 72(6): 571–6. doi: 10.1111/aji.12302
- Bennett S.A., Bagot C.N., Appiah A., Johns J., Ross J., Roberts L.N., Patel R.K., Arya R. Women with unexplained recurrent pregnancy loss do not have evidence of an underlying prothrombotic state: Experience with calibrated automated thrombography and rotational thromboelastometry. Thromb Res. 2014; 133: 892–9. doi:10.1016/j.thromres.2014.02.002
- Soshitova N.P., Karamzin S.S., Balandina A.N., Fadeeva O.A., Kretchetova A.V., Galstian G.M., et al. Predicting prothrombotic tendencies in sepsis using spatial clot growth dynamics. Blood Coagul Fibrinol. 2012; 23 (6): 498–507. doi: 10.1097/mbc.0b013e328352e90e.
- Sinauridze E.I., Gorbatenko A.S., Seregina E.A., Lipets E.N., Ataullakhanov F.I. Moderate plasma dilution using artificial plasma expanders shifts the haemostatic balance to hypercoagulation. Sci Rep. 2017; 7: 843. doi:10.1038/s41598-017-00927-w.
- Tutwiler V., Litvinov R.I., Lozhkin A.P., Peshkova A.D., Lebedeva T., Ataullakhanov F.I., et al. Kinetics and mechanics of clot contraction are governed by molecular and cellular blood composition. Blood. 2016; 127(1): 149–59. doi: 10.1182/blood-2015-05-647560.
- Tutwiler V., Peshkova A.D., Andrianova I.A., Khasanova D.R., Weisel J.W., Litvinov R.I. Blood clot contraction is impaired in acute ischemic stroke. Arter Thromb Vasc Biol. 2017; 37(2): 271–9. doi: 10.1161/ATVBAHA.116.308622
- Peshkova A.D., Malyasyov D.V., Bredikhin R.A., Le Minh G., Andrianova I.A., Tutwiler V., et al. Reduced contraction of blood clots in patients with venous thromboembolism is a possible thrombogenic and embologenic mechanism. TH Open. 2018; 2(1): e104-e115. doi:10.1055/s-0038-1635572.
- Le Minh G., Peshkova A.D., Andrianova I.A., Sibgatullin T.B., Maksudova A.N, Weisel J.W., Litvinov R.I. Impaired contraction of blood clots is a novel prothrombotic mechanism in systemic lupus erythematosus. Clini Sci. 2018; 232(2): 243–54. doi: 10.1042/CS20171510.
- Peshkova A.D., Malyasev D.V., Bredikhin R.A., Le Minh G., Litvinov R.I. Contraction of blood clots is impaired in deep vein thrombosis. BioNanoScience. 2016; 6(4): 457–9. doi:10.1007/s12668-016-0251-8
- Ланг Т., Альтман Д. Основы описания статистического анализа в статьях, публикуемых в биомедицинских журналах. Руководство «Статистический анализ и методы в публикуемой литературе (САМПЛ)». Медицинские технологии. Оценка и выбор. 2014; 1(15): 11–16. [Lang T., Altman D. Basic statistical reporting for articles published in clinical medical journals: the SAMPL Guidelines. In: Smart P., Maisonneuve H., Polderman A. (eds). Science Editors’ Handbook, European Association of Science Editors, 2013 (In Russ.)].
- Confidence intervals for a sample (Pearson) coefficient value. https://www.psyctc.org/stats/R/CI_correln1.html
- Соснова Е.А., Болевич С.Б., Покаленьева М.Ш. Патофизиологическая роль свободнорадикальных процессов при невынашивании беременности. Архив акушерства и гинекологии им. В.Ф. Снегирева. 2016; 3(3): 136–40.[Sosnova E.A., Bolevich S.B., Pokaleneva M.Sh. Pathophysiological role of free radical processes in pregnancies end in miscarriage. V.F. Snegirev Archives of Obstetrics and Gynecology, Russian journal. 2016; 3(3): 136–140.(In Russ.)]. doi:10.18821/2313-8726-2016-3-3-136-140
- Bigdeli R., Younesi M.R., Panahnejad E., Asgary V., Heidarzadeh S., Mazaheri H., Aligoudarzi S.L. Association between thrombophilia gene polymorphisms and recurrent pregnancy loss risk in the Iranian population. Syst. Biol Reprod Med. 2018; 64(4): 274–82. doi: 10.1080/19396368.2018.1456576.
- Никитина Т.В., Жегалина Д.И., Саженова Е.А., Толмачева Е.Н., Скрябин Н.А., Лебедев И.Н. Неслучайное распределение кариотипов эмбрионов у женщин с привычным невынашиванием беременности. Медицинская генетика. 2018; 17(1): 50–56. [Nikitina T.V., Zhigalina D.I., Sazhenova E.A., Tolmacheva E.N., Skryabin N.A., Lebedev I.N. Non-random distribution of embryonic kariotypes in women with recurrent pregnancy losses. Medicinskaja genetika. 2018; 17(1): 50–56. (in Russian)]. doi: 10.25557/2073-7998.2018.01.50-56.
- Карп Говард Дж.А., ред. Привычное невынашивание беременности. Причины, версии и контраверсии. Лечение. Пер. с англ. под ред. В.Е. Радзинского. М.: ГЭОТАР-МЕДИА, 2017. 589 с. [Karp Howard, J.A., ed. Habitual miscarriage. Reasons, versions and contraversions. Treatment. Per. from English under the editorship of V.E. Radzinsky. M.: GEOTAR-MEDIA, 2017.589 s. (in Russian)]. ISBN 978-5-9704-4170-1
- Holmes V.A., Wallace J.M. Haemostasis in normal pregnancy: a balancing act? Biochem Soc Trans. 2005; 33(2): 428–32. doi: 10.1042/BST0330428
- Romagnuolo I., Attanasio M., Cozzolino M., Paladino E., Castaman G., Coccia M.E., Fatini C. Thrombin potential and traditional coagulation assay: are they useful in exploring recurrent pregnancy loss risk? Blood Coagul Fibrinolysis. 2018; 29(2): 160–6. doi: 10.1097/MBC.0000000000000675.
- Kovac M.K., Lalic-Cosic S.Z., Dmitrovic J.M., Djordjevic V.J., Radojkovic D.P. Thrombin generation, D-dimer and protein S in uncomplicated pregnancy. Clin Chem Lab. 2015; 53(12): 1975–9. doi: 10.1515/cclm-2014-1030.
- Liu L., Sun D. Pregnancy outcomes in patients with primary antipholipid syndrome: A systematic review and meta-analysis. Medicine (Baltimore). 2019; 98(20): e.15733. doi: 10.1097/MD.0000000000015733.
- Kutteh W.H. Inherited and Acquired Thrombophilias and Adverse Pregnancy Outcomes. In: Bashiri A., Harlev A., Agarwal A. (eds) Recurrent Pregnancy Loss. Springer, Cham; 2016: 67–73. doi:10.1007/978-3-319-27452-2_5.
- Chang J.C. Thrombocytopenia in critically ill patients due to vascular microthrombotic disease: Pathogenesis based on «two activation theory of the endothelium». Vascul Dis Ther. 2017; 2(5): 1–7. doi: 10.15761/VDT.1000132
- Khizroeva J., Bitsadze V., Makatsariya A. Catastrophic antiphospholipid syndrome and pregnancy. Clinical report. J Matern Fetal Neonatal Med. 2019; 32(12): 2091–4. doi: 10.1080/14767058.2017.1422715.
- Reger B., Peterfalvi A., Litter I., Poto L., Mozes R., Toth O. et al. Challenges in the evaluation of D-dimer and fibrinogen levels in pregnant women. Thromb Res. 2013; 131(4): e183-e187. doi: 10.1016/j.thromres.2013.02.005
- Szecsi P.B., Jørgensen M., Klajnbard A., Andersen M.R., Colov N.P., Stender S. Haemostatic reference intervals in pregnancy. Thromb Haemost. 2010; 103(4): 718–27. doi: 10.1160/TH09-10-0704
- Pasquier E., De Saint Martin L., Bohe C. Unexplained pregnancy loss: a marker of basal endothelial dysfunction? Fertil Steril. 2013; 100(4): 1013–7. doi: 10.1016/j.fertnstert.2013.06.008
- Boilard E., Blanco P., Nigrovic P.A. Platelets: active players in the pathogenesis of arthritis and SLE. Nat Rev Rheumatol. 2012; 8: 534–42. doi:10.1038/nrrheum.2012.118.
- Lukanov T.H., Veleva G.L., Konova E.I., Ivanov P.D., Kovacheva K.S., Stoykow D.J. Levels of platelet-Leukocyte aggregates in women with both thrombophilia and recurrent pregnancy loss. Clin Appl Thromb Hemost. 2011; 17(2): 181–7. doi: 10.1177/1076029609350891.
- Gerhardt A., Scharf R.E., Greer I.A., Zotz R.B. Hereditary risk factors for thrombophilia and probability venous thromboembolism during pregnancy and the puerperium. Blood. 2016;128(19): 2343–9. doi: 10.1182/blood-2016-03-703728
- Ke R.W. Endocrine basis for recurrent pregnancy loss. Obstet Gynecol Clin North Am. 2014; 41(1): 103–12. doi: 10.1016/j.ogc.2013.10.003.
Received 30.05.2019
Accepted 21.06.2019
About the Authors
Alina D. Peshkova, PhD student, Department of Biochemistry and Biotechnology, Institute of Fundamental Medicine and Biology, Kazan Federal University;Junior Researcher, Laboratory «Protein-Cell Interaction», Kazan Federal University. E-mail: alinapeshkova26@gmail.com; http://orcid.org/0000-0002-8790-1818
420012, 76 Karl Marx St., Kazan, Russian Federation,
Svetlana I. Safiullina, MD, PhD, Assistant Professor, Department of General Medical Practice, Kazan State Medical University.
E-mail: Svetlana.ild.safiullina@gmail.com; http://orcid.org/0000-0003-4657-0140
420073, 8а Kyrskaya St., Kazan, Russian Federation; 420012, 49 Butlerov St., Kazan, Russian Federation.
Dinare G. Asarova., MS, Department of Biochemistry, Biotechnology and Pharmacology, Institute of Fundamental Medicine and Biology, Kazan Federal University.
E-mail:didi-lady@yandex.ru; https://orcid.org/0000-0002-8969-7873
420012, 76 Karl Marx St., Kazan, Russian Federation.
Aigul F. Hafizova, MD, obstetrician and gynecologist at the University Hospital, Kazan Federal University.
E-mail: ai12022102@gmail.com; https://orcid.org/0000-0002-2033-7693
420043, 44 Dostoevsky St., Kazan, Russian Federation.
Fazoil I. Ataullakhanov, PhD., Dr. Sci., Scientific Director of the Center for Theoretical Problems of Physico-Chemical Pharmacology, Russian Academy of Sciences. E-mail:ataullakhanov.fazly@gmail.com; https://orcid.org/0000-0003-3403-181X
119991, 4 Kosygina St., Moscow, Russian Federation.
Rustem I. Litvinov, MD, PhD, Dr. Sci., Senior Research Investigator, Department of Cell and Developmental Biology, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA; Adjunct Professor, Department of Biochemistry, Biotechnology and Pharmacology, Institute of Fundamental Medicine and Biology, Kazan Federal University; Chief Researcher, Head of the Laboratory «Protein-Cell Interactions», Kazan Federal University.
E-mail: litvinov@pennmedicine.upenn.edu; http://orcid.org/0000-0003-0643-1496
421 Curie Blvd., BRB II/III, Room 1116, Philadelphia, PA 19104-6058, USA; 420012, 76 Karl Marx St., Kazan, Russian Federation
For citation: Peshkova A.D., Safiullina S.I., Asarova D.G., Khafizova A.F., Ataullakhanov F.I., Litvinov R.I. Assessment of hemostatic function in women with a history of recurrent pregnancy loss using thrombodynamics and blood clot contraction tests.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 12: 111-9. (In Russian).
https://dx.doi.org/10.18565/aig.2019.12.111-119



