Platelet function and blood clotting during normal pregnancy
Objective: To investigate the changes in hemostasis during healthy pregnancy.Safiullina S.I., Evtugina N.G., Peshkova A.D., Litvinov R.I.
Materials and methods: Platelet contractile function assessed as a degree of blood clot contraction was studied in 38 healthy pregnant women, in relation to the markers of platelet activation, hemogram, hemostatic tests, and analysis of prothrombotic genetic polymorphisms.
Results: From the first week of normal pregnancy to delivery, there was moderate but statistically significant progressive impairment of blood clot contraction. A decrease in the ability of blood clots to shrink may be associated with physiological thrombocytopenia as well as partial continuous activation and exhaustion of platelets against a background of hypercoagulability and hyperfibrinogenemia. In 23/28 (82%) pregnant women, a transient reduction in the degree of clot contraction below the reference values was observed between 18 and 24 weeks of pregnancy. This reduction corresponds to the timing of placentation, suggesting local intraplacental mechanisms of platelet involvement during normal pregnancy.
Conclusion: The findings demonstrate the value of laboratory evaluation of platelet hemostasis, including platelet contractile function, during pregnancy.
Authors' contributions: Litvinov R.I., Safiullina S.I. – study conception and design, drafting of the manuscript; Safiullina S.I. – collection of clinical data; Evtugina N.G., Peshkova A.D. – performance of laboratory tests; Litvinov R.I., Evtugina N.G., Peshkova A.D. – data processing and analysis.
Conflicts of interest: The authors have no conflicts of interest to declare.
Funding: This work was supported by the Strategic Academic Leadership Program of Kazan Federal University (PRIORITET-2030).
Acknowledgements: The authors would like to thank Professor Fazoil Inoyatovich Ataullakhanov, Academician of the Russian Academy of Sciences, for his support and valuable discussions. The authors would also like to thank HemaCore LLC (Moscow) for providing the Thrombodynamics Analyzer used in this study.
Ethical Approval: The study was reviewed and approved by the Research Ethics Committee of the Kazan Federal University
(Ref. No.3 as of 23.03.17).
Patient Consent for Publication: All patients provided informed consent for the publication of their data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Safiullina S.I., Evtugina N.G., Peshkova A.D., Litvinov R.I.
Platelet function and blood clotting during normal pregnancy.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2023; (6): 51-59 (in Russian)
https://dx.doi.org/10.18565/aig.2023.135
Keywords
Physiological pregnancy is characterized by the perturbation of the hemostasis system, which adapts to prepare for blood loss during childbirth and the early postpartum period while maintaining normal function of the fetoplacental complex [1]. This adaptation results in hypercoagulability, which can be detected through global hemostatic tests, an increase in the levels of coagulation factors, and a decrease in physiological anticoagulants and enzymes of the fibrinolytic system [2–4]. The physiological hypercoagulability reaches its peak during childbirth and returns to normal 4-6 weeks after delivery [5].
Platelet levels may remain unchanged [4] or display moderate thrombocytopenia [6, 7] owing to increased circulating blood volume [8]. Another possible mechanism of the thrombocytopenia is the enhanced sequestration and consumption of platelets in the uteroplacental circulation and deposition in the spleen, whose perfusion increases during pregnancy. Changes in platelet functionality and their role during normal pregnancy have been much less studied than those of blood clotting, and are characterized by controversial results [9–12].
It is important to note that despite the local activation and secretory activity of platelets at the implantation site [13], there is no evidence of microthrombosis in the normal placenta. This suggests that the main platelet function during pregnancy may not be associated with their hemostatic and thrombogenic properties but may rather comprise something different, for example, delivery of cytokines to the site of chorion invasion through secretion of the α-granule content [14]. Therefore, the platelet function during physiological pregnancy is not fully understood, and further research is needed to investigate quantitative and qualitative changes in platelets in the blood of healthy women at different stages of normal pregnancy.
Thus, the aim of this study was to compare platelet function with coagulation hemostasis during physiological pregnancy.
Materials and methods
General characterization of the clinical material
The study was conducted using blood samples from 38 pregnant women aged 23 to 41 (mean age 29 (4) years) with an uncomplicated obstetric history. All women signed an informed consent form to participate in the study, which was reviewed and approved by the Research Ethics Committee of Kazan Federal University (Ref. No.3 as of 23.03.17). The inclusion criteria for the study were no personal or family history of thrombosis and no use of anticoagulants or antiplatelet drugs during pregnancy. The study was conducted before pregnancy and at different stages of pregnancy, with the total 3 to 11 blood draws and testing in each woman, and the test results were grouped into trimesters. When the results were summarized, some women were included only in the first-trimester group, some in the first- and second-trimester groups, some in the second- and third-trimester groups, etc. Thus, the groups were heterogeneous in members and considered independent (unpaired) for the statistical analysis. The clinical and genetic characteristics of the women enrolled in the study are shown in Table 1.
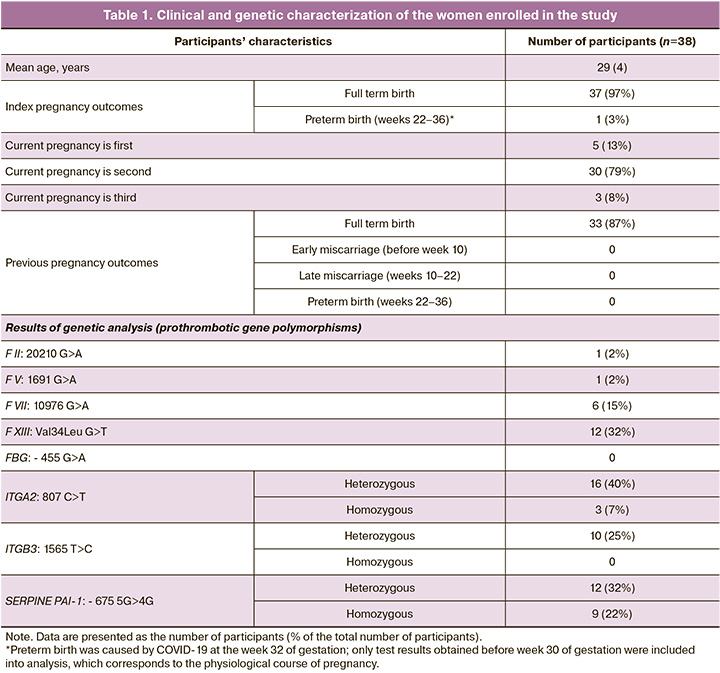
Of note is the relatively high frequency of ITGA2 19/40 (47%) and PAI-1 22/40 (55%) polymorphisms, which is consistent with previously published data on the prevalence of these polymorphisms in the population [15].
Clinical and laboratory investigations
Blood from fasting women was drawn from antecubital vein into a Vacutainer with 3.8% sodium citrate, mixed at a ratio of 9:1 by volume, and divided into a number of samples as previously described [16]. The first sample was used to study blood clot contraction. The second blood sample was centrifuged at 200g for 10 min at room temperature and platelet-rich plasma was collected and used for flow cytometry of platelets. Part of the platelet-rich plasma was re-centrifuged at 2000g for 10 min, and platelet-poor plasma was collected and used for routine hemostatic tests. Platelet-free plasma used for the thrombodynamics assay was obtained by additional centrifugation of the platelet-poor plasma for 5 min at 10,000g at room temperature. A separate blood sample was stabilized with EDTA (final concentration 1.6 mg/ml) for hematological and genetic analyses. Stabilized blood was stored and processed at room temperature and used within 4 hours after collection.
The degree of blood clot contraction was determined using an original method based on the dynamic optical registration of the size of a blood clot during shrinkage [17]. The thrombodynamics assay was performed according to a previously described protocol [18]. Both methods were performed using the T-2 Thrombodynamics Analyzer (HemaCore, Moscow). The fibrin clot formation was recorded optically every 6 s for 30 min. The resulting images were processed automatically with the calculation of the following parameters: 1) lag time, i.e., the time required for fibrin formation to begin from the moment when the plasma contacts the activating surface; 2) initial clot growth rate, i.e., the average clot growth rate calculated in the range of 2–6 min after the start of clot growth; 3) stationary clot growth rate, i.e., the average clot growth rate calculated in the range of 15–25 min after the start of clot growth; 4) the size of the fibrin clot 30 min after plasma came in contact with the activating insert; 5) optical density of the clot, i.e., the intensity of light scattering, which is directly proportional to the concentration of fibrin(ogen).
Platelets were isolated by gel filtration of platelet-rich plasma on Sepharose 2B equilibrated with Tyrode's buffer and examined within 3 hours of blood drawing. Platelet viability was approximately 97% based on the mitochondrial potential retention. Platelets were counted in a hemocytometer (Goryaev chamber) using a PrimoStar microscope (Zeiss, Germany) at a 400× magnification. The functional state of isolated platelets was assessed by flow cytometry to measure the fractions of platelets expressing P-selectin (antigen CD62p) and active integrin αIIbβ3 (determined by its ability to bind to fibrinogen) before and after activation by the peptide (TRAP-6), which activates the platelet thrombin receptor PAR1.
The state of the hemostasis system was assessed using an automated coagulometer Sysmex CA-1500 (Sysmex, Japan). The following coagulation parameters were determined: activated partial thromboplastin time (APTT), prothrombin time, thrombin time, Clauss fibrinogen concentration, and the levels of proteins S, C and antithrombin III. D-dimer levels were measured using an Immulite 2000XPi immunochemical analyzer (Siemens Healthcare Diagnostics Inc., Germany). XIIa-dependent fibrinolysis was determined using a commercial kit (NPO Renam, Russia). Thromboelastometry was performed using a ROTEM analyzer (LaRoche, France) in the native coagulation test (NATEM) by mixing a citrated whole blood sample with CaCl2, without adding clotting activators. The following parameters were determined: CT, blood clotting time; CFT, clot formation time; α, curve slope; and MCF, maximum clot strength. Hematological analysis was performed using a Sysmex XN analyzer (Sysmex, Japan). Genetic analysis was performed by real-time polymerase chain reaction using equipment and diagnostic kits from DNA Technologies (Russia).
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 8 software package. The distribution of continuous variables was tested for normality using the D'Agostino–Pearson, Kolmogorov–Smirnov, and Shapiro–Wilk tests. Numerical variables were not normally distributed; therefore, statistical differences for pairwise comparisons of blood clot contraction parameters were assessed using the nonparametric Mann–Whitney U test. For multivariate analysis of independent groups (parameters of contraction, thrombodynamics, functional state of platelets, and routine laboratory parameters), the nonparametric Kruskal–Wallis test was used, followed by pairwise comparisons using the Dunn’s test. Correlation analysis was conducted by calculating the Spearman's rank correlation coefficients. Categorical variables were compared using the chi-square test. Differences were considered statistically significant at p<0.05.
Results
Contractile function of platelets during physiological pregnancy
The contractile function of platelets is an important and relatively understudied characteristic of their functional activity [19]. In our study, the degree of shrinkage (contraction, retraction) of clots formed in blood treated with exogenous thrombin was a quantitative measure for platelet contractile function. The median degree of blood clot contraction decreases during pregnancy. The results of a pairwise comparison between the groups of patients at different stages of pregnancy, shown in Figure (A), reveal that the median degrees of blood clot contraction were significantly lower in the II and III trimesters than in the same women at the stage of pregnancy planning. The greatest difference was observed in the III trimester of pregnancy, when the median degree of contraction was 41% (36;45), which is significantly lower than the control values (45% (41;48), p=0.02) determined in the same women shortly before pregnancy. Thus, during normal pregnancy, there is a moderate but statistically significant decrease in the contractile function of platelets.
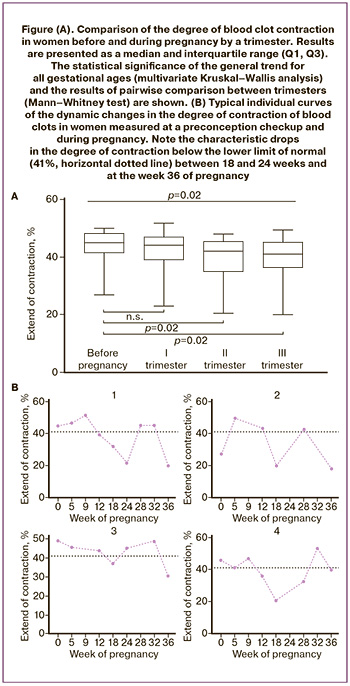
Individual changes in platelet contractile function during physiological pregnancy
Analysis of individual variations of the extent of clot contraction during pregnancy complemented substantially the averaged changes in platelet contractility. Figure (B) shows the most typical individual variations in the degree of blood clot contraction in the course of pregnancy, which reveal the following pattern: in 23/28 (82%) women examined, the degree of clot contraction decreased below the reference values (<41%) between 18 and 24 weeks of gestation, followed by normalization to the baseline (>41%). In 12/19 (63%) cases, the same patients showed another decrease in the degree of contraction at about 36 weeks of pregnancy.
The functional state of platelets, determined by the expression of activation markers
To investigate the potential relationship between the reduced degree of contraction during pregnancy and the functional state of platelets, platelet (re)activity was studied by flow cytometry to measure the expression levels of P-selectin (antigen) and active integrin αIIbβ3, which binds labeled fibrinogen. These molecular markers characterize the extent of platelet activation, which largely determines their ability to contract.
We studied both the background activity of resting platelets and their ability to respond to a biochemical stimulus, which was a peptide (TRAP-6) that activates a thrombin receptor. The absence of significant differences in the average fractions of platelets expressing P-selectin and active integrin αIIbβ3 before and during pregnancy indicates a low level of background platelet activation and preservation of their high reactivity during physiological pregnancy (Table 2).
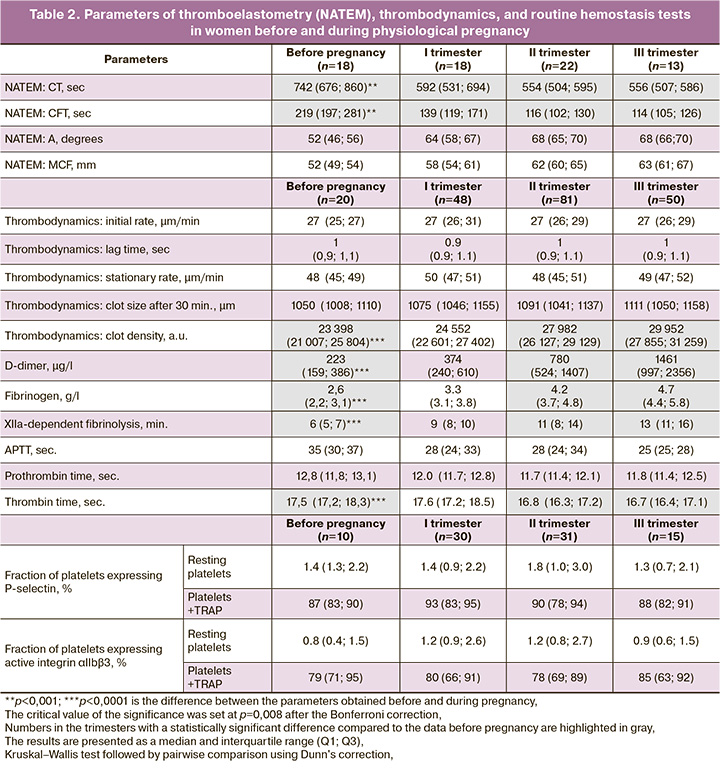
To identify the causal relationships between the parameters studied, a correlation analysis was performed, which included the main parameters presented in Table 2. Cross-correlation analysis using the Spearman method revealed the only significant correlation, namely: the reduced blood clot contraction in the third trimester was inversely related to the increased background activation of αIIbβ3 integrin (r=-0.606; 95% confidence interval (CI) -0.85; -0.14, p=0.01). This result suggests that the decrease in platelet contractile activity could originate from moderate spontaneous activation of platelets in the bloodstream, accompanied by their partial exhaustion and a decrease in their contractility [19].
Changes in hemogram
According to the hemogram, physiological pregnancy is associated with moderate anemia (a significant decrease in hematocrit, RBC count, and hemoglobin levels), most pronounced in the third trimester, which is likely due to increasing hemodilution in the course of physiological pregnancy. Unlike erythrocytes, the WBC count in the blood of pregnant women increased progressively beginning from the second trimester, reaching a level of moderate but significant leukocytosis in the third trimester, which indicates an adaptive inflammatory response. The median platelet count, including the immature platelet fraction, did not change compared to those in the preconception period and remained constant at different stages of pregnancy. At the same time, a decrease in the platelet count below 150×109/l was detected in 14/50 (7%) cases in the first trimester, in 17/81 (14%) cases in the second trimester and in 32/50 (16%) cases in the III trimester, which is consistent with the literature [5]. In the third trimester, among the women with thrombocytopenia (<150×109/l), a decrease in the degree of contraction below the normal level (41%) was observed significantly more often than in women without thrombocytopenia (p=0.026, chi-square test). This finding suggests that physiological thrombocytopenia is a possible mechanism for the decreased contraction of blood clots in the late pregnancy (see the Figure).
Changes in hemostasis according to the global tests: thromboelastometry and thrombodynamics
For an integral assessment of the state of hemostasis and its comparison with platelet function during physiological pregnancy, we studied the parameters of thromboelastometry and thrombodynamics. According to the NATEM data, progressive chronometric hypercoagulability in terms of shortened clotting time (CT) and clot formation time (CFT) was revealed, starting from the first trimester, with maximum changes in the second and third trimesters (Table 2). According to the thrombodynamics data, only the optical density of the clot increased significantly during pregnancy, starting from the second trimester, with a maximum in the third trimester, which apparently reflects the growing hyperfibrinogenemia. The kinetic parameters of thrombodynamics, such as the initial and stationary rates of clot formation along with the lag time, did not differ from the preconception period and remained unchanged during pregnancy, which corresponded to the reference values [2, 4].
Changes in routine laboratory parameters of hemostasis
According to the routine laboratory tests of hemostasis (Table 2), there were changes in the chronometric coagulation parameters characteristic of a varying extent of hypercoagulability. The fibrinogen level also increased significantly beginning from the second trimester and reaching a maximum in the third trimester. Despite a significant increase in D-dimer levels, starting from the second trimester of pregnancy with a maximum at full term, the values corresponded to the reference values [20]. An increase in the clot lysis time in the XIIa-dependent fibrinolysis assay (hypofibrinolysis) was observed starting from the second trimester with a maximum value in the third trimester. These findings indicate progressive chronometric and structural hypercoagulability during physiological pregnancy, which is consistent with the existing notions [21–23].
Discussion
To prevent complications of pregnancy, it is important to recognize the boundary between physiological and pathological changes in the hemostasis system at different stages of pregnancy. Decompensated activation of blood coagulation during pregnancy can lead to various complications ranging from miscarriage at the early stages to severe obstetric complications, such as preeclampsia or HELLP syndrome [24]. The role of platelets during physiological pregnancy is the least studied hemostatic component; therefore, this work includes not only the study of coagulation hemostasis but also a comprehensive characterization of platelets during normal pregnancy. Both traditional and relatively new methodological approaches have been used, including the assessment of platelet contractile function based on the ability to induce contraction (retraction) of blood clots.
It has been shown for the first time that a moderate but statistically significant progressive decrease in platelet contractility occurs during normal pregnancy. In the vast majority of women examined, the wave-like changes in the extent of clot contraction were observed, including a decrease below the reference values between 18 and 24 weeks of pregnancy (in 23/28 (82%) of patients) followed by a repeated decrease at 36 weeks in 12/19 (63%) cases. Remarkably, the waves of reduced platelet contractility observed during pregnancy coincide with important physiological processes, such as the end of the placentation period and the formation of the placenta at 16–18 weeks [25] as well as the prenatal readiness of the hemostasis system for blood loss with the peak of physiological hypercoagulation [4, 5]. These findings suggest a possible relationship between platelet contractile activity and local and systemic changes in the expectant mothers’ bodies.
The explanation for the reduced contraction of clots should be sought, first, in quantitative and/or qualitative changes in platelets. The observed progressive decrease in platelet counts with a maximum decrease in the third trimester is consistent with a decrease in the overall contractile potential of platelets and a decrease in the degree of blood clot contraction. However, moderate thrombocytopenia was found in a relatively small group of pregnant women (7-16% depending on gestational age), while a decrease in the completeness of blood clot contraction was detected in the vast majority of cases – 23/28 (82%). Therefore, quantitative changes in platelets can only partly explain the decrease in the extent of blood clot contraction during normal pregnancy.
As for the qualitative changes in platelets, we did not find statistically significant differences in the average expression levels of P-selectin and active integrin αIIbβ3 before and during pregnancy, which indicates the absence of an apparent background activation of platelets and the preservation of their high reactivity during physiological pregnancy. At the same time, based on a pronounced (r=-0.606; 95% CI -0.85; -0.14) and statistically significant (p=0.01) correlation between increased background activation of integrin αIIbβ3 and a decrease in the degree of contraction in pregnant women, we conclude that a moderate spontaneous activation of platelets in the bloodstream is likely, which is typically associated with the elevated expression of the active integrin αIIbβ3 [16, 26]. The probability of the background platelet activation in the bloodstream during normal pregnancy is high because of progressive physiological hypercoagulability, which is always accompanied by more or less pronounced thrombinemia, which is a pre-requisite for platelet activation. In our study, the physiological hypercoagulability is evidenced by coagulation parameters, such as an increase in fibrinogen and D-dimer levels with simultaneous hypofibrinolysis, and corresponding changes in the thromboelastometry parameters (Table 2). These results are consistent with the published data, in which the progressive hypercoagulability during physiological pregnancy was detected by both thrombodynamics [2] and thromboelastographic [3] assays. Taken together, our data show that during healthy pregnancy platelet functionality does not exhibit pronounced changes. However, in some pregnant women, background activation and subsequent exhaustion of platelets in combination with moderate thrombocytopenia may occur, which is accompanied by a decrease in the contractile potential of platelets and a decrease in the degree of blood clot contraction. Hyperfibrinogenemia can also contribute to the inhibition of blood clot contraction because the blood fibrinogen concentration is inversely related to the extent and rate of clot contraction [27].
In addition to the well-known hemostatic functions, there is growing evidence for a less obvious role for platelets in the regulation of implantation and remodeling of spiral arteries, placentation, angiogenesis, and maintenance of placental hemoperfusion [7, 14, 28]. From the beginning of implantation, the embryo modifies the endometrium, including reactions of aseptic inflammation, which is one of the mechanisms of placentation [29]. Activated platelets and procoagulant cellular microvesicles are known to mediate blood coagulation and inflammation. Microvesicles have been shown to cause aseptic inflammation during placentation by activating maternal platelets [30]. In the light of the important role of inflammation in the processes of implantation and placentation, changes in the platelet contractility may be of particular importance, as the inflammation, including aseptic inflammation, has been shown to be associated with impaired blood clot contraction [19]. From a more general standpoint, changes in the contractility of platelets representing their integral functional state may reflect the possibility of platelets being an effector element of innate immunity. Therefore, the mechanical platelet contraction may be a cellular response and/or an adaptive mechanism unrelated to the platelet hemostatic function(s).
Conclusions
Normal pregnancy is characterized by a moderate decrease in platelet contractility as revealed by progressive impairment of blood clot contraction. Changes in clot contraction are somewhat associated with the physiological thrombocytopenia and partial background platelet activation, resulting from physiological hypercoagulability. The peak of changes in the platelet contractile activity during pregnancy coincides with the end of placentation, suggesting a possible connection between platelets and local intraplacental processes, suggesting importance of non-hemostatic platelet function(s) during normal pregnancy. These findings expand and deepen the concept of pregnancy as a physiological process involving both plasma coagulation and platelet components of hemostasis. This study also highlights the practical importance of incorporating the in vitro clot contraction assay in a set of laboratory tests for a comprehensive assessment of hemostatic status and monitoring the course of pregnancy.
References
- Holmes V.A., Wallace J.M. Haemostasis in normal pregnancy: a balancing act? Biochem. Soc. Trans. 2005; 33(Pt 2): 428-32. https://dx.doi.org/10.1042/BST0330428.
- Ворошилина Е.С., Овсепян Р.А., Плотко Е.Э., Герасимова О.Б., Баскова О.Ю., Будыкина Т.С., Вуймо Т.А. Диапазоны нормальных значений для параметров стандартных коагулологических тестов и теста тромбодинамики при физиологической беременности на разных сроках гестации. Вестник РГМУ. 2015; 4: 40-5. [Voroshilina E.S., Ovsepyan R.A., Plotko E.E., Gerasimova O.B., Baskova O.Yu., Budykina T.S., Vuimo T.A. Reference ranges for standart coagulation tests and thrombodynamics assay during normal pregnancy at various gestational ages. Bulletin of RSMU. 2015; (4): 40-5.(in Russian)].
- Antony K.M., Mansouri R., Arndt M., Rocky Hui S.K., Jariwala P., Mcmullen V.M. et al. Establishing thromboelastography with platelet-function analyzer reference ranges and other measures in healthy term pregnant women. Am. J. Perinatol. 2015; 32(6): 545-54. https://dx.doi.org/10.1055/s-0034-1396700.
- Ataullakhanov F.I., Koltsova E.M., Balandina A.N., Serebriyskiy I.I., Vuimo T.A., Panteleev M.A. Classic and global hemostasis testing in pregnancy and during pregnancy complications. Semin. Thromb. Hemost. 2016; 42(7): 696-716. https://dx.doi.org/10.1055/s-0036-1592303.
- Reese J.A., Peck J.D., Deschamps D.R., McIntosh J.J., Knudtson E.J., Terrell D.R. et. al. Platelet counts during pregnancy. N. Engl. J. Med. 2018; 379(1): 32-43. https://dx.doi.org/10.1056/NEJMoa1802897.
- Costantine M.M. Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 2014; 5: 65. https://dx.doi.org/10.3389/fphar.2014.00065.
- Abduljalil K., Furness P., Johnson T.N., Rostami-Hodjegan A., Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin. Pharmacokinet. 2012; 51(6): 365-96.https://dx.doi.org/10.2165/11597440-000000000-00000.
- Janes S.L. Thrombocytopenia in pregnancy. Postgrad. Med. J. 1992; 68(799): 321-6. https://dx.doi.org/10.1136/pgmj.68.799.321.
- Burke N., Flood K., Murray A., Cotter B., Dempsey M., Fay L. et al. Platelet reactivity changes significantly throughout all trimesters of pregnancy compared with the nonpregnant state: a prospective study. BJOG. 2013; 120(13): 1599-604. https://dx.doi.org/10.1111/1471-0528.12394.
- Sheu J.R., Hsiao G., Lin W.Y., Chen T.F., Chien Y.Y., Lin C.H., Tzeng C.R. Mechanisms involved in agonist induced hyperaggregability of platelets from normal pregnancy. J. Biomed. Sci. 2002; 9(1): 17-25.https://dx.doi.org/10.1007/BF02256574.
- Gatti L., Tenconi P.M., Guarneri D., Bertulessi C., Ossola M.W., Bosco P., Gianotti G.A. Hemostatic parameters and platelet activation by flow-cytometry in normal pregnancy: a longitudinal study. Int. J. Clin. Lab. Res. 1994; 24(4): 217-9. https://dx.doi.org/10.1007/BF02592466.
- Valera M.C., Parant O., Vayssiere C., Arnal J.F., Payrastre B. Physiologic and pathologic changes of platelets in pregnancy. Platelets. 2010; 21(8): 587-95. https://dx.doi.org/10.3109/09537104.2010.509828.
- Bódis J., Papp S., Vermes I., Sulyok E., Tamás P., Farkas B. et al. "Platelet-associated regulatory system (PARS)" with particular reference to female reproduction. J. Ovarian Res. 2014; 7: 55. https://dx.doi.org/10.1186/1757-2215-7-55.
- Al Obaidly M., Regan C., Lwaleed B., Moran N. A role for platelets in normal pregnancy. The non-thrombotic role of platelets in health and disease. InTech, November 18th 2015. https://dx.doi.org/5772/61210.
- Сафиуллина С.И., Котова Я.Н., Ворошилина Е.С., Илизарова Н.А., Ягудина Л.Ш., Вуймо Т.А. Сочетание полиморфизмов генов PAI–1-675 5G>4G, FXIII: Val34Leu C>T и ITGA2: 807 С>T у женщин с бесплодием может рассматриваться как предиктор неудач вспомогательных репродуктивных технологий. Тромбоз, гемостаз, реология. 2019; 2: 57-62. [Safiullina S.I., Kotova Ya.N., Voroshilina E.S., Ilizarova N.A., Yagudina L.Sh., Vujmo T.A. The combination of polymorphisms of genes PAI-1 –675 5G > 4G, FXIII: Val34Leu G > T and ITGA2: 807 C > T in women with infertility can be considered as a predictor of failures of assisted reproductive technologies. Thrombosis, Hemostasis and Rheology. 2019; (2): 57-62. (in Russian)].https://dx.doi.org/10.25555/THR.2019.2.0881.
- Пешкова А.Д., Сафиуллина С.И., Асарова Д.Г., Хафизова А.Ф., Атауллаханов Ф.И., Литвинов Р.И. Изменения гемостаза по данным тестов тромбодинамики и контракции сгустков крови у женщин с привычным невынашиванием беременности в анамнезе. Акушерство и гинекология. 2019; 12: 111-9. [Peshkova A.D., Safiullina S.I., Asarova D.G., Khafizova A.F., Ataullakhanov F.I., Litvinov R.I. Assessment of hemostatic function in women with a history of recurrent pregnancy loss using thrombodynamics and blood clot contraction tests. Obstetrics and Gynecology. 2019; (12): 111-9. (in Russian)]. https://dx.doi.org/10.18565/aig.2019.12.111-119.
- Ложкин А.П., Пешкова А.Д., Атауллаханов Ф.И., Литвинов Р.И. Разработка и применения нового метода контракции (ретракции) сгустка крови. Гены & Клетки. 2014; 9(3): 99-104. [Lozhkin A.P., Peshkova A.D., Ataullakhanov F.I., Litvinov R.I. Development and applications of a new technique to study blood clot contraction (retraction). Genes & Cells. 2014; 9(3): 99-104. (in Russian)].
- Sinauridze E.I., Vuimo T.A., Tarandovskiy I.D., Ovsepyan R.A., Surov S.S., Korotina N.G. et al. Thrombodynamics, a new global coagulation test: Measurement of heparin efficiency. Talanta. 2018; 180: 282-91.https://dx.doi.org/10.1016/j.talanta.2017.12.055.
- Litvinov R.I., Weisel J.W. Blood clot contraction: mechanisms, pathophysiology, and disease. Res. Pract. Thromb. Haemost. 2023; 7(1): 100023.https://dx.doi.org/10.1016/j.rpth.2022.100023.
- Siennicka A., Kłysz M., Chełstowski K., Tabaczniuk A., Marcinowska Z., Tarnowska P. et al. Reference values of D-dimers and fibrinogen in the course of physiological pregnancy: the potential impact of selected risk factors - A pilot study. Biomed. Res. Int. 2020; 2020: 3192350.https://dx.doi.org/10.1155/2020/3192350.
- Bhave A.A. Coagulopathies in pregnancy: what an obstetrician ought to know! J. Obstet. Gynaecol. India. 2019; 69(6): 479-82. https://dx.doi.org/10.1007/s13224-019-01290-8.
- Ronenson A.M., Shifman E.M., Kulikov A.V., Raspopin Y.S., Görlinger K., Ioscovich A.M. et al. Rotational thromboelastometry reference range during pregnancy, labor and postpartum period: A systematic review with meta-analysis. J. Obstet. Anaesth. Crit. Care. 2022; 12: 105-15.https://dx.doi.org/10.4103/JOACC.JOACC_21_22.
- Chan W.S., Lee A., Spencer F.A., Chunilal S., Crowther M., Wu W. et al. D-dimer testing in pregnant patients: towards determining the next 'level' in the diagnosis of deep vein thrombosis. J. Thromb. Haemost. 2010; 8(5): 1004-11.https://dx.doi.org/10.1111/j.1538-7836.2010.03783.x.
- Bhutani N., Jethani V., Jethani S., Ratwani K. Coagulation profile and platelet parameters in pregnancy induced hypertension cases and normotensive pregnancies: A cross-sectional study. Ann. Med. Surg. (Lond). 2022; 80: 104124. https://dx.doi.org/10.1016/j.amsu.2022.104124.
- Радзинский В.Е., Оразмурадов А.А., ред. Беременность ранних сроков. От прегравидарной подготовки к здоровой гестации. М.: StatusPraesens; 2018. 800с. [Radzinsky V.E., Orazmuradov A.A., ed. Early pregnancy. From pregravidar preparation to healthy gestation. Moscow: StatusPraesens; 2018. 800p. (in Russian)].
- Peshkova A.D., Safiullina S.I., Evtugina N.G., Baras Y.S., Ataullakhanov F.I., Weisel J.W. et al. Premorbid hemostasis in women with a history of pregnancy loss. Thromb. Haemost. 2019; 119(12): 1994-2004. https://dx.doi.org/10.1055/s-0039-1696972.
- Пешкова А.Д., Ложкин А.П., Фатхуллина Л.С., Малясёв Д.В., Бредихин Р.А., Литвинов Р.И. Зависимость контракции (ретракции) сгустка от молекулярного и клеточного состава крови. Казанский медицинский журнал. 2016; 97(1): 70-7. [Peshkova A.D., Lozhkin A.P., Fathullina L.S., Malyasev D.V., Bredikhin R.A., Litvinov R.I. Dependence of clot contraction (retraction) on the molecular and cellular blood composition. Kazan Medical Journal. 2016; 97(1): 70-7. (in Russian)]. https://dx.doi.org/10.17750/KMJ2016-70.
- Church B., Wall E., Webb J.R., Cameron C.E. Interaction of treponema pallidum, the syphilis spirochete, with human platelets. PLoS One. 2019; 14(1): e0210902. https://dx.doi.org/10.1371/journal.pone.0210902.
- Griffitha O.W., Chavan A.R., Protopapas S., Maziarz J., Romero R., Wagner G.P. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl. Acad. Sci. USA. 2017; 114(32): E6566-75. https://dx.doi.org/10.1073/pnas.1701129114.
- Kohli S., Isermann B. Placental hemostasis and sterile inflammation: New insights into gestational vascular disease. Thromb. Res. 2017;151(Suppl. 1):S30-3. https://dx.doi.org/10.1016/S0049-3848(17)30063-4.
Received 24.05.2023
Accepted 07.06.2023
About the Authors
Svetlana I. Safiullina, M.D. Ph.D., Associate Professor, Department of Internal Diseases, Institute of Fundamental Medicine and Biology, Kazan Federal University,Svetlana.ild.safiullina@gmail.com, https://orcid.org/0000-0003-4657-0140, 420012, 76 Karl Marx str., Kazan, Russian Federation,
420081, 8а Kyrskaya str., Kazan, Russian Federation.
Natalia G. Evtugina, PhD student, Department of Biochemistry and Biotechnology, Institute of Fundamental Medicine and Biology, Kazan Federal University,
natalja.evtugyna@gmail.com, https://orcid.org/0000-0002-4950-3691, 420008, 18 Kremlevskaya str., Kazan, Russian Federation.
Alina D. Peshkova, PhD, Senior Teacher, Department of Biochemistry and Biotechnology, Institute of Fundamental Medicine and Biology, Kazan Federal University, alinapeshkova26@gmail.com, https://orcid.org/0000-0002-8790-1818, 420008, 18 Kremlevskaya str., Kazan, Russian Federation.
Rustem I. Litvinov, M.D., Ph.D., Dr. Sci., Senior Research Investigator, Department of Cell and Developmental Biology, University of Pennsylvania School of Medicine,
421 Curie Blvd., BRB II/III, Room 1116, Philadelphia, PA 19104-6058, USA, rustempa@gmail.com, https://orcid.org/0000-0003-0643-1496
Corresponding author: Svetlana I. Safiullina, svetlana.ild.safiullina@gmail.com



