Time to pregnancy after pre-gestational allogeneic immunotherapy and peripheral blood cell cytokine profile in women with a history of recurrent spontaneous abortion
An optimal ratio of inflammatory (Th1) and anti-inflammatory (Th2) immune responses is required for successful embryo implantation and subsequent fetal development. Allogenic lymphocyte immunization also referred to as cell immunotherapy (CIT) before conception and in the early stages of pregnancy, effectively corrects immunoregulation in women with recurrent spontaneous abortion. However, the time to pregnancy (TTP) after pre-gestational CIT varies significantly.Nikolaeva M.A., Arefieva A.S., Stepanova E.O., Golubeva E.L., Vtorushina V.V., Tetruashvili N.K., Krechetova L.V.
Aim. To investigate the relationship between Th1/Th2 cytokine balance after pre-gestational CIT and the TTP in women with a history of recurrent spontaneous abortion.
Materials and methods. The study included 37 women who underwent CIT as monotherapy. TTP was defined as the duration of time expressed in the number of menstrual cycles from contraception discontinuation until conception occurred. Production of pro-inflammatory (IFN-γ, IL-2, IL-6, IL-8, IL-12, IL-18, TNF-α, IL-1β) and anti-inflammatory (IL-4, IL-5, IL-10) cytokines after mitogen-induced activation of maternal peripheral blood cells were analyzed by flow cytometry using FlowCytomix ™ kits (eBioscience).
Results. In 22 (59.5%) women, pregnancy occurred within six menstrual cycles, and five (13.5%) women conceived between 7 to 12 menstrual cycles. Ten women (27%) failed to achieve pregnancy within 12 menstrual cycles. Compared to women with TTP ≤ 6 menstrual cycles, those with 6 < ТТР ≤ 12 menstrual cycles and who was unable to conceive had reduced Th1/Th2 cytokine ratio.
Conclusion. The TTP after allogeneic immunotherapy may depend on the Th1/Th2 cytokine ratio.
Keywords
The most critical condition for the successful pregnancy progression is the maternal immune system's capacity to prevent immune response against the fetus carrying paternal antigens. One of the immune tolerance induction methods widely used in organ transplantation is pre-transplant recipient immunization with donor transplantation antigens. Based on the positive experience of allogenic immunization in transplantology, it has been proposed to use immunization with leukocytes from a woman partner, also referred to as cell immunotherapy (CIT), to prevent pregnancy losses in patients with a history of recurrent spontaneous abortion [1–4].
The cytokine profile of immunocompetent cells plays a fundamental role in providing immune tolerance to the developing fetus. Many studies have shown that impaired immune tolerance and the risk of graft rejection are associated with inflammatory reactions accompanied by increased production of proinflammatory cytokines [5–7]. The functional state of T helper cells (Th) determines the pro- or anti-inflammatory direction of immune responses. T-helper cells of the first type (Th1), producing the cytokines interferon (IFN) -γ, interleukin (IL) -2 and tumor necrosis factor (TNF) -α, stimulate inflammatory reactions. T helper cells of the second type (Th2), which produce IL-10, IL-4, and IL-5, prevent inflammation. Physiological pregnancy develops against the background of anti-inflammatory Th2 reactions [5, 8–10]. Several studies have shown that CIT's immunomodulatory effect is associated with the Th2-polarization of the immune response, making it possible to consider this method of preventing recurrent miscarriage as a reasonable and practical approach [11–14]. It was assumed that multiple pre-gestational immunizations could improve the anti-inflammatory background during pregnancy.
However, current evidence suggests that implantation and the early stages of the first trimester of physiological pregnancy occur against the background of pro-inflammatory Th1 reactions. Simultaneously, an anti-inflammatory Th2 environment is formed at later pregnancy stages [10, 15–17].
Therefore, the development of excessive anti-inflammatory reactions after CIT may prevent pregnancy. The optimal therapeutic effect of CIT can be observed only with “fine-tuning” of Th1/Th2 balance, ensuring the predominance of the pro-inflammatory environment during implantation and in the early stages of pregnancy and its timely transition to the anti-inflammatory environment at later stages.
Therefore, the Th1/Th2 balance assessment during pre-gestational immunotherapy and pregnancy timing is of particular relevance. Examining factors influencing implantation and early pregnancy often involves determining the time from attempting to conceive until the onset of pregnancy (TTP, time to pregnancy) is widely used [18–22]. We assumed that pregnancy occurring during the first six months after pre-gestational CIT is associated with the timely development of balanced Th1/Th2 reactions. TTP longer than six months may be related to the dominance of Th2 responses. Therefore, TTP can be an indicator reflecting the features of the Th1/Th2 balance during CIT.
The present study aimed to investigate the relationship between Th1/Th2 cytokine balance after pre-gestational CIT and the TTP in women with a history of recurrent spontaneous abortion.
Materials and methods
The study included 37 women with recurrent spontaneous abortion (ICD-10 N96). The inclusion criteria were woman's age from 20 to 40, spontaneous loss of two or more clinical pregnancies before 20 completed weeks of gestational age with the same partner. There must be a normal karyotype of abortus and partners, normozoospermia in the partner, spontaneous onset of previous pregnancy, the absence of anatomical, autoimmune, hormonal disorders, acute pelvic inflammatory diseases, signed informed consent to participate in the study.
CIT was administered to all women before a planned pregnancy as monotherapy. The CIT procedure was performed using the spouse's lymphocytes in the setting of antenatal care in the middle of the follicular phase of two consecutive menstrual cycles and during the onset of pregnancy at 5–6 weeks and 8–9 weeks. The CIT methodology was approved by the Academic Council of the V.I. Kulakov NMRC for OG&P, Ministry of Health of Russia (protocol No. 19 dated December 25, 2012).
Blood samples were drawn from the antecubital vein into a sterile disposable plastic 50 ml tube with 200 μl of heparin solution as an anticoagulant (the concentration of heparin solution was 5000 IU/ ml). Blood samples were incubated at 37°C for 1–1.5 hours. After the separation of blood into two layers, the upper layer was transferred into a sterile centrifuge tube and centrifuged. After removing the supernatant, the residue was thoroughly mixed in distilled water to ensure osmotic lysis and removal of erythrocytes. Then dibasic sodium phosphate buffer was added. After washing, the isolated cells were resuspended in 2 ml of sterile saline, and the concentration and viability of leukocytes were assessed (trypan blue test) using a hemocytometer. Cell viability was at least 95%. The suspension containing 50×106 leukocyte cells was injected intradermally into the forearm's palmar surface (10–12 points, 0.2 ml each).
During antenatal care, barrier contraception was recommended. From the third menstrual cycle, contraception was discontinued. The onset of pregnancy was recorded during 12 subsequent menstrual cycles. TTP was defined as the duration of time expressed in the number of menstrual cycles from the first cycle after contraception discontinuation until conception occurred.
Production of pro-inflammatory (IFN-γ, IL-2, IL-6, IL-8, IL-12, IL-18, TNF-α, IL-1β) and anti-inflammatory (IL-4, IL-5, IL-10) cytokines after mitogen-induced activation of female whole blood cells using Cytokin-Stimul-Best kits (Vector-Best) were assessed by flow cytometry using multiplex analysis with standard FlowCytomix ™ kits (eBioscience). Cytokine analysis was performed on blood samples obtained two weeks after the second immunization.
Statistical analysis
Statistical analysis was performed using the MedCalc v12.3.0 software. The distribution of continuous variables was tested for normality using the Shapiro-Wilk test. Numerical variables that were not normally distributed were summarized as medians and maximum and minimum values. The statistical significance of differences between two independent groups with not normally distributed continuous variables was tested with the Mann–Whitney test. Categorical variables were compared by the χ2-test. Differences were considered significant at p<0.05. ROC (Receiver Operating Characteristics) curves were constructed to define the sensitivity and specificity of cytokines level measurements for predicting TTP after CIT. Time to event data was calculated using the Kaplan–Meier method with the log-rank test for curves comparisons.
 Results
Results
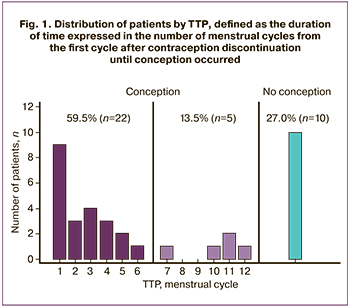
Of the 37 women included in the study, 22 (59.5%) women conceived within six menstrual cycles, and in five (13.5%) women, pregnancy occurred between 7 to 12 menstrual cycles. Ten women (27%) failed to achieve pregnancy within 12 menstrual cycles (Fig. 1). Twenty three out of 27 (85.2%) women gave birth at full-term to live children. Two women (7.4%) had missed miscarriages at 6–8 weeks, and another two (7.4%) at 14–16 weeks.
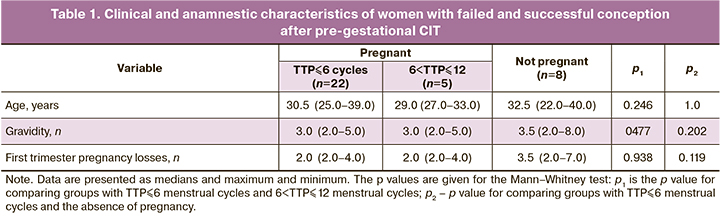
Based on TTP analysis, patients were divided into three comparison groups, including a group with TTP not exceeding six cycles, a group with 6 <TTP ≤ 12 cycles, and a group of patients who failed to achieve pregnancy within 12 cycles. At the same time, patients with failed and successful conception after CIT did not differ significantly regarding clinical and anamnestic characteristics (Table 1).
The study findings allowed for assessing the relationship between TTP and pregnancy outcome. Among women with a successful treatment outcome (full-term pregnancy), the mean time to pregnancy was two menstrual cycles and more than eight menstrual cycles among women with first-trimester pregnancy loss (p=0.007). In women with TTP> 6 menstrual cycles, pregnancy losses rate was significantly higher than in the group with TTP ≤ 6 menstrual cycles amounting to 60.0% versus 4.7%, respectively (p=0.002).
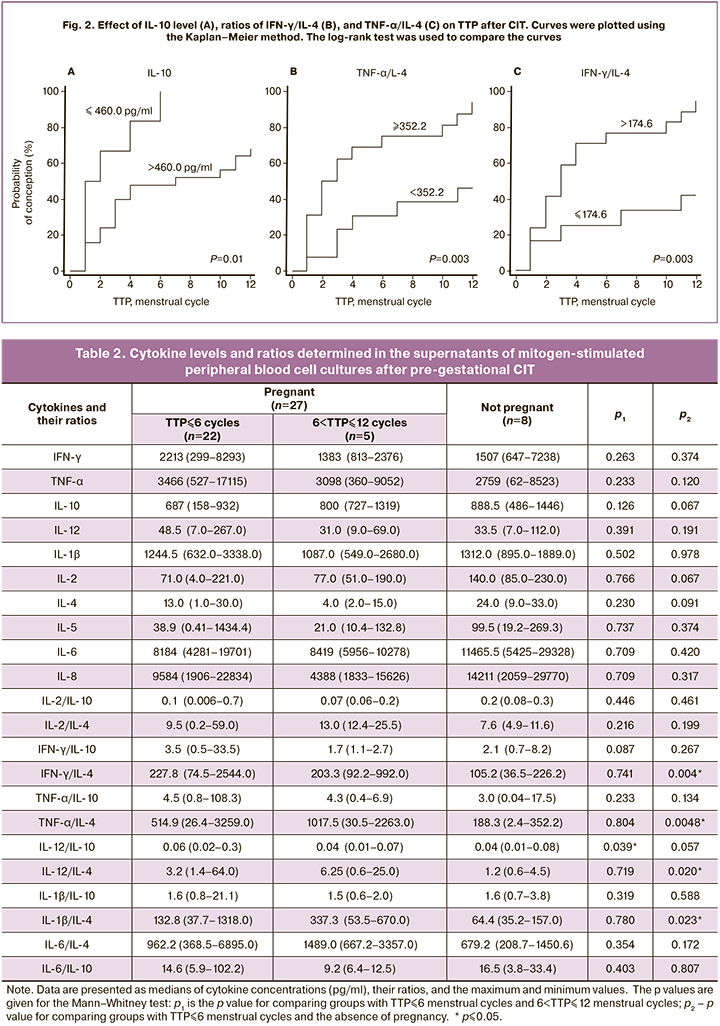
A retrospective analysis revealed no TTP dependence on the production of peripheral blood cell cytokines in patients after double immunization (Table 2). However, there was a decrease in the IL-12/IL-10 ratio and a pronounced tendency to the IFN-γ/IL-10 ratio reduction in women with 6 <TTP ≤ 12 menstrual cycles compared to women with TTP ≤ 6 menstrual cycles. (p=0.039 and p=0.087, respectively). A decrease in the Th1/Th2 balance was also revealed in the group with failed pregnancy compared with women with TTP ≤ 6 menstrual cycles. IFN-γ/IL-4, TNF-α/IL-4, IL-12/ IL4, and IL-1β/IL-4 ratios in the group with 6 <TTP ≤ 12 menstrual cycles were significantly lower than in the group with TTP ≤ 6 menstrual cycles (p=0.004, p=0.005, p=0.020 and p=0.023, respectively). There was also a pronounced tendency towards a decrease in the IFN-γ/IL-10 ratio among women with failed conception compared with the group with TTP ≤ 6 menstrual cycles.
We compared cytokines' production in patients with TTP ≤ 6 menstrual cycles and the combined group, including the group with 6<TTP ≤ 12 menstrual cycles and a group with no pregnancy within 12 menstrual cycles. These findings allowed assessing the cytokine profile's prognostic significance for predicting pregnancy onset within six menstrual cycles. Using RОС analysis showed the high significance of assessing cytokine IL-10 concentration and ratios of IFN-γ/ IL-4, TNF-α/IL-4, and IL-12/IL-10 for predicting pregnancy (Table 3).
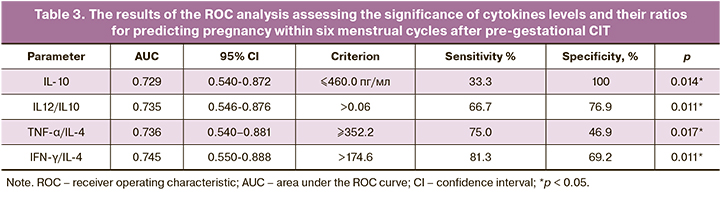
We analyzed the relationship between the ТТР after CIT and the level of prognostically significant variables (Fig. 2). Both the concentration of the cytokine IL-10 and the ratio of IFN-γ/IL-4 and TNF-α/IL-4 were found to affect TTP significantly (p=0.01, p=0.003 и р=0.003).
Discussion
The study findings confirmed that CIT is a highly effective approach to treat a recurrent miscarriage. However, pregnancy did not occur within a year after pre-gestational immunization in 27% of patients with a history of recurrent miscarriage. This is consistent with the literature reporting pregnancy rates from 50.5% [4] to 84.8% [14] in patients undergoing CIT.
The current literature is lacking sufficient coverage of the association between CIT and TTP. Most studies on CIT for the treatment of recurrent spontaneous abortion have followed the protocol proposed by J.F. Mowbray (1985) [4], suggesting one injection of lymphocytes before and after pregnancy onset. The authors did not find the effect of pre-gestational immunization on TTP. Still, they did demonstrate an increase in the percentage of full-term pregnancies among women with recurrent miscarriage after CIT compared with placebo. After 1985, CIT has been widely used to treat miscarriage, but the immunization protocols were significantly different from those proposed in J.F. Mowbray. The number of pre-gestational immunizations varied, and in some cases, reached 8 [23]; however, only a few studies compared TTP in CIT and placebo groups, and none since 1985 analyzed TTP.
Our study's design provided a unique opportunity for an objective assessment of TTP associated with a high motivation of the study participants to treat recurrent miscarriage and immediately visit a doctor in case of pregnancy onset. Thus, our approach to assessing TTP differs from the previously used subjective methods of evaluating this parameter using questionnaire surveys [21, 22].
Our findings suggest that the Th1/Th2 ratio can significantly affect the formation of the immunity environment necessary to achieve pregnancy after pre-gestational CIT. It was found that an increase in the pro-inflammatory background is favorably associated with the onset of pregnancy, which is consistent with the literature.
It cannot be ruled out that in some cases, CIT may lead to reversible suppression of the Th1 inflammatory background, leading to impaired implantation. The onset of pregnancy occurs with a weakening of anti-inflammatory reactions. The TTP is determined by the time required to normalize the Th1/Th2 imbalance resulting from pre-gestational immunization.
These assumptions are consistent with previously published data that one of the mechanisms of immunological tolerance is anergy (non-responsiveness) of Th1 and Th2 cells, which manifests itself in inhibition of Th1 cell activity [24].
In the presented work, a comparison was made of immunological parameters in groups of patients with pregnancy occurring within six menstrual cycles after immunization (TTP≤6 menstrual cycles) and in the absence of pregnancy within 12 menstrual cycles. Earlier, a multicenter study conducted in 5 European countries found that in 62–79% of fertile women, pregnancy occurs within six menstrual cycles and 80–85% of women within 12 menstrual cycles [25]. Therefore, it is highly probable that our data on the features of the preimplantation immunological background in the group of women with TTP ≤ 6 menstrual cycles and full-term pregnancy can match the characteristics of the immune system's state during the physiological onset of pregnancy in fertile women. At the same time, among patients with the onset of pregnancy at six or more months after CIT, as well as the group without pregnancy, differ in immunological parameters from the group with TTP ≤ 6 menstrual cycles. Moreover, the ratio of Th1/Th2 cytokines in both groups is lower than in the group with TTP ≤ 6 menstrual cycles
Our data allowed us to assess the significance of determining the cytokine profile for predicting the onset of pregnancy within six menstrual cycles. It was found that the assessment of IL-10 production and IL-12/IL-10, TNF-α/IL-4 and IFN-γ/IL-4 ratios can be used to predict the onset of pregnancy within six menstrual cycles. It was previously shown that in 81.7% of women, implantation disorders occur against the background of changes in the cytokine profile of endometrial lymphocytes [26]. According to modern concepts, immune cell migration significantly contributes to forming the preimplantation immune profile of the endometrium [27]. Therefore, it cannot be ruled out that cytokine production by peripheral blood cells after pre-gestational CIT may determine the nature of preimplantation immunological changes in the endometrium.
The dependence of pregnancy onset on cytokine production was most pronounced in the cycle of conception. Therefore, the cytokine profile assessment in each of the menstrual cycles preceding the cycle of conception could make a significant contribution to understanding the mechanisms of immunoregulation of embryo implantation. The lack of such an assessment is one of the limitations of the presented study.
The high level of proinflammatory reactions among patients with TTP ≤ 6 menstrual cycles and its decrease in groups with delayed onset of pregnancy and with no pregnancy is consistent with the modern concept that an increase in the proinflammatory immune responses ensures endometrial receptivity during the implantation window [10]. The proinflammatory status of immunocompetent cells present in the endometrium after successful implantation has been confirmed in numerous studies [7, 15, 28, 29]. For example, Saito showed that the ratio of Th1/Th2 cells in the endometrium during the secretory phase was significantly higher than that in peripheral blood cells, which indicates the formation of a local proinflammatory background before implantation [27].
The positive association between pregnancy rate and the increase in the endometrial TNF-α concentration confirms the significance of Th1 reactions during implantation [30]. It has been found that mechanical damage to the endometrium during biopsy stimulates the production of proinflammatory cytokines, including TNF-α, and thus increases pregnancy rates in the IVF program [31]. It has been found that IFN-γ, TNF-α, and IL-1β stimulate the production by endometrial stromal and epithelial cells of RANTES. This chemokine plays a crucial role in enhancing the migration of immunocompetent cells into the endometrium. At the same time, IL-4 suppresses RANTES production induced by TNF-α [32, 33]. Several studies have reported that an increase in the production of TNF-α and IFN-γ is necessary for the onset of pregnancy since it also activates uterine NK cells playing an essential role in angiogenesis and placentation [34]. A high level of TNFα-mRNA was found during the implantation window [35].
The mechanisms underlying changes in the immune system after CIT remain unclear and require further study. It cannot be ruled out that both the subpopulation composition of lymphocytes present in the leukocyte fraction used for immunization [36] and differences in the intensity of reactions to CIT associated with the immune system's characteristics can have a significant effect on the formation of the pre-gestational immunological environment. Therefore, it cannot be asserted with certainty that the identified features of immunity are due to the CIT procedure. The formation of secondary infertility in women with miscarriage can be due to several reasons. They include complications of surgical interventions resulting in endometrial damage and immunomodulatory effects of antibiotics that inhibit the production of proinflammatory cytokines [37] and other factors. In our work, there was no association between women's age and the cytokine profile of peripheral blood cells, and the timing of pregnancy. Sufficiently powered prospective studies assigning women to groups according to age are needed. The causes of reproductive dysfunction in women with a history of recurrent miscarriage require further research.
The study findings also offer insights into the relationship between TTP and pregnancy outcome. In women with TTP > 6 menstrual cycles, the rate of miscarriages was significantly higher than in the group with TTP ≤ 6 menstrual cycles. These observations are consistent with the modern concept that an increase in TTP is a risk factor for some reproductive disorders. A positive correlation was previously found between TTP and the incidence of ectopic pregnancy, miscarriage, and preterm birth [18]. There has also been a correlation between TTP and the risk of neonatal complications [38] and even neonatal mortality risk [39].
Therefore, immune dysfunction, which determines an increase in TTP, can be a pathogenetic factor for various reproductive pathologies. Investigation of mechanisms underlying the late onset of pregnancy in the presence of CIT can help understand the role of immune factors in reproductive physiology and pathology and select optimal strategies to correct immunological abnormalities.
Conclusion
Our results indicate that the therapeutic effect of double pre-gestational CIT can be achieved only with an optimal balance of Th1/Th2 cytokines and pro-inflammatory background during implantation. Assessment of the cytokine profile of peripheral blood cells helps predict pregnancy within six menstrual cycles after double allogeneic immunization. An individualized selection of optimal allogeneic immunization schemes based on monitoring changes in patients' peripheral blood cells' cytokine profile during CIT may improve recurrent spontaneous abortion treatment results.
References
- Taylor C., Faulk W.P. Prevention of recurrent abortions with leukocyte transfusions. Lancet. 1981; 29(8237): 68-70. https://dx.doi.org/10.1016/s0140-6736(81)90413-x.
- Патент CCCР SU925346A1, МПК А61К 39/00 (2006.01). Говалло В.И. Способ профилактики самопроизвольных выкидышей. Говалло В.И.; заявитель и патентообладатель Центральный ордена Трудового Красного Знамени научно-исследовательский институт травматологии и ортопедии им. Н.И. Приорова. № 2950719/28-13; Заявка: 04.07.1980; Опубликовано: 07.05.1982; Бюллетень № 17. 2с. [USSR patent SU925346A1, IPC A61K 39/00 (2006.01). Method of prevention of spontaneous miscarriages / Govallo V.I.;applicant and patent holder of the Central Order of the Red Banner of Labor Research Institute of Traumatology and Orthopedics named after N.I. Priorov. No. 2950719/28-13; declared on 04.07.1980; published on 07.05.1982; Bull. No. 17. 2 p. (in Russian)].
- Говалло В.И., Сидельникова В.М. Иммунизация беременных женщин аллогенными лимфоцитами мужа как метод профилактики самопроизвольных выкидышей. Акушерство и гинекология. 1983; 59(12): 25-8. [Govallo V.I., Sidelnikova V.M. Immunization of pregnant women with allogeneic lymphocytes of the husband as a method of prevention of spontaneous miscarriages. Obstetrics and gynecology. 1983; 59(12): 25-8. (in Russian)].
- Mowbray J.F., Gibbings C., Liddell H., Reginald P.W., Underwood J.L., Beard R.W. Controlled trial of treatment of recurrent spontaneous abortion by immunization with paternal cells. Lancet. 1985; 1(8435): 941-3. https://dx.doi.org/10.1016/s0140-6736(85)91723-4.
- Wegmann T.G., Lin H., Guilbert L., Mosmann T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today. 1993; 14(7): 353-6. https://dx.doi.org/ 10.1016/0167-5699(93)90235-D.
- Raghupathy R. Th-1 type immunity is incompatible with successful pregnancy. Immunol. Today. 1997; 18(10): 478-82. https://dx.doi.org/ 10.1016/s0167-5699(97)01127-4.
- Dekel N., Gnainsky Y., Granot I., Mor G. Inflammation and implantation. Am. J. Reprod. Immunol. 2010; 63(1): 17-21. https://dx.doi.org/ 10.1111/j.1600-0897.2009.00792.x.
- Makhseed M., Raghupathy R., Azizieh F., Omu A., Al-Shamali E., Ashkanani L. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum. Reprod. 2001; 16(10): 2219-26. https://dx.doi.org/10.1093/humrep/16.10.2219.
- Hossein H., Mahroo M., Abbas A., Firouzeh A., Nadia H. Cytokine production by peripheral blood mononuclear cells in recurrent miscarriage. Cytokine. 2004; 28(2): 83-6. https://dx.doi.org/10.1016/j.cyto.2004.07.002.
- Mor G., Cardenas I., Abrahams V., Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann. N.Y. Acad. Sci. 2011; 1221(1): 80-7. https://dx.doi.org/10.1111/j.1749-6632.2010.05938.x.
- Hayakawa S., Karasaki-Suzuki M., Itoh T., Ishii M., Kanaeda T., Nagai N. et al. Effects of paternal lymphocyte immunization on peripheral Th1/Th2 balance and TCR V beta and V gamma repertoire usage of patients with recurrent spontaneous abortions. Am. J. Reprod. Immunol. 2000; 43(2): 107-15. https://dx.doi.org/10.1111/j.8755-8920.2000.430207.x.
- Pandey M.K., Thakur S., Agrawal S. Lymphocyte immunotherapy and its probable mechanism in the maintenance of pregnancy in women with recurrent spontaneous abortion. Arch. Gynecol. Obstet. 2004; 269(3): 161-72. https://dx.doi.org/10.1007/s00404-003-0560-3.
- Yokoo T., Takakuwa K., Ooki I., Kikuchi A., Tamura M., Tanaka K. Alteration of TH1 and TH2 cells by intracellular cytokine detection in patients with unexplained recurrent abortion before and after immunotherapy with the husband's mononuclear cells. Fertil. Steril. 2006; 85(5): 1452-8. https://dx.doi.org/10.1016/j.fertnstert.2005.10.058.
- Nonaka T., Takakuwa K., Ooki I., Akashi M., Yokoo T., Kikuchi A. et al. Results of immunotherapy for patients with unexplained primary recurrent abortions - prospective non-randomized cohort study. Am. J. Reprod. Immunol. 2007; 58(6): 530-6. https://dx.doi.org/10.1111/j.1600-0897.2007.00536.x.
- Chaouat G. The Th1/Th2 paradigm: still important in pregnancy? Semin. Immunopathol. 2007; 29: 95-113. https://dx.doi.org/10.1007/s00281-007-0069-0.
- Saini V., Arora S., Yadav A., Bhattacharjee J. Cytokines in recurrent pregnancy loss. Clin. Chim. Acta. 2011; 412(9-10): 702-8. https://dx.doi.org/ 10.1016/j.cca.2011.01.002.
- Dekel N., Gnainsky Y., Granot I., Racicot K., Mor G. The role of inflammation for a successful implantation. Am. J. Reprod. Immunol. 2014; 72(2): 141-7. https://dx.doi.org/10.1111/aji.12266.
- Joffe M., Li Z. Association of time to pregnancy and the outcome of pregnancy. Fertil. Steril. 1994; 62(1): 71-5. https://dx.doi.org/10.1016/s0015-0282(16)56818-6.
- Axmon A., Hagmar L. Time to pregnancy and pregnancy outcome. Fertil. Steril. 2005; 84(4): 966-74. https://dx.doi.org/10.1016/j.fertnstert.2005.04.030.
- Gnoth C., Godehardt E., Frank-Herrmann P., Friol K., Tigges J., Freundl G. Definition and prevalence of subfertility and infertility. Hum. Reprod. 2005; 20(5): 1144-7. https://dx.doi.org/10.1093/humrep/deh870.
- Tehrani H.G., Allameh Z.S., Mehrabi A.K. Relation between time to pregnancy and pregnancy outcome. Adv. Biomed. Res. 2014; 3: 175. https://dx.doi.org/10.4103/2277-9175.139411.
- Wise L.A., Mikkelsen E.M., Sørensen H.T., Rothman K.J., Hahn K.A., Riis A.H. et al. Prospective study of time to pregnancy and adverse birth outcomes. Fertil. Steril. 2015; 103(4): 1065-73. e2. https://dx.doi.org/ 10.1016/j.fertnstert.2015.01.024.
- Matsubayashi H., Maruyama T., Ozawa N., Izumi S.I., Sugi T., Yoshikata K. et al. Anti-paternal antibodies by flow cytometry in the management of alloimmunization on recurrent miscarriages. Am. J. Reprod. Immunol. 2000; 44(5): 284-8. https://dx.doi.org/10.1111/j.8755-8920.2000.440506.x.
- Ebihara M., Hattori M., Yoshida T. Distinctly different sensitivity in the induction and reversal of anergy of Th1 and Th2 cells. Biosci. Biotechnol. Biochem. 2007; 71(1): 130-7. https://dx.doi.org/10.1271/bbb.60403.
- Juul S., Karmaus W., Olsen J. Regional differences in waiting time to pregnancy: pregnancy-based surveys from Denmark, France, Germany, Italy and Sweden. The European Infertility and Subfecundity Study Group. Hum. Reprod. 1999; 14(5): 1250-4. https://dx.doi.org/10.1093/humrep/14.5.1250.
- Lédée N., Petitbarat M., Chevrier L., Vitoux D., Vezmar K., Rahmati M. et al. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am. J. Reprod. Immunol. 2016; 75(3): 388-401. https://dx.doi.org/10.1111/aji.12483.
- Saito S., Tsukaguchi N., Hasegawa T., Michimata T., Tsuda H., Narita N. Distribution of Th1, Th2, and Th0 and the Th1/Th2 cell ratios in human peripheral and endometrial T cells. Am. J. Reprod. Immunol. 1999; 42(4): 240-5. https://dx.doi.org/10.1111/j.1600-0897.1999.tb00097.x.
- Ticconi C., Pietropolli A., Di Simone N., Piccione E., Fazleabas A. Endometrial immune dysfunction in recurrent pregnancy loss. Int. J. Mol. Sci. 2019; 20(21): 5332. https://dx.doi.org/10.3390/ijms20215332.
- Yoshinaga K. Review of factors essential for blastocyst implantation for their modulating effects on the maternal immune system. Semin. Cell Dev. Biol. 2008; 19(2): 161-9. https://dx.doi.org/10.1016/j.semcdb.2007.10.006.
- Boomsma C.M., Kavelaars A., Eijkemans M.J., Lentjes E.G., Fauser B.C., Heijnen C.J. et al. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum. Reprod. 2009; 24(6): 1427-35. https://dx.doi.org/10.1093/humrep/dep011.
- Gnainsky Y., Granot I., Aldo P.B., Barash A., Or Y., Schechtman E. et al. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertil. Steril. 2010; 94(6): 2030-6. https://dx.doi.org/10.1016/j.fertnstert.2010.02.022.
- Altman G.B., Gown A.M., Luchtel D.L., Baker C. RANTES production by cultured primate endometrial epithelial cells. Am. J. Reprod. Immunol. 1999; 42(3): 168-74. https://dx.doi.org/10.1111/j.1600-0897.1999.tb00481.x.
- Arima K., Nasu K., Narahara H., Fujisawa K., Matsui N., Miyakawa I. Effects of lipopolysaccharide and cytokines on production of RANTES by cultured human endometrial stromal cells. Mol. Hum. Reprod. 2000; 6(3): 246-51. https://dx.doi.org/10.1093/molehr/6.3.246.
- Croy B.A., Esadeg S., Chantakru S., van den Heuvel M., Paffaro V.A., He H. et al. Update on pathways regulating the activation of uterine Natural Killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. J. Reprod. Immunol. 2003; 59(2): 175-91. https://dx.doi.org/10.1016/s0165-0378(03)00046-9.
- Hunt J.S., Chen H.L., Hu X.L., Tabibzadeh S. Tumor necrosis factor-alpha messenger ribonucleic acid and protein in human endometrium. Biol. Reprod. 1992; 47(1): 141-7. https://dx.doi.org/10.1095/biolreprod47.1.141.
- Clark D.A. Cell-surface CD200 may predict efficacy of paternal mononuclear leukocyte immunotherapy in treatment of human recurrent pregnancy loss. Am. J. Reprod. Immunol. 2009; 61(1): 75-84. https://dx.doi.org/10.1111/j.1600-0897.2008.00665.x.
- Morikawa K., Watabe H., Araake M., Morikawa S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob. Agents Chemother. 1996; 40(6): 1366-70. https://dx.doi.org/ 10.1128/AAC.40.6.1366.
- Raatikainen K., Harju M., Hippeläinen M., Heinonen S. Prolonged time to pregnancy is associated with a greater risk of adverse outcomes. Fertil. Steril. 2010; 94(3): 1148-51. https://dx.doi.org/10.1016/j.fertnstert.2009.10.058.
- Basso O., Olsen J. Subfecundity and neonatal mortality: longitudinal study within the Danish national birth cohort. BMJ. 2005; 330(7488): 393-4. https://dx.doi.org/10.1136/bmj.38336.616806.8F.
Received 20.08.2020
Accepted 05.10.2020
About the Authors
Marina A. Nikolaeva, Dr. Bio. Sci., Leading Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation.Tel.: +7(495)438-11-83. E-mail: nikolaeva_ma@mail.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Alla S. Arefieva, Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation. Tel.: +7(495)438-11-83.
E-mail: arefeva.oparina4@gmail.com. 4, Oparina str., Moscow, 117997, Russian Federation.
Elena O. Stepanova, Junior Researcher at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation.
Tel.: +7(495)438-11-83. E-mail: elena2404.07@mail.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Elena L. Golubeva, Ph.D., Clinical Pathologist at the Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation.
Tel.: +7(495)438-11-83. E-mail: e_golubeva@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Valentina V. Vtorushina, Ph.D., Clinical Pathologist at the, Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation. Tel.: +7(495)438-11-83. E-mail: vtorushina@inbox.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Nana K. Tetruashvili, Dr. Med. Sci., Head of the 2nd Obstetric Department of Pregnancy Pathology, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation. Tel.: +7(495)438-11-83. E-mail: tetrauly@mail.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
Lyubov V. Krechetova, Dr. Med. Sci., Head of Laboratory of Clinical Immunology, V.I. Kulakov NMRC for OG&P, Ministry of Health of the Russian Federation.
Tel.: +7(495)438-11-83. E-mail: l_krechetova@oparina4.ru. 4, Oparina str., Moscow, 117997, Russian Federation.
For citation: Nikolaeva M.A., Arefieva A.S., Stepanova E.O., Golubeva E.L., Vtorushina V.V., Tetruashvili N.K., Krechetova L.V. Time to pregnancy after pre-gestational allogeneic immunotherapy and peripheral blood cell cytokine profile in women with a history of recurrent spontaneous abortion.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2021; 1: 79-87 (in Russian)
https://dx.doi.org/10.18565/aig.2021.1.79-87



