Последние годы ознаменовались разработкой и внедрением в клиническую практику методов неинвазивного пренатального тестирования анеуплоидий на основе анализа внеклеточной ДНК плода (пДНК) [1]. В настоящее время определение пДНК активно используется в неинвазивной пренатальной диагностике анеуплоидий плода, определении RhD принадлежности и заболеваний, сцепленных с полом плода [2].
Выделяется пДНК из общей внеклеточной ДНК (оДНК), в состав которой входит также ДНК матери (мДНК). Основным источником пДНК в материнской крови являются клетки трофобласта, подвергающиеся апоптозу [3, 4]. Под влиянием гипоксии и окислительного стресса значительно усиливается апоптоз клеток трофобласта, что, как известно, является важным патогенетическим звеном в развитии осложнений беременности, таких как преэклампсия и задержка роста плода [5]. Тем самым, определение концентрации внеклеточной пДНК в крови женщины может также служить маркером патофизиологических изменений в плаценте и предиктором осложнений беременности, ассоциированных с плацентарной дисфункцией. Клиническое применение данного маркера с целью прогнозирования осложнений беременности не может быть реализовано без учета потенциальных факторов, которые могут оказывать влияние на концентрацию внеклеточной пДНК в различные сроки беременности. Так, имеются данные о влиянии возраста женщины, индекса массы тела (ИМТ), курения, массы плода и плаценты. Некоторые исследователи, такие как M. Haghiac и соавт. (2012), продемонстрировали, что концентрация оДНК повышена у женщин с ожирением, в отличие от пДНК [6]. Напротив, O. Lapaire и соавт. (2009) не нашли корреляции концентрации, как оДНК с увеличением ИМТ женщины, так и пДНК [7]. M. Smid и соавт. (2003) обнаружили существенную положительную корреляцию между увеличением массы плаценты и концентрацией пДНК, в то время как другие исследователи аналогичную связь не обнаружили [7, 8]. Кроме того, представляет интерес определение содержания внеклеточной ДНК у женщин с измененным клеточным составом крови вследствие анемии. В литературе имеется только одно исследование, в котором изучали концентрацию пДНК при анемии беременных, однако нет сведений об оДНК и мДНК [9]. Таким образом, существуют противоречивые данные о влиянии материнских и плодовых факторов на уровни внеклеточной мДНК и пДНК в крови матери.
В связи с этим целью данной работы стало определение концентрации внеклеточной ДНК и ее фракций в динамике беременности с учетом возможного влияния ИМТ, анемии беременных, массы плода и плаценты.
Материал и методы исследования
Исследование проводили в ФГБУ Научный центр акушерства, гинекологии и перинатологии им. академика В.И. Кулакова Минздрава России с 15 января 2016 г. по 15 апреля 2017 г.
В исследование включены 24 женщины с одноплодной беременностью. Все женщины были соматически здоровы и имели неотягощенный акушерско-гинекологический анамнез. Критериями исключения из исследования послужили такие осложнения беременности, как преэклампсия, преждевременные роды, задержка роста плода, гестационная артериальная гипертензия, гестационный сахарный диабет, хромосомные аномалии и пороки развития плода, аномалии прикрепления и расположения плаценты, острая фаза и обострение хронических инфекционных заболеваний. У 13 женщин беременность протекала без осложнений, у 11 женщин была выявлена анемия легкой степени во II (n=2) и III (n=11) триместрах беременности. На проведение исследования получено разрешение комиссии по этике ФГБУ Научный центр акушерства, гинекологии и перинатологии им. академика В.И. Кулакова Минздрава России. Перед забором биологического материала все женщины подписали информированное согласие на участие в клиническом исследовании.
Забор венозной крови для определения внеклеточной ДНК производили трижды: в промежутках 11–14, 24–26 и 30–32 недели. Было собрано по пять мл периферической крови в вакуумные пробирки, содержащие ЭДТА, и обработано в течение часа после забора. Плазма крови была выделена центр фугированием в два этапа при 4°С: первый этап – 10 мин, 200 g, второй этап – 10 мин, 4500 g. Образцы плазмы хранили при температуре -80°С. Уровень оДНК был оценен количественной полимеразной цепной реакцией (ПЦР) путем определения концентрации гена RASSF1A. Оценка уровня пДНК основана на данных о том, что в клетках плаценты ген RASSF1A гиперметилирован, и, соответственно, концентрация его гиперметилированной части в крови матери будет соответствовать количеству геномных единиц пДНК [10]. оДНК выделяли из 1000 мкл плазмы с использованием магнитных частиц (Силекс, Россия) согласно рекомендациям изготовителя. Полученную ДНК переосаждали этанолом с соосадителем Satellite Red (Евроген, Россия) с предварительной очисткой хлороформом, далее разводили в 13 мкл воды. 10 мкл раствора ДНК использовали в реакции метилчувствительной рестрикции для выделения гиперметилированной части гена RASSF1A. Для этого использовали фермент BstUI (NEB, England) 60 Е. Реакцию рестрикции проводили 6 ч при 60°С, после чего ДНК осаждали этанолом с предварительным удалением рестриктаз с помощью хлороформа. Полученную ДНК растворяли в 10 мкл воды. 2 мкл полученного раствора использовали в реакции ПЦР для контроля рестрикции с праймерами к гену ACTB. В случае отсутствия ответа оставшийся раствор ДНК использовали в реакции ПЦР с праймерами к гену RASSF1A. ПЦР проводили одновременно с пятью различными концентрациями стандарта ДНК, который изготовили из ДНК, выделенной из крови, с использованием магнитных частиц (Силекс, Россия). Концентрацию стандарта ДНК определили с помощью спектрофотометра (DeNovix, USA). Для проведения ПЦР использовали амплификатор CFX96 (BioRad, USA). Программа ПЦР: 95°C – 3 мин, 45 циклов: 95°C 10 с, 60°C 15 с, 72°C 30 с. Последовательности праймеров и зондов представлены в табл. 1.
Статистическую обработку данных производили в среде R 3.4.0 с использованием пакета ggplot2 для визуализации данных. Для оценки значимости связи между количественными переменными были построены модели линейной регрессии, для оценки различий между группами в количественных переменных использован тест Манна–Уитни. Различия считались статистически значимыми при p<0,05.
Результаты исследования
Клинико-анамнестические данные женщин представлены в табл. 2. Средний возраст на момент родов составил 30,04 (3,88) года. Первородящие женщины составили 54,17% (n=13), повторнородящие – 45,83% (n=11). Все женщины родоразрешены в доношенном сроке беременности: 13 беременных через естественные родовые пути, 11 – путем операции кесарева сечения. Показаниями к операции кесарева сечения служили: тазовое предлежание плода, рубец на матке после миомэктомии и операций кесарева сечения, неготовность родовых путей и преждевременное излитие околоплодных вод, а также клинически узкий таз (1 наблюдение) и острая гипоксия плода (2 наблюдения). Только у 1 новорожденного оценка состояния по шкале Апгар на 1-й минуте была менее 8 баллов и составила 7 баллов, на 5-й минуте у всех новорожденных состояние оценили на 8 и более баллов. Масса тела детей при рождении составила от 2770 до 3860 г (в среднем 3390±337,58 г), а масса плаценты – от 383 до 598 г (в среднем 473±62,87 г). Родились 8 мальчиков и 16 девочек.

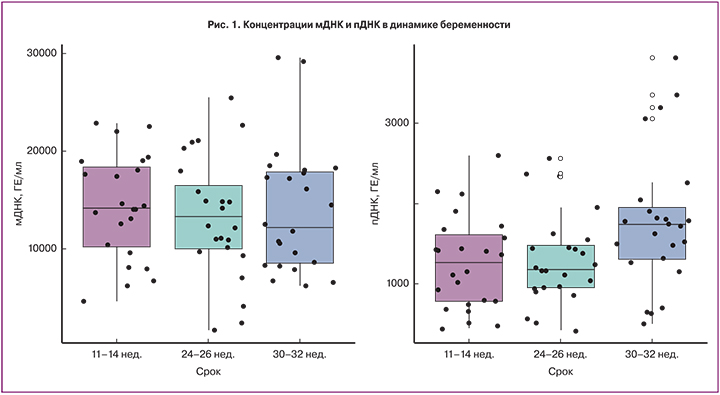
Концентрация как оДНК, так и мДНК в 11–14, 24–26 и 30–32 недели достоверно не различалась (рис. 1). В 11–14 и 24–26 недель концентрация пДНК существенно не различалась, однако в 30–32 недели была значимо выше (p<0,05).
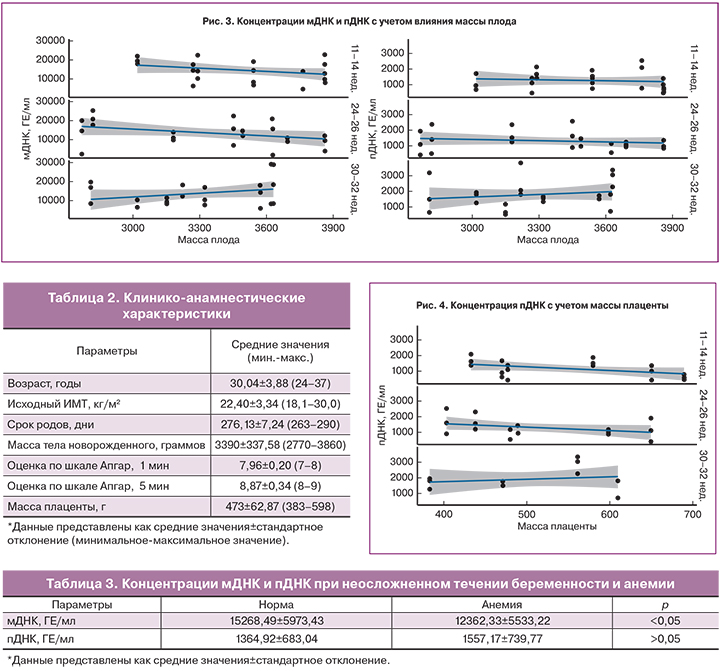
Полученные результаты показали, что концентрации мДНК и пДНК в изученные сроки беременности не зависели от исходного ИМТ женщины (рис. 2).
Также не было обнаружено зависимости между концентрацией фракций внеклеточной ДНК и массой тела новорожденного и плаценты (рис. 3, 4).
Для определения влияния анемии на концентрацию мДНК и пДНК беременные были разделены на 2 группы: с анемией (n=11) и без (n=13). В I триместре беременности анемии не было ни в одной группе женщин и гемоглобин крови (Hb) составлял в среднем 126,54±7,26 г/л. Во II триместре анемия была диагностирована у 2 женщин (Hb в среднем составил 113,73±4,58 г/л). В III триместре анемия имела место у 11 беременных. У этих женщин Hb крови составил в среднем 104,82±3,49 г/л, что достоверно ниже, чем в группе женщин без анемии (124,08±11,29 г/л) (p<0,05). Установлено, что при анемии концентрация пДНК существенно не различалась между группами (табл. 3). Однако следует отметить, что концентрация мДНК у пациенток с анемией была достоверно ниже, чем у женщин без анемии (p<0,05).
Обсуждение
В мировой литературе на протяжении нескольких лет обсуждается возможность определения концентрации внеклеточной пДНК в качестве маркера, прогнозирующего осложнения беременности. Важным аспектом является понимание патофизиологических механизмов, приводящих к изменению уровней внеклеточных нуклеиновых кислот в материнской крови, а также совокупности факторов, влияющих на концентрацию данного маркера. На сегодняшний день среди исследователей нет однозначного мнения.
В данной работе, проанализировав концентрацию внеклеточной оДНК в динамике беременности, мы не обнаружили существенных различий, что коррелирует с данными других исследователей [11]. Однако представляет интерес оценка фракций внеклеточной ДНК (материнской и плодовой).
В исследовании R.J. Levine и соавт. (2004) концентрация пДНК оставалась низкой до III триместра беременности, далее наблюдался значительный подъем с каждой неделей гестации [12]. S. Galbiati и соавт. (2005) отметили значительный прирост уровня пДНК во II и III триместрах беременности, что совпадает с данными Y. Zhou и соавт. (2015) об относительно стабильной концентрации пДНК до 22 недели с последующим быстрым повышением по мере прогрессирования беременности [13, 14]. Недостатком представленных исследований является ограниченность выборки, так как пДНК определялась лишь у женщин с плодом мужского пола по Y-хромосоме.
В нашем исследовании обнаружена положительная корреляция между концентрацией пДНК и сроком беременности. При этом пДНК оставалась относительно стабильной до середины срока гестации и значительно возрастала к 30–32 неделям беременности. Использованный нами метод выделения пДНК при помощи гиперметилированной части RASSF1A гена позволяет определить концентрацию пДНК с ранних сроков беременности, а также не ограничивает выборку по полу плода или резус принадлежности [10]. При использовании этого метода M.J. Kim и соавт. (2013) обнаружили, что уровень пДНК значительно повышается в III триместре беременности, что соответствует нашим данным [15].
Еще меньше данных имеется о мДНК в динамике беременности. Только в работе Y.M. Lo и соавт. (1999) было обнаружено, что концентрация мДНК увеличивается со сроком беременности [16]. Авторы предположили, что возможным объяснением является снижение выведения внеклеточной ДНК в связи с физиологическими изменениями в организме беременной женщины. В нашей выборке мы не обнаружили существенных колебаний мДНК в течение неосложненной беременности.
Среди факторов, оказывающих влияние на концентрацию внеклеточной ДНК, наиболее изучаемым является ИМТ. Большинство исследователей пришли к выводу, что с увеличением массы тела женщины концентрация пДНК уменьшается [14, 17, 18]. Объясняется данная обратная корреляционная зависимость увеличением объема циркулирующей крови и эффектом гемодилюции у женщин с большей массой тела. S.L. Kinnings и соавт. (2015) описали зависимость между изменением ИМТ беременной и концентрацией пДНК: происходит снижение пДНК на 1,17% при увеличении ИМТ на 5 кг/м2 у женщин с ИМТ 20–40 кг/м2; при ИМТ приблизительно равном 50 кг/м2 концентрация ДНК плода остается относительно постоянной [19]. В исследовании T. Wataganara и соавт. (2004) отмечено отсутствие зависимости между концентрацией пДНК и массой тела женщины в I триместре беременности [20]. В литературе отсутствуют данные о влиянии ИМТ на концентрацию мДНК. Полученные нами результаты показали, что в изученные сроки концентрации мДНК и пДНК не зависели от ИМТ женщины.
Как известно, основным источником пДНК являются клетки трофобласта [3]. Предполагается наличие положительной корреляции между массой плаценты и уровнем внеклеточной пДНК в кровотоке женщины. M. Smid и соавт. (2003), дважды определив уровень пДНК в течение одноплодной и многоплодной беременности, обнаружили существенную положительную корреляцию между увеличением массы плаценты и концентрацией пДНК [8]. Однако T. Wataganara и соавт. (2005), измеряя массу плаценты с помощью трехмерного ультразвукового исследования в I триместре беременности, не обнаружили зависимости [21]. В результате полученных данных авторы предположили, что процесс реализации внеклеточной пДНК в кровоток женщины обусловлен иными механизмами, не зависящими от объема плаценты [21]. Нами также не обнаружено корреляции пДНК с массой плаценты при неосложненном течении беременности или легкой анемии.
В нашем исследовании при анализе концентрации пДНК и массы тела новорожденного статистически значимых зависимостей не обнаружено. O. Lapaire и соавт. (2009) также не обнаружили зависимости, что возможно подтверждает плацентарное происхождение данного маркера [7].
Во время беременности происходит снижение Hb крови ко II триместру. Данный феномен связан с увеличением объема плазмы и физиологической гемодилюцией. Повышение объема циркулирующей крови обеспечивает лучшую плацентарную перфузию. Анемия же во время беременности может сопровождаться ухудшением оксигенации тканей, вследствие чего возможно усиление апоптоза клеток трофобласта. В 2013 г. M. Zamanpoor и соавт. впервые провели оценку влияния анемии беременных на концентрацию пДНК [9]. Отмечены более низкие значения пДНК при анемии беременных по сравнению с нормой во II и III триместрах, однако данные не были статистически значимыми (p=0,114).
Нами также не обнаружено достоверных различий уровней пДНК в зависимости от наличия анемии беременных. Возможно, полученные данные обусловлены тем, что при анемии беременных легкой степени наблюдается компенсаторное увеличение количества капилляров, ворсин хориона, а также объема плаценты, что не сопровождается повышением апоптоза клеток трофобласта [22]. В доступной литературе нет данных о содержании мДНК при анемии у беременных. Проведенное нами исследование выявило достоверное уменьшение концентрации мДНК при анемии. В процессе созревания эритроцита, когда клетка находится в составе эритробластного островка или мигрирует через стенку кровеносных сосудов костного мозга в кровоток, происходит выталкивание ядра [23]. Поскольку в крови у взрослого человека циркулируют (25–30)×1012 эритроцитов, 1% из которых ежедневно обновляется, в составе ядер в кровь может попадать около 1,2 г ДНК [23]. Часть ДНК остается циркулировать в кровотоке. Сниженное количество клеток эритробластного островка может являться одной из причин уменьшения количества внеклеточной ДНК в крови. Однако для объяснения нашего наблюдения необходимы дальнейшие исследования источников мДНК в крови матери.
Преимуществом нашей работы является то, что было проведено исследование концентрации фракций внеклеточной ДНК в динамике, которое указывает на увеличение содержания пДНК во второй половине беременности. Нами впервые изучено содержание мДНК у женщин с анемией беременных и установлена статистически значимая связь. Недостатками нашего исследования являются малый объем выборки, оценка ИМТ только на момент наступления беременности.
Заключение
Полученные данные подтверждают значение определения концентрации внеклеточной ДНК с учетом срока гестации для прогнозирования осложнений беременности. Однако для исключения влияния других материнских и плодовых факторов необходимо продолжение исследований.



