Predictive value of critical disturbances in fetoplacental circulation in intrauterine growth restriction pregnancies
Objective. To analyze the indicators of fetoplacental hemodynamics in intrauterine growth restriction (IUGR) pregnancies and to assess the effect of fetoplacental circulation on the neonatal outcome.Kuznetsova N.B., Bushtyrova I.О., Zabanova Е.А., Barinova V.V., Gugueva А.V.
Materials and methods. The analysis of data was based on 138 birth records of women with IUGR who delivered infants with a weight less than 2000 g.
Results. The evaluation of the perinatal outcomes and their correlation with the disturbances of fetoplacental circulation revealed that the risk of neonatal death is three times higher if there are severe disturbances in fetoplacental hemodynamics.
Conclusion. The results of the study have demonstrated the significance of regular ultrasound Doppler evaluation of fetoplacental circulation in IUGR pregnancies in order to control intrauterine fetal condition and improve perinatal outcomes.
Keywords
Intrauterine growth restriction (IUGR) is a pregnancy complication characterized by inability of the fetus to achieve its genetically determined potential growth [1–3]. This definition reflects the mechanisms of development of this pathological condition, emphasizing the difference between the term ‘true’ intrauterine growth restriction and the term small for gestational age, which is characterized by a lower weight of a constitutionally small fetus with no other abnormal clinical features in utero. There is still no consensus regarding the criteria for making IUGR diagnosis which is mainly based on a decrease in the estimated fetal weight or the abdominal circumference of the fetus less than 10th percentile. Nowadays, the most common diagnostic criteria include not only deviations in ultrasound fetometry, but also a number of factors associated with adverse perinatal outcomes, primarily those that characterize disturbances of fetoplacental circulation [3, 4].
IUGR affects up to 10% of all pregnancies and contributes significantly to perinatal mortality and morbidity pattern [1]. In pregnancy complicated by IUGR, there is a significant increase in the risk of both antenatal fetal death [5, 6] and early neonatal fetal death [5, 7]. In most cases, antenatal death occurs during the period when the infant is mature enough for extrauterine survival and further normal development.
Chronic fetal malnutrition, especially at low gestational age, affects the structure and function of a number of organs and systems (mainly the central nervous system and respiratory organs). It also leads to a decrease in immunological reactivity and metabolic disorders. Respiratory distress syndrome, intraventricular hemorrhages, bronchopulmonary dysplasia, and septic complications prevail in infant morbidity pattern [8–10]. Reproductive losses and costs of caring and rehabilitating infants with IUGR cause considerable social and economic damage [11, 12].
Adaptive mechanisms triggered in the fetus with IUGR can have negative influence if they last too long. Thus, under the conditions of chronic hypoxemia caused by placental insufficiency and nutrient deficiency, fetal cardiac output is redistributed in order to support the brain (brain-sparing effect). There is a change in the structure of cerebral vessels and a decrease in vascular reactivity, which leads to the disturbances of cerebral autoregulation and destruction of an important link in the protection of the brain from hypoperfusion or hyperperfusion [13, 14]. All this increases the frequency of lesions of the central nervous system in infants with IUGR: increased neuro-reflex excitability, hypertension-hydrocephalic syndrome, depression syndrome, decreased cognitive functions, persistent severe lesions of the central nervous system such as cerebral palsy, progressive hydrocephalus, and oligophrenia [15].
Under the conditions of placental insufficiency and intrauterine hypoxia, adaptation of the fetus to longterm nutritional and oxygen deficiency allows it to survive during its antenatal development. However, it leads to the changes at the molecular and physiological level that have long-term consequences. For example, there is increasing evidence that long-term exposure to adverse prenatal conditions changes the profile of risk factors for chronic noncommunicable diseases in adulthood (Barker’s hypothesis of fetal programming) [16–19].
One of the methods for evaluating fetal condition is Doppler assessment of fetoplacental blood flow; it is mainly used to measure the pulsation index in the umbilical artery and ductus venosus [3, 20–23]. Pathological flow velocity waveforms in the umbilical artery are characterized by a decrease in the end-diastolic blood flow rate, which indicates a significant increase in peripheral vascular resistance of the fetal placenta and is expressed in an increase in vascular resistance indices above the standard values. Absent and reversed blood flow in the umbilical artery and disorders in the ductus venosus are signs of a critical disturbance of the fetoplacental circulation, which can be followed by antenatal fetal death. In case of detecting the combination of disorders in the umbilical artery with absent or reversed blood flow in the ductus venosus, the issue of early delivery is considered. There is no agreement about the question of choosing the optimal time for delivery, depending on the state of fetoplacental hemodynamics. The criteria for delivery, depending on the term, vary in different countries, guidelines, scientific schools and medical institutions [7, 24–27].
The purpose of this study was to analyze indicators of fetoplacental hemodynamics in IUGR pregnancies and to assess the effect of fetoplacental circulation on the neonatal outcome.
Materials and Methods
In this study a retrospective analysis of birth records was conducted. The study was carried out between 2016 and 2019 in Rostov Regional Perinatal Centre, Russia. All pregnant women received treatment in accordance with the order of the Russian Ministry of Health No. 572 dated 01.11.2012 “On Approval of the Order of Medica l Assistance on the Obstetrics and Gynecology Profile”.
The analysis of data was based on 138 birth records of women with confirmed IUGR who delivered preterm infants with a weight less than 2000 g. The patients were divided into two groups: group I consisted of women whose children died antenatally, intranatally, or within 28 days after birth (n=22), group II included women with neonatal survivors (n=116).
IUGR was diagnosed on the basis of ultrasound and Doppler studies using percentile curves (Fenton growth charts for premature boys and girls) [28]. Before delivery or on the delivery day all pregnant women were performed ultrasound assessment including traditional fetometry and evaluation of the fetal anatomy, a thorough study of the placental localization, Doppler assessment of the uterine arteries, umbilical artery, ductus venosus and fetal middle cerebral artery. Women who had multiple pregnancies or confirmed genetic and chromosomal abnormalities in the fetus were not eligible for inclusion in the study.
Statistical processing
Statistical processing of the obtained data was performed using the software package IBM SPSS Statistics 21, criteria of Kolmogorov-Smirnov test for estimating whether the sampling corresponds to the normal law of distribution, Student’s t-test and Mann-Whitney U test, calculation of relative risks (RR). When comparing two independent groups based on the same parameter, nonparametric statistical methods were used (Fisher’s exact test, Pearson’s χ2 test).
When describing characteristics that had a normal distribution, the data are presented as medians, standard deviation – M (SD). To analyze the data, the Student’s t-test was used for independent samples after preliminary testing of the hypothesis of variances equality using the Levene’s test (thus, the comparison of the average delivery period and the weight of newborns in the study groups was performed).
When describing characteristics with distribution beyond the normal, the data are presented as medians Me, quantiles (Q1; Q3). To analyze these data, the Mann-Whitney U test was used for independent samples (thus, the comparison of the average period of pregnancy prolongation in case of detecting critical disturbances of fetoplacental circulation and the duration of neonatal resuscitation was carried out).
Differences in data were considered statistically significant at p<0.05, which is consistent with the criteria adopted in biomedical studies. If the p value was less than 0.001, then p value was registered as p<0.001.
Results and Discussion
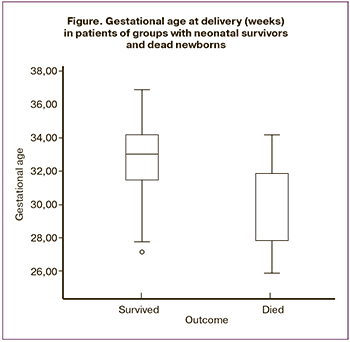 Pregnant women of the study groups were comparable in age, baseline body mass index, concomitant pathology, and obstetric and gynecological history. The analysis of the perinatal losses in group I revealed that the majority of newborns (n=20, 90.9%) died during the neonatal period, four of them died during the first 7 days of life. One woman was delivered due to antenatal fetal death (4.5%), and one patient’s child died intranatally (4.5%). All of the women included in the study with IUGR were delivered prematurely, on average, at 32.3 (2.3) weeks of pregnancy, M (SD), Figure. Gestational age at delivery in the group with dead infants was 30.0 (2.3) weeks, and it was 32.8 (2.0) weeks in the group with neonatal survivors; there were no statistically significant differences in the delivery period (p=0.37).
Pregnant women of the study groups were comparable in age, baseline body mass index, concomitant pathology, and obstetric and gynecological history. The analysis of the perinatal losses in group I revealed that the majority of newborns (n=20, 90.9%) died during the neonatal period, four of them died during the first 7 days of life. One woman was delivered due to antenatal fetal death (4.5%), and one patient’s child died intranatally (4.5%). All of the women included in the study with IUGR were delivered prematurely, on average, at 32.3 (2.3) weeks of pregnancy, M (SD), Figure. Gestational age at delivery in the group with dead infants was 30.0 (2.3) weeks, and it was 32.8 (2.0) weeks in the group with neonatal survivors; there were no statistically significant differences in the delivery period (p=0.37).
When evaluating the body weight of newborns in the study groups, the statistically significant difference (p<0.001) was revealed: in group I the body weight of newborns at birth was 969.1 (214.8) g, M (SD), and it was 1332.4 (275.7) g in group II. Since the gestational age at delivery was statistically insignificantly different, the difference in weight of newborns was likely to be due to more remarkable and/or longer IUGR, accompanied by more severe disorders in the fetoplacental circulation. The majority of patients were delivered by cesarean section, 17 out of 22 (77.3%) in the group with dead children, and 105 out of 116 (90.5%) in the group with neonatal survivors, p=0.075. The analyzed groups were comparable in the indications for termination of pregnancy. Most often women were delivered due to severe preeclampsia, 9 women (40.9%) in group I and 45 women (38.8%) in group II, and due to fetoplacental insufficiency, 6 women (27.3%) in group I, and 48 patients (41.4%) in group II, including decompensated placental insufficiency.
When comparing the indicators of fetoplacental hemodynamics, the following results were obtained using Doppler assessment: the number of pregnant women with critical disturbances of fetoplacental circulation was statistically significantly higher in group II (Table 1). Patients with critical disturbances of fetoplacental hemodynamics included women who had absent or reversed end-diastolic blood flow in the umbilical artery, as well as those who had absent or reversed blood flow in the ductus venosus. Besides, the hemodynamics were assessed on the basis of the most dramatic disturbances of fetoplacental blood flow. Thus, in the presence of absent end-diastolic blood flow in the umbilical artery, but orthograde blood flow in the ductus venosus, women were categorized as having absent end-diastolic blood flow in the umbilical artery; in the presence of reversed end-diastolic blood flow in the umbilical artery accompanied by absent blood flow in the ductus venosus, women were categorized as having absent flow in the ductus venosus.
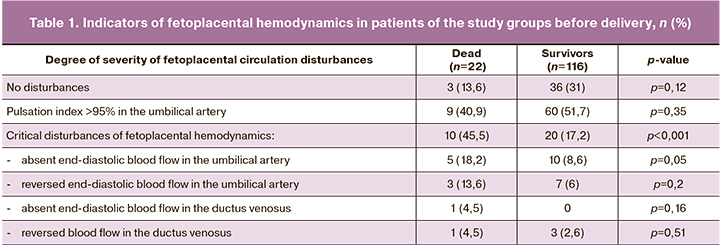
Thus, the proportion of critical disturbances of fetoplacental circulation was statistically significantly higher (p<0.001) in the group where newborns did not survive (10 infants, 45.5%), compared to the group with neonatal survivors (20 infants, 17.2%). Thus, the mortality rate among infants with critical disturbances of circulation (n=30) was 33.3% (n=10), which was statistically significantly (p=0.003) higher than one among newborns without critical disturbances, 11.1% (n=20), OR 3.0, 95% CI: 1.3–6.62. Among critical disturbances of fetoplacental hemodynamics (in terms of severity), absent or reversed end-diastolic blood flow in the umbilical cord arteries prevailed. Disturbances in the ductus venosus (reversed blood flow) were somewhat more common (but statistically insignificant, p=0.51) in group I.
When assessing neonatal mortality depending on the severity of disturbances of fetoplacental hemodynamics, it was found that neonatal mortality was 7.7% (3 of 39 newborns) among patients without disturbances of fetoplacental blood flow; in the presence of disturbances that did not reach critical values (the pulsation index in the umbilical artery was more than 95%) mortality rate was 13% (9 of 69 newborns). Among patients with critical disturbances of fetoplacental hemodynamics, the highest mortality of newborns was observed in the group with disturbances in the ductus venosus, namely absent or reversed blood flow, 40% (two out of five children). In the presence of absent and reversed end-diastolic blood flow in the umbilical artery, the mortality rate was 30% in each of the subgroups (3 out of 10 children with absent blood flow and 5 out of 15 children with reversed end-diastolic blood flow in the umbilical artery). Thus, we observe a progressive increase in perinatal mortality rate depending on the increase in severity of fetoplacental circulation disturbances; however, mortality rate remains consistently high in case of critical disturbances with varying degrees of severity.
In detected critical disturbances of fetoplacental blood flow, the duration of pregnancy prolongation was higher (but statistically insignificant, p=0.88) in the group where infants died, 1.0 (0; 6.3) days (Me (Q1; Q3)); this indicator was 1.0 (0.3; 1) days in the group with neonatal survivors.
Almost all pregnant women with critical disturbances of fetoplacental hemodynamics, whose infants survived the neonatal period, were delivered on the same day when these disturbances were revealed, or the following day (except two patients).
The analysis of the condition of infants at birth showed that newborns who had critical disturbances of fetoplacental hemodynamics had a lower score on the Apgar scale: infants had a score of 4.5 (3.0; 5.0) on the first minute Apgar, Me (Q1; Q3), and 6.0 (5.0; 6.0) on the fifth minute, compared to the newborns without critical disturbances of fetoplacental circulation - 6.0 (5.0; 6.0) on the first minute and 6.0 (6.0; 7.0) on the fifth minute, the difference was statistically significant (p<0.001 on the first minute and p<0.001 on the fifth minute). Children with critical disturbances of fetoplacental hemodynamics needed less time (p=0.93) for resuscitation – 12.0 (9.0; 25.0) days, compared to children without critical disturbances of fetoplacental blood flow – 15.0 (6.3; 26.8) days. This result can be explained by a higher mortality rate among infants with critical blood flow disturbances. Thus, their stay in the intensive care unit was less prolonged due to their death. Before delivery, most of the patients were treated for fetal respiratory distress syndrome with a course of dexamethasone intramuscularly, its cumulative dose was 24 mg. Fetal lung immaturity was prevented in 15 pregnant women (68.2%) of group I and in 82 women (70.7%) of group II with neonatal survivors (p = 0.81).
Conclusion
This clinical study demonstrated the significance of regular ultrasound Doppler evaluation of fetoplacental circulation in IUGR pregnancies. In pregnant women, whose newborns subsequently died, critical disturbances of fetoplacental hemodynamics were detected statistically significantly more often during pregnancy. The results of our study showed that at the time of delivery there was a three-fold increase in the risk of antenatal death, intranatal death, or death of the infant within the first 28 days due to the critical fetoplacental hemodynamic disturbances.
The presence of critical disturbances of the fetoplacental circulation regardless of their severity increases the risk of giving birth to a child in a critical condition (lower Apgar score) and the duration of resuscitation, especially in long-term prolongation of pregnancy after detection of critical disturbances of fetoplacental hemodynamics.
A significant factor that determines the outcome for a newborn with IUGR is the birth weight of the fetus: the lower the fetal mass due to long-term placental abnormalities, the higher the risk of perinatal mortality.
The results of this study demonstrate the importance of regular Doppler assessment of the fetoplacental blood flow in detecting IUGR in order to control intrauterine fetal condition and improve perinatal outcomes. There is no doubt that there is a need to develop national clinical recommendationsforthemanagementofpregnantwomen with placental insufficiency and IUGR to regulate the choice of the optimal moment of delivery. However, the approach to delivery of patients with IUGR should be exclusively personalized taking into account the increased risk of stillbirth and more severe hypoxia in case of delayed delivery, especially if it occurs after prolonged pregnancy with critical disturbances of fetoplacental hemodynamics; the risk also increases in emergency delivery due to severe immaturity and low birth weight.
References
- Стрижаков А.Н., Игнатко И.В., Тимохина Е.В., Белоцерковцева Л.Д. Синдром задержки роста плода: патогенез, диагностика, лечение, акушерская тактика. М.: ГЭОТАР-Медиа; 2014. 120 с. [Strizhakov A. N., Ignatko I. V., Timokhina E. V., Belotserkovtseva L. D. Fetal growth retardation. Pathogenesis, diagnosis, treatment, obstetric tactics. Moscow: GEOTAR-Media; 2014. (in Russian)].
- Unterscheider J., Daly S., Geary M.P., Kennelly M.M., Mc Auliffe F.M., O’Donoghue K. et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO Study. Am. J. Obstet. Gynecol. 2013; 208(4): 290. e1-6. https://dx.doi.org/10.1016/j.ajog.2013.02.007.
- Figueras F., Gratacós E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn. Ther. 2014; 36(2): 86-98. https://dx.doi.org/10.1159/000357592.
- Gordijn S.J., Beune I.M., Ganzevoort W. Building consensus and standards in fetal growth restriction studies. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 49: 117-26. https://dx.doi.org/10.1016/j.bpobgyn.2018.02.002.
- Serena C., Marchetti G., Rambaldi M.P., Ottanelli S., Di Tommaso M., Avagliano L. et al. Stillbirth and fetal growth restriction. J. Matern. Fetal Neonatal Med. 2013; 26(1): 16-20. https://dx.doi.org/10.3109/14767058.2012.718389.
- Gardosi J., Madurasinghe V., Williams M., Malik A., Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013; 346: f108. https://dx.doi.org/10.1136/bmj.f108.
- American College of Obstetricians and Gynecologists. ACOG Practice bulletin No. 134: fetal growth restriction. Obstet. Gynecol. 2013; 121(5): 1122-33. https://dx.doi.org/10.1097/01.AOG.0000429658.85846.f9.
- Mendez-Figueroa H., Truong V.T., Pedroza C., Khan A.M., Chauhan S.P. Small-for-gestational-age infants among uncomplicated pregnancies at term: a secondary analysis of 9 Maternal-Fetal Medicine Units Network studies. Am. J. Obstet. Gynecol. 2016; 215(5):628. e1-628. e7. https://dx.doi.org/10.1016/j.ajog.2016.06.043.
- Malhotra A., Allison B.J., Castillo-Melendez M., Jenkin G., Polglase G.R., Miller S.L. Neonatal morbidities of fetal growth restriction: pathophysiology and impact. Front. Endocrinol. (Lausanne). 2019; 10: 55. https://dx.doi.org/10.3389/fendo.2019.00055.
- Lio A., Rosati P., Pastorino R., Cota F., Tana M., Tirone C. et al. Fetal Doppler velocimetry and bronchopulmonary dysplasia risk among growth-restricted preterm infants: an observational study. BMJ Open. 2017; 7(7): e015232. https://dx.doi.org/10.1136/bmjopen-2016-015232.
- Афанасьева Н.В., Стрижаков А.Н. Исходы беременности и родов при фетоплацентарной недостаточности различной степени тяжести. Вопросы гинекологии, акушерства и перинатологии. 2004; 3(2): 7-13. [Afanasyeva N.V., Strizhakov A.V. Outcomes of pregnancy and childbirth with fetoplacental insufficiency of various severity. Problems of Gynecology, Obstetrics and Perinatology. 2004; 3(2): 7-13. (in Russian)].
- Katz J., Lee A.C., Kozuki N., Lawn J.E., Cousens S., Blencowe H. et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013; 382(9890): 417-25. https://dx.doi.org/10.1016/S0140-6736(13)60993-9.
- Cohen E., Baerts W., van Bel F. Brain-sparing in intrauterine growth restriction: considerations for the neonatologist. Neonatology. 2015; 108(4): 269-76. https://dx.doi.org/10.1159/000438451.
- Baschat A.A. Neurodevelopment after fetal growth restriction. Fetal Diagn. Ther. 2014; 36(2): 136-42. https://dx.doi.org/10.1159/000353631.
- Leitner Y., Fattal-Valevski A., Geva R., Eshel R., Toledano-Alhadef H., Rotstein M. et al. Neurodevelopmental outcome of children with intrauterine growth retardation: a longitudinal, 10-year prospective study. J. Child Neurol. 2007; 22(5): 580-7. https://dx.doi.org/10.1177/0883073807302605.
- Barker D.J. In utero programming of chronic disease. Clin. Sci. (Lond.). 1998; 95(2): 115-28.
- Colella M., Frérot A., Novais A.R.B., Baud O. Neonatal and long-term consequences of fetal growth restriction. Curr. Pediatr. Rev. 2018; 14(4): 212-8. https://dx.doi.org/10.2174/1573396314666180712114531.
- Ojeda N.B., Grigore D., Alexander B.T. Intrauterine growth restriction: fetal programming of hypertension and kidney disease. Adv. Chronic Kidney Dis. 2008; 15(2): 101-6. https://dx.doi.org/10.1053/j.ackd.2008.01.001.
- Crispi F., Crovetto F., Gratacos E. Intrauterine growth restriction and later cardiovascular function. Early Hum. Dev. 2018; 126: 23-7. https://dx.doi.org/10.1016/j.earlhumdev.2018.08.013.
- Trudinger B.J., Cook C.M. Doppler umbilical and uterine flow waveforms in severe pregnancy hypertension. Br. J. Obstet. Gynaecol. 1990; 97(2): 142-8. https://dx.doi.org/10.1111/j.1471-0528.1990.tb01739.x.
- Hecher K., Bilardo C.M., Stigter R.H., Ville Y., Hackelöer B.J., Kok H.J. et al. Monitoring of fetuses with intrauterine growth restriction: a longitudinal study. Ultrasound Obstet. Gynecol. 2001; 18(6): 564-70. https://dx.doi.org/10.1046/j.0960-7692.2001.00590.x.
- Hecher K., Snijders R., Campbell S., Nicolaides K. Fetal venous, intracardiac, and arterial blood flow measurements in intrauterine growth retardation: relationship with fetal blood gases. Am. J. Obstet. Gynecol. 1995; 173(1): 10-5. https://dx.doi.org/10.1016/0002-9378(95)90161-2.
- Schwarze A., Gembruch U., Krapp M., Katalinic A., Germer U., Axt-Fliedner R. Qualitative venous Doppler flow waveform analysis in preterm intrauterine growth-restricted fetuses with ARED flow in the umbilical artery – correlation with short-term outcome. Ultrasound Obstet. Gynecol. 2005; 25(6): 573-9. https://dx.doi.org/10.1002/uog.1914.
- Royal College of Obstetricians and Gynaecologists. The investigation and management of the small-for-gestational-age fetus. Green-Top Guideline No. 31. 2nd ed. 22.03.2013.
- New Zealand Maternal Fetal Medicine Network. Guideline for the management of suspected small for gestational age singleton pregnancies and infants after 34 wk’ gestation. New Zealand Maternal Fetal Medicine Network ; 2014.
- Institute of Obstetricians and Gynaecologists Royal College of Physicians of Ireland. Fetal growth restriction–recognition, diagnosis management. Clinical Practice Guideline No. 28. 2017.
- Vayssière C., Sentilhes L., Ego A., Bernard C., Cambourieu D., Flamant C. et al. Fetal growth restriction and intra-uterine growth restriction: guidelines for clinical practice from the French College of Gynaecologists and Obstetricians. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015; 193: 10-8. https://dx.doi.org/10.1016/j.ejogrb.2015.06.021.
- Fenton T.R., Kim J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013; 13: 59. https://dx.doi.org/10.1186/1471-2431-13-59.
Received 17.01.2020
Accepted 21.03.2020
About the Authors
Natalia B. Kuznetsova, Doctor of Medical Sciences, Professor at the Department of Simulation education of RostSMU.Tel.: +7(928)770-97-62. E-mail: lauranb@inbox.ru. 29 Nahichevansky ave., Rostov-on-Don, 344022, Russian Federation.
Irina O. Bushtyreva, Doctor of Medical Sciences, Full Professor. Tel.: +7(863)288-00-00, E-mail: kio4@mail.ru.
58/7 A Sobornyi ave., Rostov-on-Don, 344010, Russian Federation.
Ekaterina A. Zabanova, Postgraduate at the Department of Simulation education of RostSMU. Tel.: +7(918)566-88-25. E-mail: rock-fe@mail.ru.
29 Nahichevansky ave., Rostov-on-Don, 344022, Russian Federation.
Victoria V. Barinova, Candidate of Medical Sciences, Assistant of the Department of Obstetrics and Gynecology №1 of RostSMU.
Tel.: +7(928)959-03-76. E-mail: victoria-barinova@yandex.ru. 29 Nahichevansky ave., Rostov-on-Don, 344022, Russian Federation.
Anna V. Gugueva, obstetrician-gynecologist, State Budgetary Institution of Rostov Region Perinatal center.
Tel.: +7(908)185-43-53. E-mail: anna-gugueva@mail.ru.
90 Bodraya ave., Rostov-on-Don, 344068, Russian Federation.
For reference: Kuznetsova N.B., Bushtyrova I.О., Zabanova Е.А., Barinova V.V., Gugueva А.V. Predictive Value of Critical Disturbances in Fetoplacental Circulation in Intrauterine Growth Restriction Pregnancies.
Akusherstvo i Ginekologiya/ Obstetrics and Gynecology. 2020; 6: 59-64 (in Russian)
https://dx.doi.org/10.18565/aig.2020.6.59-64



