A study of polymorphisms rs3020434, rs11742635, rs124577644, rs12637801, rs2861221, and rs17677069 in women with uterine leiomyomas and a family history of the disease
Objective. To optimize diagnosis, patient management tactics and to predict the risk of recurrence, by searching for genetic markers for uterine fibroids.Sogoyan N.S., Kuznetsova M.V., Lolomadze E.A., Mikhailovskaya G.V., Mishina N.D., Trofimov D.Yu., Adamyan L.V.
Subjects and methods. The investigators collected tissue specimens from 329 uterine myomas and blood aliquots from 106 study group patients and 24 blood samples from control group patients. They isolated DNA, amplified exon 2 of the MED 12 gene and genotyped single nucleotide polymorphisms (SNP) (rs3020434, rs11742635, rs124577644, rs12637801, rs2861221, and rs17677069) by six ESR1, FBN2, CELF4, and KCWMB2 loci.
Results and discussion. Statistical analysis showed that the frequencies of rare alleles of the SNP rs3020434, rs11742635, rs2861221, and rs176069 were lower among women with uterine fibroids and practically did not occur in the group of patients with a positive family history as compared with the controls and the general population, which can probably suggest that these alleles play a protective role; therefore, it is possible to use them as markers for predicting the probability of development and recurrence of uterine fibroids.
Conclusion. The rare alleles of the gene polymorphisms rs3020434, rs11742635, rs2861221, and rs17677069 are not found in the groups with a positive family history, which is likely to allow them to be used in the future for a comprehensive diagnosis of the probability of developing uterine fibroids, as well as for predicting the risk of recurrence.
Keywords
In recent decades, there has been a significant increase in the incidence of hormone-dependent tumors worldwide. Uterine leiomyoma (UL) is the most common hormone-dependent tumor of the female reproductive system.
Uterine leiomyoma, also known as uterine fibroids, is a benign monoclonal, well-demarcated, encapsulated tumor arising from smooth muscle compartment (myometrium) of the uterus or cervix. The estimated incidence rate of UL among reproductive-age women varies from 30 to 70% [1, 2].
Although ULs are considered benign, they are accompanied by quite diverse clinically significant symptoms, including pelvic organ dysfunction, abnormal uterine bleeding, anemia, and pain. These symptoms are associated with high rates of surgical interventions for UL in gynecological hospitals with especially high percentage of hysterectomies in reproductive-age women with UL [1, 3]. ULs can also cause infertility, adversely affect the course of pregnancy, and cause problems not only during gestation but also during childbirth [4].
Due to the facts mentioned above, UL poses a considerable socio-economic impact on reproductive-age women with UL and determine the relevance of further investigation of the pathogenesis of uterine fibroids, clinical diagnostic decision-making, patient management strategy, and prediction of recurrence risk.
Currently, the etiopathogenetic mechanisms of UL development remain debatable and poorly understood. However, some evidence suggests that reproductive age and ethnicity may contribute to the development of UL (for example, in the USA, ULs occur in higher percentages in women of African American descent). Risk factors also include family history, increased body mass index (BMI) [5], stress, dysfunction of the immune system, pregnancy, hormonal status, and genetic mechanisms [6–8].
The presence of family history in 5-10% of women with UL [6] indicates the key role of genetic mechanisms in the pathogenesis of this disease.
Thus, over the past few years, there has been an ongoing search for genetic markers of UL development. The most significant discovery in this area was the description of somatic mutations in the MED12 gene, which encodes one of the mediator complex subunits of RNA polymerase II.
In 2015, a group of authors [9] reported that the integration of the mutant allele of the MED12 gene into the mice genome leads to the development of UL. Following the publication of this study, considerable research attention has been devoted worldwide to this gene in women with UL with various nodule locations, their sizes, and numbers [10, 11].
In our previous study, we found that somatic MED12 exon 2 mutations were found in 50% of tissue specimens obtained from uterine fibroids of women in Russia’s general female population [12].
In a subsequent study of somatic MED12 gene mutations, we identified the relationship between these mutations and a family history of ULs. According to our data, somatic mutations were found in exon 2 of the MED12 gene in 61% and 48% of nodules obtained from women with and without a family history of ULs, respectively (p = 0.109) [13]. Also during the study, we described several unique somatic mutations that were not previously mentioned in the literature and databases.
The development of genetic marker panels is still one of the most knowledge-based areas in reproductive genetics. The use of such panels can help optimize management strategy, early diagnosis, and predict the risks of recurrence of many common gynecological diseases.
The present study is aimed to identify associations between polymorphisms in rs3020434, rs11742635, rs124577644, rs12637801, rs2861221, rs17677069 genes and a family history of ULs with further detection of genes contributing to the development of fibroids and protective genes preventing their occurrence.
Materials and methods
From 2017 to 2018, a total of 329 uterine fibroid tissue samples and blood aliquots were collected from 106 patients (1 to 5 UL nodules per patient) at the Department of Operative Gynecology of V.I. Kulakov NMRC for OG&P; 24 blood aliquots were obtained from patients in the control group.
All patients underwent a complete clinical examination, including history taking, general and gynecological examinations, pelvic ultrasound, and laboratory testing. Endoscopic surgery was performed by a standardized technique using endovideosurgical Karl Storz equipment (Germany). Uterine fibroid tissue samples were obtained directly during myomectomy or hysterectomy, depending on the size and number of nodules. Tissue fragments were placed in saline and sent to the Biobank of V.I. Kulakov NMRC for OG&P; they were frozen at -70°C for subsequent storage in the collection. The samples of each nodule were also subjected to histological examination to confirm that they consist exclusively of myomatous tissue without capsular or myometrial tissue.
Twenty patients with a family history of ULs and MED12 gene somatic mutations (Pat subgroup) and 14 patients in the control group (Con subgroup), underwent microarray analysis on SNP 6.0 chips (Affymetrix, USA); the samples were genotyped at 906,600 single nucleotide polymorphisms (rs), evenly distributed throughout the genome, and the most significant of them were identified by statistical analysis.
The selected most statistically significant candidate rs were tested on the entire sample (130 patients) by genotyping using the Sanger sequencing with a further comparison of allele frequencies by study groups.
The study comprised 130 patients, divided into 4 groups: group 1 (control group) included postmenopausal women without a history of UL (n=24), group 2 consisted of women with a family history of first-degree relatives diagnosed with UL (n=51); group 3 included women without a family history of ULs (information about family history we received during patients’ interview) (n=36), group 4 included patients who could not provide information about their family history of UL (n=19).
To identify the most statistically significant of the potential 906600 rs, we used genotyping results of 34 samples (subgroups Pat and Con), which can be interpreted and presented as specific variants of alleles for each sample and each of the studied rs. Accordingly, considering all the samples, it is possible to calculate the frequencies of alleles and genotypes in the studied sample separately for the control group (Con) and the group of patients (Pat).
For each rs, two alleles were determined as A - reference (major) and B - alternative (minor), and their frequencies in the subgroups PatA, PatB, ConA, ConB. Possible genotypes were also identified, including homozygous AA, BB, and heterozygous AB and their frequencies in the subgroups PatAA, PatBB, PatAB, ConAA, ConBB, and ConAB.
The significance of rs was assessed by the Fisher exact, which was used 4 times independently to assess the statistical significance of the following factors: the frequencies of A and B alleles in the subgroup (test1), the frequencies of the AA genotype in the subgroup (test2), the frequencies of the AB genotype in the subgroup (test3), the frequency of the AB genotype in the subgroup (test4). Contingency tables are given in table 1.
Upon completion of the calculations by the Fisher exact test, we obtained four estimates of p-value significance for each rs: the significance of alleles and genotypes.
At the stage of filtering and isolating significant rs, population data were also taken into account. Information on the occurrence frequency of the alleles for the studied rs was downloaded from the dbSNP database both for European (EUR) and some other populations (East Asian EAS, South Asian SAS, American AMR, and African AFR).
Another limitation of rs filtering was the coordinate proximity on the genome: from the vicinity of 1000 bp, no more than 1 rs could be isolated - a necessary measure for filtering putatively linked polymorphisms.
The selection of the most interesting candidate rs was based on the following features: p-value of 4 Fisher tests, occurrence in the European population, and coordinate proximity on the genome.
As a result, 6 candidate polymorphisms were identified for further testing of the entire study sample: rs3020434, rs11742635, rs124577644, rs12637801, rs2861221, and rs17677069 located in the genes ESR1, FBN2, CELF4, KCWMB2 that had statistically significantly different frequencies. Then, we genotyped the entire sample of UL patients at these loci with a further comparison of allele frequencies by study groups.
At the second stage of the study, candidate rs were analyzed in an extended sample of patients with UL divided into groups according to the anamnesis. Statistical analysis was carried out using the Fisher exact test (with expected counts in the contingency table of less or equal than 5); otherwise, the Pearson χ2 test was used.
Statistical analysis was performed using Microsoft Excel 2013, SciPy library, and Pandas for the Python 2.7 programming language.
Results
Statistical analysis of genotyping data using a microarray study of 20 patients with a family history of ULs and the MED12 gene somatic mutations and 14 patients of the control group (Pat and Con subgroups) allowed the isolation of 6 polymorphisms rs3020434, rs11742635, rs124577644, rs12637801, rs2861221, and rs17677069 in genes ESR1, FBN2, CELF4, and KCWMB2 that had significantly different frequencies in women with a family history of ULs and women in the control group (Table 2).
The frequencies of these polymorphisms were compared with that in the general population (Table 3). We selected rare allelic variants that could further serve as predictors of the risk of uterine fibroids.
Detection rates of the rare allele in all 6 polymorphisms were significantly higher in the control group than among patients with UL.
Further work was devoted to the genotyping of a larger sample of patients to identify the association between the representation of alleles and the family history of women with UL.
For each polymorphism, we determined the frequencies by alleles in each study group: the control group, the group of women with a family history of UL, without a family history of UL, and in the group of women who did not provide information about their history of UL; we compared the data of the group with the entire population and a common group of women with UL.
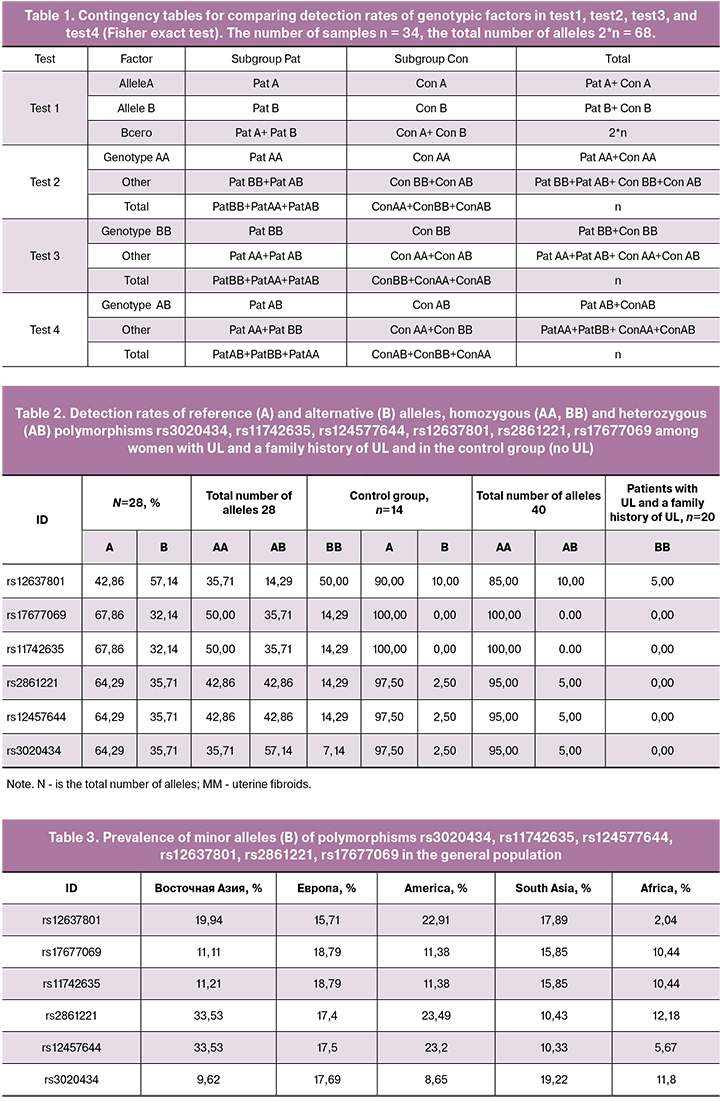
The homozygous variant TTof polymorphism rs3020434 - ESR1 (Table 4, Figure 1) was absent in patients with a history of UL, and it was detected in 5% of all patients with this disease. Therefore, this variant of the allele may be protective, while the homozygous variant of SS was detected in 73% of patients with a family history of UL. This allele may be associated with an increased risk of developing UL.
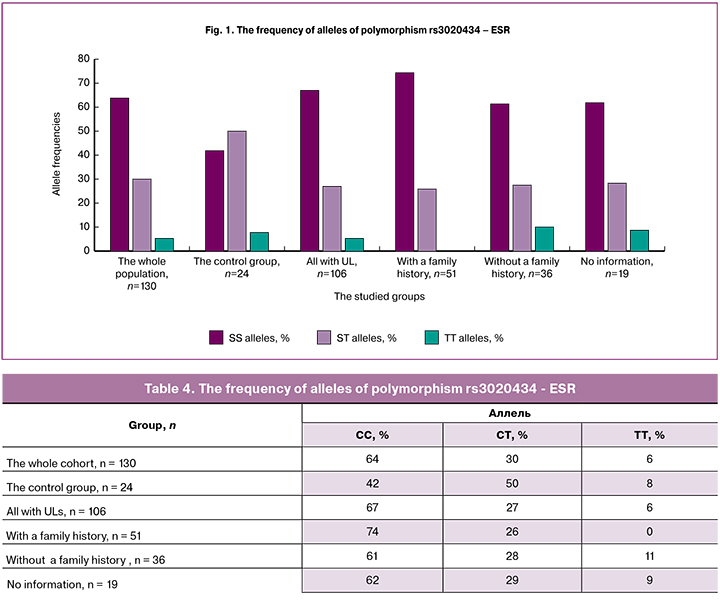
The homozygous variant TT of polymorphism rs11742635 - FBN2 (Table 5, Figure 2) was absent in patients with a family history of UL, and it was detected in 3% of all patients with UL; therefore, this variant of the allele may be protective. The homozygous variant of SS was detected in 82% of patients with a family history of UL, suggesting that this allele may be associated with an increased risk of UL.
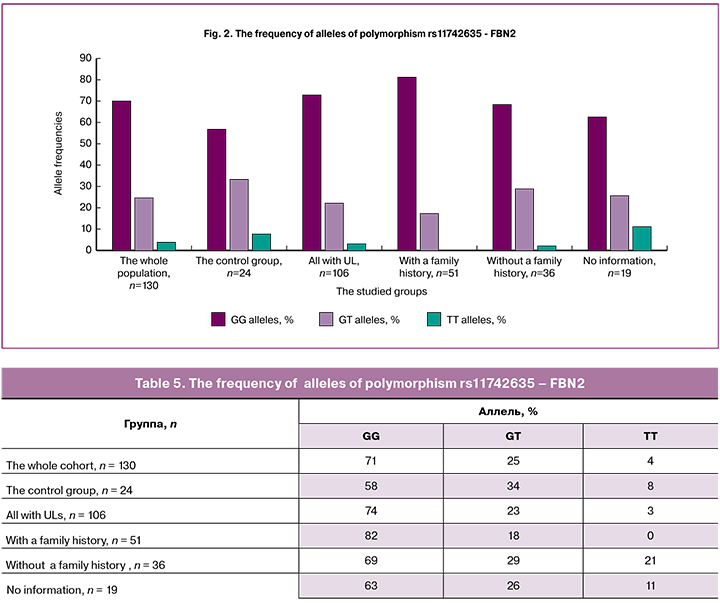
The detection rate of homozygous variant AA of polymorphism rs124577644 - CELF4 (Table 6, Figure 3) was the lowest (6%) among patients with a family history of UL compared with other groups, and, therefore, this variant of the allele may be protective. The detection rate of the homozygous variant of GG in this group was the highest (78%), i.e., this allele may be associated with an increased risk of developing UL.
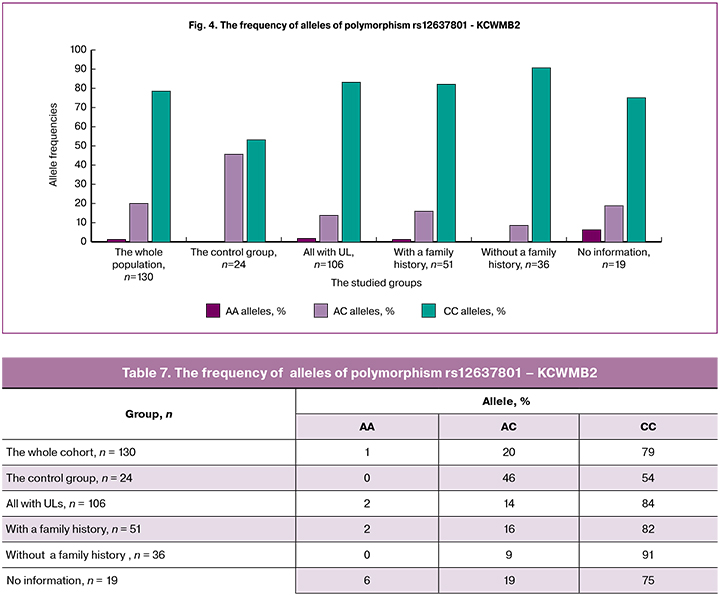
The detection rate of the homozygous variant CC of polymorphism rs12637801 - KCWMB2 (Table 7, Figure 4) was the highest (84%) among patients with a family history of UL compared with other groups, i.e., this allele may be associated with an increased risk of developing UL.
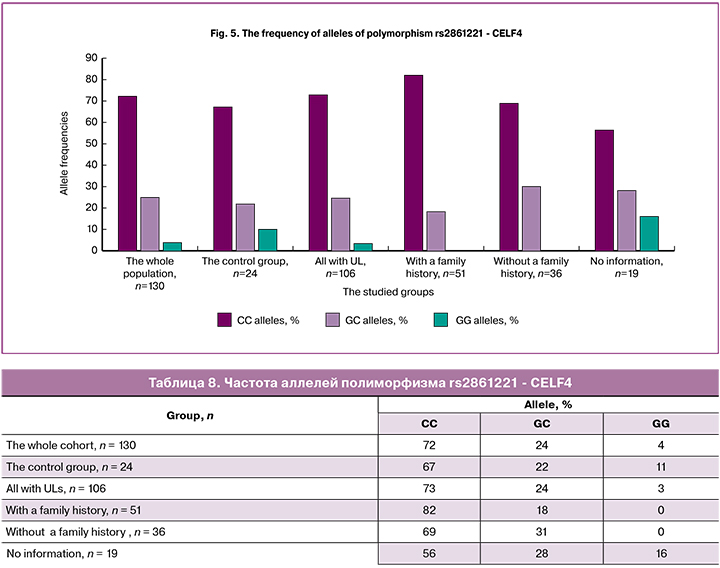
The homozygous variant GG of polymorphism rs2861221 - CELF4 (Table 8, Figure 5) was not found in patients with a family history of UL compared with other groups; therefore, this variant of the allele may be protective. The detection rate of the homozygous variant of CC was the highest (82%), indicating the possible role of this allele as a risk factor for UL.
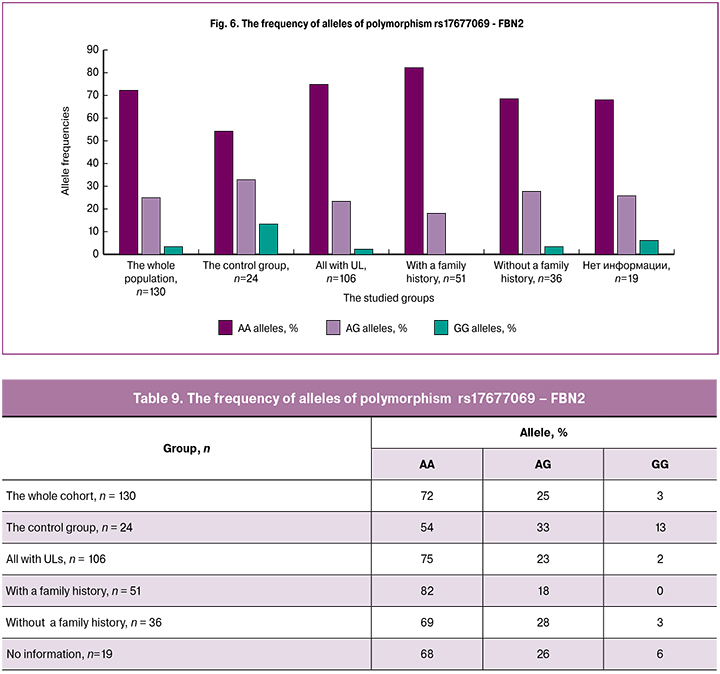
The homozygous variant of the GG polymorphism rs17677069 - FBN2 (Table 9, Figure 6) was not found in patients with a family history of UL. Among women with UL regardless of family history, i.e. in all patients, the GG detection rate was 2%. Therefore, this allele variant may be protective. The detection rate of homozygous variant of AA was the highest (82%) in patients with a family history of UL, indicating that this genotype may be a risk factor for UL.
In the case of 4 polymorphisms rs3020434, rs11742635, rs2861221, and rs17677069 in the group of women with a family history of UL, one of the rare alleles, a homozygous variant, was completely absent, which statistically distinguished this group from the others. Considering this fact, we can assume the protective role of the rare allele in the pathogenesis of UL, in particular, in the familial form of the disease. However, the higher detection rate of the second homozygous variant of the alleles of these polymorphisms, on the contrary, may indicate the role of frequent variants of polymorphism in UL pathogenesis. This assumption can also be confirmed by the fact that two of the detected polymorphisms, rs11742635 and rs17677069, are linked, i.e. are located in one gene, which can be directly or indirectly involved in the pathological process.
Statistical analysis of the data obtained by comparing the control group with all patients with ULs regardless of the family history revealed high statistical significance in the case of 4 of the 6 studied loci (Table10).
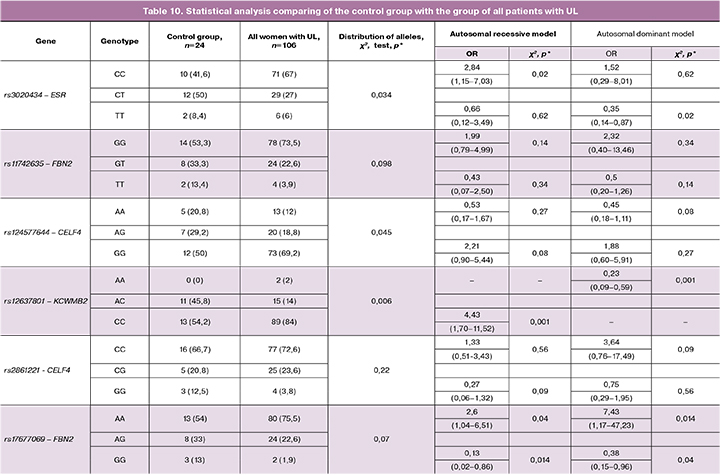
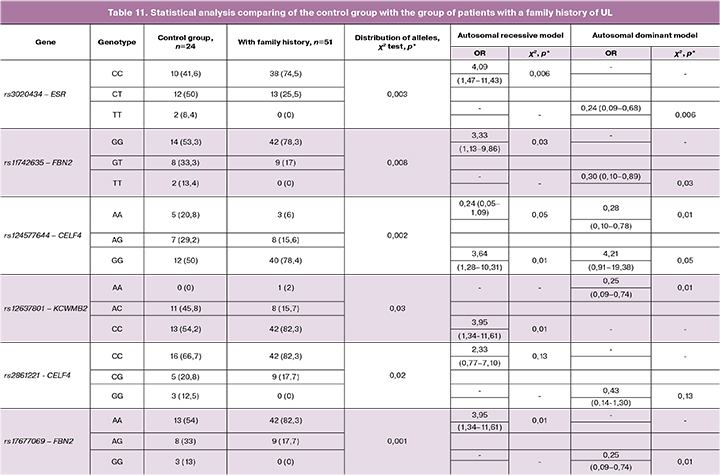
Comparison of the control group with the group of patients with a family of history of UL, which was twice smaller than the general group of women with UL, revealed a statistically significant difference in the detection rates of protective variants of polymorphisms and alleles, which confirms the participation of detected polymorphisms in the pathogenesis of familial form of uterine fibroids (Table11) .
Discussion
Currently, the search for genetic markers associated with the socially significant gynecological diseases is being conducted to create genetic panels for early diagnosis, the prognosis of recurrence, optimization of management strategy, and the development of new targeted drugs for the treatment of these diseases.
Contemporary genomic disease-specific databases (Breast Cancer Information Core (BIC) Database, International Cancer Research Partnership (ICRP) Database, Cancer Genomics Hub (CGHub) Database, Online Mendelian Inheritance in Man (OMIM) contain data on genes and their allelic variants, which can be involved in the pathogenesis of malignancies. The marker genes associated with malignant neoplasms of female reproductive organs include the BRCA (Breast cancer antigen) genes BRCA1 and BRCA2, located on 17 and 13 somatic chromosomes, respectively. In the international Breast Cancer, the Information Core database (BIC) has aggregated1809 mutations and polymorphisms in the BRCA1 gene and 2019 in the BRCA2 gene.
BRCA1 gene, responsible for a hereditary predisposition to breast and ovarian cancers, consists of 22 coding and two non-coding exons separated by introns, was identified by Miki et al. [14] on the long arm of chromosome 17 (17q21). BRCA2 gene was discovered by Wooster et al. [15] as the second breast cancer-predisposing gene located on chromosome 13 (13q12).
The BRCA1 and BRCA2 genes are expressed in normal cells and involved in maintaining the genomic stability. Germinal mutations in these genes are associated with 20–25% of all hereditary breast cancers and 15% of ovarian cancers [16]. More than 1000 BRCA1 and BRCA2 gene mutations have been identified, many of which are associated with an increased risk of cancer (especially breast cancer in women).
Today, there are various diagnostic panels based on the detection of various mutations in the genes associated with breast cancer, which are being actively implemented into practice to identify the risks of developing this malignant neoplasm, predict metastasis and recurrence.
The very first gene expression panel approved by the Food and Drug Administration in February 2007 is a microchip that allows profiling of 70 classification genes and identifies a difference in expression patterns between patients with a high and low 10-year recurrence risk [ 17].
There is an ongoing search for genetic markers associated with other gynecological diseases. A meta-analysis by Nilufer Rahmioglu et al. showed a direct relationship between six genetic loci rs12700667 in 7p15.2, rs7521902-WNT4, rs10859871-VEZT, rs1537377-CDKN2B-AS1, rs7739264-ID4, rs13394619 in GREB1 and endometriosis in female populations of Australia, Belgium, Italy, Great Britain, USA, and Japan. Five of the six loci were frequently associated with stage III-IV endometriosis. This association emphasizes the importance of further research in this area to identify the effect of variants of alleles of various genes on the pathogenesis of endometriosis [18].
A study aimed at identifying genetic markers of endometriosis was conducted at the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology, and Perinatology. This study findings showed a positive association between the heterozygous genotype of the C/G locus at rs4703908 and the risk of OEC (OR=1.78; 95% CI: 0.88-3.62; p > 0.05) and deep infiltrating endometriosis (OR=2.02; 95% CI: 0.85-4.73; p > 0.05). There was a positive association between the C/G locus at rs4703908 and the risk of peritoneal pelvic endometriosis (OR=1.24; 95% CI: 0.49-3.16; p > 0.05); however, OR did not exceed 1.5. There was a positive association between the homozygous genotype of the CC locus at rs10859871 and the risk of OEC (OR=5.61; 95% CI: 1.8-17.49; p < 0.01), and deep infiltrating endometriosis (OR=5.92; 95% CI: 1.68-20.83; p < 0.01) and pelvic peritoneal endometriosis (OR=4.44; 95% CI: 1.15-17.07; p < 0.05) compared with the apparently healthy women in the control group [19].
For the first time, familial and ethnic predisposition to UL was shown in a study of American geneticists, who genotyped and analyzed 261 white UL-affected sister-pair families [20]. Immunohistochemical and genetic studies of this group of patients revealed a UL risk allele, a gene encoding fatty acid synthase (FASN) in 17q25.3.
In a recently published work, Mackinen et al. reported 27 novel genomic loci associated with UL. On very extensive (tens of thousands) samples, the authors compared rs frequencies in women with and without UL. Candidate genes were also divided into two categories: genomic regions containing characteristic tumor suppressors and oncogenes, and genes involved in hormonal signaling pathways previously associated with endometriosis. The high frequency of polymorphisms in the identified genes in women with UL and common variants of the polymorphisms of these genes in patients with UL and endometriosis may indicate similar molecular mechanisms of development and common progenitor cells of these two socially significant gynecological diseases [21].
A group of Japanese scientists conducted a genome-wide association study in which 457044 SNPs were analyzed in 1607 women with clinically diagnosed UL and 1428 female controls. SNPs showing suggestive associations (P < 5 × 10−5) were further genotyped in 3466 additional cases and 3245 female controls. Three loci on chromosomes 10q24.33, 22q13.1, and 11p15.5 revealed genome-wide significant associations with UL. The SNPs showing the most significant association in a combination analysis at each of these loci were rs7913069 (P = 8.65 × 10−14, OR = 1.47), rs12484776 (P = 2.79 × 10−12, OR = 1.23) and rs2280543 (P = 3.82 × 10−12, OR = 1.39), respectively. The authors concluded that further investigation of these regions would be necessary to identify the causal variants [22].
In our study, we genotyped 906600 single nucleotide polymorphisms from patients with UL and women with a family history of first-degree relatives diagnosed with UL and a control subject without a history of UL. As a result of genotyping, we identified 6 polymorphisms rs3020434, rs11742635, rs124577644, rs12637801, rs2861221, rs17677069 of the genes ESR1, FBN2, CELF4, KCWMB2, the frequencies of which were statistically different in both groups compared with other single nucleotide polymorphisms; they were higher in women with a family history of UL compared with the control group. Further we examined only the above six polymorphisms, which may be associated with the development of UL. Perhaps our strategy for investigating gene polymorphism will be able to explain the pathogenesis of familial form of UL and help develop a genetic diagnostic panel for this disease.
Conclusion
In our study, among 906,600 single nucleotide polymorphisms, we identified six SNPs (rs3020434, rs11742635, rs124577644, rs12637801, rs2861221, rs17677069), which were statistically different from other study groups, and had higher detection rates in the group of women a family history of UL compared with the control group. Therefore, they may be associated with the development of UL.
Analysis of polymorphisms in women with UL who had a family history of the disease allowed the detection of statistically significant differences in allele frequencies compared with the control group and the general population group. In particular, rare alleles of rs3020434, rs11742635, rs2861221, and rs17677069 polymorphisms were absent in patients with a family history, which may indicate a protective role of these allele variants in the development of UL, in particular, familial forms of UL, and the high frequency of homozygous allele variants, possibly suggest their involvement in the development of UL.
Further studies of the newly identified polymorphisms should be aimed at detecting possible molecular mechanisms of developing UL, which can lead to the development of test systems for early diagnosis, predicting the recurrence risk, and better management of UL patients using genetic markers.
References
- Адамян Л.В. Миома матки: диагностика, лечение и реабилитация. М.: ФГБУ "Научный центр акушерства, гинекологии и перинатологии им. В.И. Кулакова" Минздрава России; 2015. 72 с. [Adamyan L.V. Uterine fibroids: diagnosis, treatment and rehabilitation. M.: Scientific Center for Obstetrics, Gynecology and Perinatology named after Academician V.I. Kulakov, Ministry of Health of Russia. 2015; 72 p. (in Russian)]
- Baird D.D., Dunson D.B., Hill M.C., Cousins D., Schectman J.M. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003; 188(1): 100-7. doi: 10.12691/ajcmr-3-1-2
- Torres-de la Roche L.A., Becker S., Cezar C., Hermann A., Larbig A., Leicher L., et al. Pathobiology of myomatosis uteri: the underlying knowledge to support our clinical practice. Arch. Gynecol. Obstet. 2017; 296(4): 701-7. doi: 10.1007/s00404-017-4494-6.
- Подзолкова Н.М., Колода Ю.А., Коренная В.В., Кайибханова К.Н. Эффективность вспомогательных репродуктивных технологий при миоме матки. Гинекология. 2015; 17(2): 60-4. [Podzolkova N.M., Koloda Yu.A., Korennaya V.V., Kayibhanova K. The efficacy of assisted reproductive technologies in patients with uterine fibroids (review). Ginekologija; 2015; 17(2): 60-4.(Russian)]. eLIBRARY ID: 23599769
- Pavone, D., Clemenza, S., Sorbi, F., Fambrini, M. & Petraglia, F. Epidemiology and risk factors of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2018; 46: 3-11. doi: 10.1016/j.bpobgyn.2017.09.004
- Адамян Л.В., Спицын В.А., Андреева Е.Н. Генетические аспекты гинекологических заболеваний. Руководство для врачей. М.: ГЭОТАР-Медиа; 2008. 215 с. [Adamyan L.V. Spitsyn V.A., Andreeva E.N. Genetic aktekty gynecological diseases. M: GEOTAR-media; 2008. 215 p.
- Chang C.C., Hsieh Y.Y., Lin W.H., Lin C.S. Leiomyoma and vascular endothelial growth factor gene polymorphisms: a systematic review. Taiwan J Obstet Gynecol. 2010; 49(3): 247-53. doi: 10.1016/S1028-4559(10)60056-3
- Mittal P., Shin Y.H., Yatsenko S.A., Castro C.A., Surti U., Rajkovic A. MED12 gain-of-function mutation causes leiomyomas and genomic instability. J. Clin. Invest. 2015; 125(8): 3280-4. DOI: 10.1172/JCI81534
- Di Tommaso S., Massari S., Malvasi A., Vergara D., Maffia M., Greco M., Tinelli A. Selective genetic analysis of myoma pseudocapsule and potential biological impact on uterine fibroid medical therapy. Expert Opin. Ther. Targets. 2015; 19(1): 7-12. doi: 10.1517/14728222.2014.975793
- Osinovskaya N.S., Malysheva O.V., Shved N.Yu., Ivashchenko T.E., Sultanov I.Yu., Efimova O.A., Yarmolinskaya M.I., Bezhenar V.F., Baranov V.S. Frequency and spectrum of MED12 Exon 2 mutations in multiple versus solitary uterine, leiomyomas from Russian patients. Int. J. Gynecol. Pathol. 2016; 35(6): 509-15. doi: 10.1097/PGP.0000000000000255.
- Кузнецова М.В., Трофимов Д.Ю., Тихончук Е.Ю., Согоян Н.С., Адамян Л.В., Сухих Г.Т. Молекулярные механизмы патогенеза миомы матки: анализ мутаций гена MED12 в российской популяции. М.; 2016. [Kuznetsova M.V., Trofimov D.Yu., Tikhonchuk E.Yu., Sogoyan N.S., Adamyan L.V., Sukhikh G.T., Molecular mechanisms of the pathogenesis of uterine fibroids: analysis of mutations in the MED12 gene in the Russian population, M., 2016. (in Russian)].
- Согоян Н.С., Кузнецова М.В., Асатурова А.В., Адамян Л.В., Трофимов Д.Ю. Соматические мутации в экзоне 2 гена MED12 у женщин с одиночной и множественной миомой матки. Акушерство и гинекология. 2018; 12: 63-70. [Sogoyan N.S., Kuznetsova M.V., Asaturova A.V., Adamyan L.V., Trofimov D.Yu. Somatic mutations in MED12 gene exon 2 in women with a single uterine fibroid or multiple ones. 2018; (12): 63-70. (in Russian)]. doi.org/10.18565/aig.2018.12.63-70.
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P.A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L.M., Ding W., et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994; 266(5182): 66-71. DOI: 10.1126/science.7545954
- Wooster R., Bignell G., Lancaster J., Swift S., Seal S., Mangion J., Collins N., Gregory S., Gumbs C., Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995; 379(6567): 749. DOI: 10.1038/378789a0
- Domchek S.M., et al, Breast cancer risks in individuals testing negative for a known family mutation in BRCA1 or BRCA2. Breast Cancer Res Treat. 2010;119(2):409-14. doi: 10.1007/s10549-009-0611-y
- Mamma Print Test. URL: https://www.breastcancer.org/symptoms/testing/types/mammaprint
- Rahmioglu N., Nyholt D.R., Morris A.P., Missmer S.A., Montgomery G.W., Zondervan K.T. Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Hum. Repr. Update. 2014, 20 (5): 702-16. DOI: 10.1093/humupd/dmu015
- Пшеничнюк Е.Ю., Кузнецова М.В., Бурменская О.В., Кочеткова Т.О., Непша О.С., Трофимов Д.Ю., Адамян Л.В. Ассоциация между частотами встречаемости однонуклеотидных полиморфизмов в генах ZNF366 и VEZT и риском развития наружного генитального эндометриоза: данные по российской популяции. Акушерство и гинекология. 2017; 6: 64-72.[Pshenichnyuk E.Yu., Kuznetsova M.V., Burmenskaya O.V., Kochetkova T.O., Nepsha O.S., Trofimov D.Yu., Adamyan L.V. An association between the frequencies of single nucleotide polymorphisms in the ZNF366 and VEZT genes and the risk of external genital endometriosis: Data on a Russian population. Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2017; (6): 64-72. (in Russian)] https://dx.doi.org/10.18565/aig.2017.6.64-72
- Eggert S.L., Huyck K.L., Somasundaram P., Kavalla R., Stewart E.A., Lu A.T., Painter J.N., Montgomery G.W., et. al. Genome-wide Linkage and Association Analyses Implicate FASN in Predisposition to Uterine Leiomyomata. Am. J. Hum. Genet. 2012; 91(4): 621-8. doi: 10.1016/j.ajhg.2012.08.009.
- Gallagher C.S., Makinen N., Harris H.R., Uimari O., Cook J.P., Shigesi N., Rahmioglu N., Ferreira et al. Genome-wide association analysis identifies 27 novel loci associated with uterine leiomyomata revealing common genetic origins with endometriosis. BioRxiv. 2018; 324905. doi: doi: https://doi.org/10.1101/324905
- Cha P.C., Takahashi A., Hosono N., Low S.K., Kamatani N., Kubo M., Nakamura Y. A genome-wide association study identifies three loci associated with susceptibility to uterine fibroids. Nature Genetics. 2011; 43(5): 447-50. doi:10.1038/ng.805.
Received 27.03.2019
Accepted 19.04.2019
About the Authors
Nelly S. Sogoyan, resident of Academician V. I. Kulakov Research Center of Obstetrics, Gynecology and Perinatology.119997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +79859158217, Е-mail: sogoyan.n@mail.ru
Maria V. Kuznetsova, PhD, research scientist, laboratory of molecular-genetic methods, of Academician V. I. Kulakov Research Center of Obstetrics, Gynecology and Perinatology. 119997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381341. Е-mail: mkarja@mail.ru
Elena. A. Lolomadze , biologist, laboratory for genome editing, FSBI «Research Center of Obstetrics, Gynecology and Perinatology of V.I. Kulakov» Ministry of Healthcare of the Russian Federation. Address: 119997, Russian Federation, Moscow, Oparin street, 4. Phone: +7(977) 380-57-49. E-mail: 6332424@gmail.com
Galina V. Mikhaylovskaya, biologist, Department of clinical and molecular genetics, FSBI «Research Center of Obstetrics, Gynecology and Perinatology of V.I. Kulakov» Ministry of Healthcare of the Russian Federation. Address: 119997, Russian Federation, Moscow, Oparin street, 4. Phone: +7(903)750-15-10. E-mail: galinavalkuz@mail.ru
Nataliia D. Mishina, junior researcher, Department of clinical and molecular genetics, FSBI «Research Center of Obstetrics, Gynecology and Perinatology of V.I. Kulakov» Ministry of Healthcare of the Russian Federation. Address: 119997, Russian Federation, Moscow, Oparin street, 4. Phone: +7(985)217-29-89. E-mail: mis7ha@gmail.com
Dmitry Yu. Trofimov, DSci, professor RAS, Head of Laboratory of molecular-genetic methods, of Academician V. I. Kulakov Research Center of Obstetrics, Gynecology and Perinatology. 119997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954381341. ORCID.org/0000-0003-0001-1765ology
Leila V. Adamyan, Academician of RAS, MD, PhD, Professor RAS, Honored Master of Science of the Russian Federation, Head Specialist in Obstetrics and Gynecology of Ministry of Healthcare of Russia, Head of the Department of Surgical Gynecology of Academician V. I. Kulakov Research Center of Obstetrics, Gynecology, and Perinatology, Ministry of Healthcare of Russia, Head of the Department of Reproductive Medicine and Surgery, Faculty of Postgraduate Education, Moscow State University of Medicine and Dentistry. 119997, Russia, Moscow, Ac. Oparina str. 4. Tel.: +74954384068. E-mail: l_adamyan@oparina4.ru
For citation: Sogoyan N.S., Kuznetsova M.V., Lolomadze E.A., Mikhailovskaya G.V., Mishina N.D., Trofimov D.Yu., Adamyan L.V. A study of polymorphisms rs3020434, rs11742635, rs124577644, rs12637801, rs2861221, and rs17677069 in women with uterine leiomyomas and a family history of the disease.
Akusherstvo i Ginekologiya/ Obstetrics and gynecology. 2019; 10: 115-28.(In Russian).
https://dx.doi.org/10.18565/aig.2019.10.115-128



