Миома матки является наиболее распространенной опухолью малого таза, поражающей около 70% женщин во всем мире [1]. Заболевание клинически проявляется только у 30% женщин репродуктивного возраста [2]. Опухоль проявляется выраженными менструальными кровотечениями, часто приводящими к тяжелой железодефицитной анемии, болями в области таза, нарушениями репродуктивной функции, включая бесплодие и осложнения беременности [1, 3]. Миома матки является основной причиной гистерэктомий, на долю которых приходится по меньшей мере одна треть от всех гистерэктомий [4].
Высокая распространенность миомы матки оказывает выраженное экономическое влияние на здравоохранение во всем мире. По данным литературы, затраты, связанные с лечением миомы матки, составляют ежегодно в США до 34,4 млрд долларов, в Германии – 348 млн долларов, во Франции – 120 млн долларов и 86 млн долларов в Англии [5]. Следует отметить, что расходы на лечение женщин, имеющих миому матки, превышают затраты на лечение женщин с такими распространенными опухолями, как рак молочной железы и рак яичников [5]. Дополнительно к прямым расходам здравоохранения, косвенные затраты вследствие временной нетрудоспособности и инвалидности женщин с миомой матки оцениваются в мире в 1,6–17,2 млрд долларов ежегодно [6].
На сегодняшний день этиологические факторы и патогенетические механизмы развития миомы матки (рисунок) в значительной степени остаются загадкой. Согласно данным литературы, генетические, эпигенетические факторы, нарушения регуляции ключевых сигнальных путей, участвующих в клеточной пролиферации, апоптозе, разрастании внеклеточного матрикса, а также реакции на стероидные гормоны, играют важную роль в формировании и росте миоматозных узлов [1–3, 7–9].
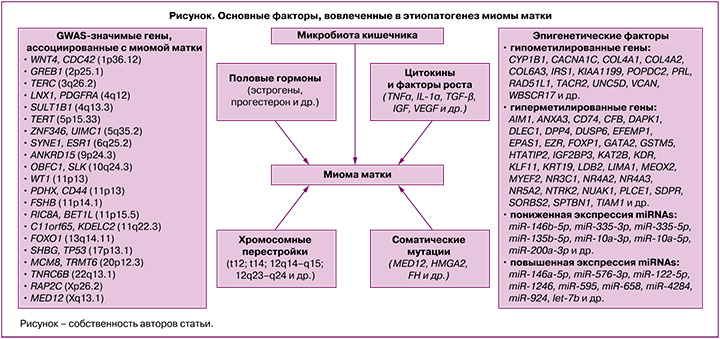
Модель этиопатогенеза миомы матки включает генетическую трансформацию единственной стволовой клетки миометрия в клетку, инициирующую опухоль, которая под влиянием эндокринных, аутокринных, паракринных факторов роста и сигнализации гормональных рецепторов «включается» в процесс клонального роста опухоли [10].
Миома матки характеризуется разнообразными хромосомными аномалиями, которые регистрируются у 40% женщин с данным заболеванием, и высокой гетерогенностью соматических мутаций [3, 7, 8]. К основным цитогенетическим изменениям в миоматозных узлах можно отнести трисомию по 12 хромосоме, транслокацию (t12; t14) (12q14–q15; 12q23–q24), делеции в 7 хромосоме (7q22–q32), локусах 3q и 1p, а также хромосомные перестройки в локусах 6p21, 10q22 и 13q21–q22 и хромотрипсис [3, 11].
Важное значение в этиопатогенезе заболевания имеют мутации ряда генов [12]. Наиболее частыми соматическими мутациями при миоме матки являются мутации в генах MED12, HMGA2 и FH [12]. В результате секвенирования 2 экзона гена MED12 показано, что до 90% миом матки содержат специфические мутации в субъединице 12 медиаторного комплекса [1]. Важно отметить, что этот показатель значительно варьирует в зависимости от этнической принадлежности пациенток [13]. В метаанализе, выполненном He C. et. al. (2022), установлено, что мутации в MED12 чаще наблюдались у афроамериканских женщин (74,5%) по сравнению с европеоидными (65,8%) и азиатскими (53,2%) пациентками с миомой матки [13]. Также в литературе имеются исследования, посвященные анализу корреляций мутаций в MED12 с размерами миоматозных узлов и их количеством. Выявлено, что мутации в MED12 чаще регистрируются при множественных миоматозных узлах с небольшой митотической активностью, субсерозной локализации и с высоким содержанием внеклеточного матрикса [13–15].
MED12 представляет собой часть медиатора мультипротеинового комплекса, который является эволюционно сохраненным регулятором транскрипции, опосредуемой РНК-полимеразой II [15]. Ген MED12 расположен в Х-хромосоме и находится в высококонсервативном участке генома. Мутации в MED12 приводят к отсоединению циклина С и CDK8/19 от основного медиатора, потере активности CDK-киназы и формированию в результате этого «уникального» паттерна экспрессии ряда генов (Axin2, cyclin D1, Myc и Wnt), вовлеченных в процессы клеточного цикла [14, 16]. Наряду с миомой матки мутации в MED12 также связаны с другими гормонозависимыми опухолями женского организма, такими как фиброаденома и филлодные опухоли молочных желез [17], аденомиома матки [18].
Другой распространенной мутацией при миоме матки является мутация в гене HMGA2, которая наблюдается примерно в 10% случаев заболевания [14]. Ген HMGA2 локализуется в 12-й хромосоме человека (q14.3) и кодирует один из представителей группы белков с высокой мобильностью. HMGA2 – хроматин-связывающий негистоновый белок, который обычно экспрессируется только в недифференцированной мезенхимальной ткани, играет важную роль в пролиферации, дифференцировке клеток и апоптозе [19, 20]. Согласно данным литературы, высокая экспрессия гена HMGA2 регистрируется только во время эмбриогенеза и снижается до необнаруживаемых уровней в тканях взрослого организма [15, 19]. Наряду с этим имеются отдельные данные о «возобновлении» экспрессии гена HMGA2 в дифференцированных тканях, которая вызывает мезенхимальный онкогенез. В основе этого «возобновления» экспрессии гена HMGA2 может лежать хромосомная перестройка в области 12q15 в клетках миометрия [21]. Исследования как in vitro, так и in vivo показали, что сверхэкспрессия HMGA2 способствует повышенной продукции факторов ангиогенеза как в нормальных клетках миометрия, так и в клетках опухоли [20]. Гену HMGA2 принадлежит одна из ключевых ролей в регуляции ангиогенеза, сверхэкспрессия этого гена коррелирует с повышенной плотностью сосудистой сети, что является важным фактором в развитии опухоли [19, 20].
Итак, мутации в генах MED12 и HMGA2 наблюдаются примерно в 80–90% миом матки, но следует отметить, что данные мутации, как правило, являются взаимоисключающими (присутствует в опухоли только одна какая-либо из этих 2 мутаций) [10], а также характеризуются разнонаправленными эффектами по отношению к опухоли: у женщин с мутацией в гене HMGA2 наблюдаются более крупные миоматозные узлы в отличие от женщин, имеющих мутацию в гене MED12, для которых характерны множественные миоматозные узлы меньшего размера [19]. Вместе с этим в исследовании, выполненном Galindo L.J. et al., показано, что в 50% опухолей одновременно наблюдались как мутации в MED12, так и сверхэкспрессия мРНК HMGA2, что позволило авторам предположить, что совместные изменения в обоих генах достаточно часты при миоме матки [22].
Третья по частоте встречаемости соматическая мутация при миоме матки – мутация в гене FH, которая составляет 1–2% случаев [1]. Данный ген, расположенный в 1-й хромосоме человека (q42.3–q43), кодирует фермент фумаратгидратазу, которая в цикле трикарбоновых кислот катализирует превращение фумарата в L-малат, участвующий в энергетическом метаболизме клетки [23]. Клетки с дефицитом фермента фумаратгидратазы теряют способность метаболизировать фумарат, и, следовательно, повышается его внутриклеточный уровень, что приводит к серии метаболических нарушений [24]. Так, в митохондриях снижается окислительное фосфорилирование, активируются аэробный гликолиз, катаболизм глютамина и основные анаболические пути белков HIF-1α и HIF-2α, которые способны индуцировать транскрипцию ключевых генов, участвующих в ангиогенезе и росте клеток [25]. Кроме того, нарушается репарация ДНК за счет изменения метилирования гистонов [23]. Еще одним онкогенным эффектом дефицита фермента фумаратгидратазы является стимулирование перехода эпителия в мезенхиму посредством подавления экспрессии микроРНК семейства miR-200 [26].
Интересным представляется тот факт, что миомы матки с мутацией FH имеют специфические гистоморфологические признаки, которые включают ядерную атипию симпластического типа, отек альвеолярного рисунка, а также заметные эозинофильные ядрышки, окруженные перинуклеолярными ореолами и эозинофильными цитоплазматическими включениями [27]. Следует отметить, что миомы матки с дефицитом фермента фумаратгидратазы не связаны с повышенным риском злокачественности [28], однако они могут характеризоваться ранним началом и множественными миоматозными узлами [29]. Важно отметить, что гетерозиготные мутации в гене FH, возникшие в зародышевой клеточной линии, приводят к развитию аутосомно-доминантного онкологического синдрома, известного как наследственный лейомиоматоз и почечно-клеточный рак (HLRCC) [28].
Наряду с хромосомными перестройками и соматическими мутациями, развитие заболевания в значительной степени зависит от полиморфизма ряда генов-кандидатов, в том числе генов половых гормонов, их рецепторов, цитокинов, факторов роста, апоптоза и др. [3]. На сегодняшний день выполнено 19 полногеномных исследований (GWAS) ассоциаций однонуклеотидных полиморфных локусов с развитием матки [https://www.ebi.ac.uk/gwas/search?query=uterine%20fibroids], а также большое количество ассоциативных исследований, направленных на изучение вовлеченности отдельных полиморфных маркеров, а также межгенных взаимодействий в формирование данного заболевания. В результате этих исследований имеются данные с риском развития заболевания более 20 GWAS-значимых локусов [https://www.ebi.ac.uk/gwas/search?query=uterine%20fibroids] и свыше 100 полиморфизмов, показавших свою вовлеченность в развитие заболевания в других ассоциативных исследованиях [3, 30, 31]. При этом результаты исследований нередко неоднозначны, что обусловлено как межэтническими генетическими различиями пациенток с миомой матки, так и многофакторной природой данного заболевания и обусловленными этим различиями в характере генно-средовых взаимодействий в различных исследуемых этно-территориальных группах населения, имеющих свои особенности как в генетической «конституции», так и в средовых факторах риска развития заболевания (их перечень, встречаемость и степень выраженности).
Важная роль в формировании и прогрессировании миомы матки принадлежит эпигенетическим факторам, влияющим на экспрессию генов, вовлеченных в патогенез данного заболевания [7, 8]. Основные эпигенетические механизмы включают метилирование ДНК, модификацию гистонов, микро-РНК (miRNAs) и длинные некодирующие РНК (lncRNAs). Метилирование ДНК является общим механизмом программирования клеток как при нормальном росте ткани, так и при развитии опухоли посредством изменения нормального профиля экспрессии мРНК в клетках миометрия [32]. В литературе имеется информация о примерно 120 генах, уровень метилирования которых (гипо- и гиперметилирование) изменен при миоме матки [14].
Микро-РНК играют значимую роль в эпигенетическом контроле экспрессии генов в различных органах, включая матку [33–35]. Недавние исследования, посвященные изучению роли miRNAs в патофизиологии миомы матки, показали, что miRNAs действуют как опухолевые супрессоры и антиапоптотические медиаторы (семейство let-7, кластер miR-17-92, miR-372-373, miR-155-BIC, miR 15/16, miR-20, miR-21, miR-26a), а также играют роль в дифференцировке, пролиферации (семейство let-7, miR-181b, miR-21), воспалении (miR-125, miR-155) и ремоделировании внеклеточного матрикса (miR-21, miR-192, miR-206, miR-1, miR-133a) [35, 36]. Таким образом, miRNAs являются важнейшими регуляторами клеточных процессов в организме при миоме матки, таких как клеточная пролиферация, апоптоз, ремоделирование внеклеточного матрикса, ангиогенез и воспаление [34, 36].
В клетках миоматозных узлов зарегистрированы изменения в экспрессии значительного числа генов lncRNAs (HOTTIP, H19, CAR10, APTR, TCONS_l2_00000923, CASC15, UC.10, XIST, LINC00890, LNCRNA-ATB, HULC, MEG3, BX640708, TSIX, UCA1, AK023096, MAMDC2AS1) в сравнении с нормальными клетками миометрия [37]. Следует отметить, что lncRNA H19 является регулятором экспрессии генов, связанных с пролиферацией, воспалением – HMGA2, MED12 (ключевые для миомы матки), TET3 и генов, ремоделирующих внеклеточный матрикс [38]. lncRNA генов APTR и XIST участвуют в гормонозависимой пролиферации клеток миомы матки, активации сигнального пути WNT и экспрессии генов COL1A1, COL3A1 и FN1 [39, 40].
Половые гормоны играют ключевую роль в регуляции роста миомы матки [41]. Однако, несмотря на большое количество исследований, посвященных изучению роли половых гормонов, в частности эстрогенов и прогестерона, в патофизиологии данного заболевания молекулярные механизмы, с помощью которых эстрогены и прогестерон способствуют развитию миомы матки, в значительной степени неизвестны [8, 42]. Миома матки считается эстроген-зависимой опухолью, основываясь на ее связи с репродуктивным возрастом женщины – отсутствие опухоли матки до менархе и низкая частота встречаемости миоматозных узлов после менопаузы [43]. Кроме того, следует отметить, что для клеток опухоли характерна сверхэкспрессия рецепторов эстрогенов, а также фермента ароматазы, катализирующей превращение андрогенов в эстрогены по сравнению с клетками миометрия [43]. Однако следует отметить, что уровень эстрогенов в сыворотке крови у женщин с миомой и без нее не имеют статистически значимых различий [43]. Кроме того, для подтверждения важной роли стероидных гормонов яичников в патогенезе опухоли служат данные исследований, показывающих замедление роста миоматозных узлов при непрерывном лечении агонистами гонадотропин-рилизинг-гормона (ГнРГ) женщин в течение 3 месяцев [41, 44]. Это обусловлено уменьшением выброса эстрогенов и прогестерона в ответ на снижение активности рецептора ГнРГ в гипофизе.
Биосинтез эстрогенов происходит в яичниках под действием лютеинизирующего и фолликулостимулирующего гормонов, которые регулируются ГнРГ [45]. Эстрогены опосредуют свои биологические эффекты на клетки-мишени несколькими путями, которые можно классифицировать как геномные и негеномные. Геномные пути зависят от модуляции транскрипционной активности генов. Комплексы эстроген – эстрогеновый рецептор могут как «напрямую» связываться с регуляторными областями «генов-мишеней» и оказывать влияние на их экспрессию, так и взаимодействовать с определенным ДНК-связывающим транскрипционным фактором (специфичный белок 1, ядерный фактор kB, CCAAT/энхансер-связывающий белок β, GATA-связывающий белок 1) [1, 43]. Конечным результатом этого является активация или подавление экспрессии целевого ряда генов, кодирующих факторы роста, белки внеклеточного матрикса и другие в «чувствительных» к эстрогенам тканях [46]. Негеномные пути, как правило, реализуются через активацию различных сигнальных каскадов (Ras–Raf–MEK–MAPK, PI3K/Akt, PLC/PKC, cAMP/PKA, Wnt/β-катенин), которые, в свою очередь, косвенно могут модулировать экспрессию определенных генов [1, 43].
Обращает на себя внимание тот факт, что рост миоматозных узлов стимулируют не только «естественные» эстрогены, образующиеся в яичниках, но и эстрогены, которые формируются за счет локального превращения андрогенов внутри самих опухолей [3, 43]. Так, тестостерон и андростендион превращаются в эстрадиол в опухолевых клетках (ферменты – ароматаза и 17β-гидроксистероиддегидрогеназа), что приводит к локальной гиперэстрогении в области миомы матки [3, 43].
Эффекты эстрогенов в организме напрямую зависят от содержания их свободных форм. При этом важная роль отводится глобулину, связывающему половые гормоны (SHBG) [47]. По данным литературы, около 38% эстрогенов связывается с SHBG, 60% – с альбумином и только 2% являются свободными, и именно эти эстрогены проявляют биологическую активность в организме женщины [48]. Таким образом, SHBG регулирует концентрацию биологически активных эстрогенов в крови, влияя на их биодоступность [47, 49]. При этом следует отметить, что, несмотря на очевидную патогенетическую значимость SHBG для миомы матки, работы, посвященные изучению связи SHBG с заболеванием, отсутствуют, хотя очевидно, что SHBG, детерминируя в значительной степени уровень свободных эстрогенов, будет таким образом и существенно влиять на фенотипические эффекты эстрогенов в организме и, в том числе, при формировании миомы матки. В литературе указывается, что одним из значимых факторов развития заболевания у женщин, имеющих ожирение, является сниженная продукция SHBG, что приводит к повышению уровня несвязанных циркулирующих эстрогенов, являющихся фактором риска для миомы матки [50].
По данным литературы, эстрогены в клетках опухоли вызывают увеличение экспрессии рецепторов прогестерона, что делает миому матки более «чувствительной» к сигналам данного гормона [8]. На животных моделях показано, что уровень экспрессии рецепторов прогестерона в миоматозных узлах выше по сравнению с рецепторами эстрогенов [51]. В исследовании, выполненном Khan K.N. et al. выявлено, что у женщин с миомой матки, не получавших терапию агонистами ГнРГ, содержание рецепторов прогестерона было значительно выше, чем рецепторов эстрогенов [44]. Однако в литературе есть и противоположные данные, демонстрирующие преобладание эстрогеновых рецепторов в опухоли [43]. Обращает на себя внимание, что митотическая активность в миоматозных клетках выше во время секреторной фазы менструального цикла (когда доминирует прогестерон), чем во время пролиферативной фазы (когда доминируют эстрогены) [52]. Таким образом, прогестерону отводится важная роль в патофизиологии миомы матки [8]. Данный гормон стимулирует пролиферацию, способствуя аномальному разрастанию внеклеточного матрикса, и, таким образом, индуцирует рост опухоли [8]. Следует отметить, что регуляция роста миомы матки, опосредованная прогестероном, происходит не только за счет повышенной пролиферации, но и за счет снижения апоптоза через индукцию экспрессии белка B-клеточной лимфомы-2 (Bcl-2) [2]. Свои биологические эффекты прогестерон реализует двумя путями, которые можно классифицировать как геномные и негеномные. Геномный механизм заключается в том, что комплекс прогестерона и рецептора прогестерона самостоятельно или через факторы транскрипции взаимодействует с ДНК и регулирует экспрессию генов-мишеней, в частности ядерного антигена пролиферирующих клеток (PCNA), эпидермального фактора роста (EGF), трансформирующего фактора роста бета (TGF-β), антиапоптотического белка (Bcl-2) [42]. Согласно негеномному пути, комплекс прогестерона и рецептора прогестерона активирует множество быстрых сигнальных путей в клетках, вовлеченных в патофизиологию миомы матки [42].
Значимую роль в патогенезе миомы матки играют гормоны передней (лютеинизирующий гормон) и задней (пролактин) долей гипофиза [4, 53]. Согласно результатам исследования, выполненного Baird D.D. et al., у женщин с более высоким уровнем лютеинизирующего гормона вероятность развития миомы матки была значительно выше [54]. Литературные данные свидетельствуют о том, что пролактин – гормон, ответственный за лактацию, присутствует и функционирует в миоматозных клетках [55]. Пролактин активирует передачу сигналов STAT5 и MAPK в клеточных линиях миометрия крысы и человека и, таким образом, участвует в регуляции развития/роста опухоли матки. Кроме того, данный гормон стимулирует экспрессию маркеров миофибробластов в клетках миометрия крысы [55].
Негормональными регуляторами развития миомы матки являются факторы роста и провоспалительные цитокины, которые «отвечают» за многочисленные аспекты клеточного цикла, включая пролиферацию, миграцию и дифференцировку [56, 57]. Трансформирующий фактор роста β (TGF-β) считается одним из наиболее значимых факторов роста и имеет ключевое значение в патогенезе ряда заболеваний, ассоциированных с фиброзом, в том числе и миомы матки [58]. Его концентрация в опухоли матки в 3–5 раз выше, чем в нормальном миометрии [58]. По данным литературы, аномальное разрастание внеклеточного матрикса при миоме матки связано с активацией сигнальных путей TGF-β [59]. В нормальных гладкомышечных клетках TGF-β действует как мощный опухолевый супрессор посредством ингибирования роста и стимуляции апоптоза. А в клетках опухоли матки, для которых характерна избыточная экспрессия TGF-β, он является индуктором роста миоматозного узла [60].
Цитокины играют важную роль во многих биологических процессах организма, включая онкогенез. Цитокины стимулируют пролиферацию клеток опухоли, регулируют ангиогенез и апоптоз, участвуют в формировании внеклеточного матрикса [61, 62]. Установлено, что ключевые провоспалительные цитокины, такие как фактор некроза опухоли (TNF)-α, интерлейкин (IL)-1β, интерферон (INF)-γ и NF-κB сверхэкспрессируются в клетках миомы матки по сравнению с нормальными клетками миометрия [63]. Кроме того, в литературе есть данные о том, что уровень TNF-α в сыворотке крови у женщин с клиническими симптомами миомы матки достоверно выше, чем в группе здоровых женщин [64]. Следует отметить, что TNFα является мощным стимулятором активности фермента ароматазы и за счет этого способствует локальной гиперэстрогении [64].
Интересные данные представлены в работе K V.K. et al., где указывается, что микробиота кишечника играет значимую роль в патогенезе миомы матки [65]. Предполагается, что дисбактериоз кишечника, характеризующийся изменением его микробиома, запускает следующие пути, важные для патофизиологии миомы матки: 1) нарушается функционирование микробиом-зависимого «эстроболома» (группа бактерий, включающая Eubacterium lentum, Bacteroides sp., Bifidobacterium sp., Streptococcus sp. и др., которые могут метаболизировать эстрогены), что приводит к гиперэстрогении, являющейся известным фактором риска развития миомы матки; 2) изменяются уровни кишечных метаболитов (короткоцепочечные жирные кислоты), что вызывает различные иммунные нарушения и, тем самым, индуцирует воспаление (потенцирует развитие гиперэстрогении) [65].
Заключение
Таким образом, современные представления об этиопатогенезе миомы матки свидетельствуют о том, что данное заболевание имеет сложную, многофакторную природу, в развитии которого задействованы генетические и эпигенетические механизмы, реакции на стероидные гормоны, нарушения регуляции ключевых сигнальных путей и др. Однако, несмотря на существенный прогресс в понимании патофизиологии миомы матки, на сегодняшний день остается больше вопросов, чем ответов.



