Exploration of protein PCP4 as a potential tumor marker in uterine leiomyoma
Kuznetsova M.V., Shevelev A.B., Pozdnyakova N.V., Samoilova D.V., Karyagina V.E., Tonoyan N.M., Trubnikova E.V., Zelensky D.V., Vishnyakova P.A.
Objective: Evaluation of the possible role of Purkinje cell protein 4 (PCP4) as a potential tumor marker of uterine leiomyoma by measurement of antibody titer against this protein in blood serum and the applicability of this technique for evaluation of proteins as potential vaccine antigens.
Materials and methods: cDNA fragment clone was derived from PCP4 gene in leiomyoma nodule. After that the soluble PCP4-GFP chimera based on E. coli was constructed, and the recombinant protein was purified. This product was used to evaluate the upper limit of titer to detect antibodies in blood serum in patients using indirect enzyme immunoassay. Serum samples (24) were collected from donors among the patients undergoing treatment at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, who gave informed consent to participate in the study. Four groups of patients were formed to study the immunological activity of sera against the 6His-PCP4hs-GFP protein. Group 1 was the control group and was composed of men. Group 2 consisted of women without diagnosed fibroids who had five or more successful pregnancies in history. Group 3 included women with fibroids, who also had five or more successful pregnancies in history. Group 4 included women with recurrent myomas.
Results: The pRSET-EmGFP expression vector containing the PCP4 gene and the reporter gene of the green fluorescent protein EmGFP was derived from the cDNA of the myoma nodule with a driver mutation in the MED12 gene. Human PCP4 in preparative amounts was obtained using this construct embedded in the E. coli genome, that was sufficient for analysis of its reaction with antibodies from blood serum in different groups of patients. Blood sera in the control group of men showed high immunological reactivity against the 6His-PCP4hs-GFP protein, whereas the difference between the groups of women was insignificant. Minimum difference between sera was found in groups 2 and 4. Moreover, the reaction of sera in women with recurrent fibroids was higher compared with women without fibroids, who had five or more successful pregnancies. Comparison of women in groups 3 and 4 showed statistically significant differences in the optical density values obtained in dilution of sera 1:1600, 1:3200 and 1:6400. At the same time the reaction of sera of women with recurrent fibroids was higher compared with sera of women with fibroids, who had five or more successful pregnancies.
Based on the level of activity, sera were divided into 3 categories. It was found that reaction of sera of men against the 6His-PCP4hs-GFP antigen was most pronounced. The proportion of high activity in sera was 60%. In the group of women without fibroids, who had five or more successful pregnancies, the proportion of low activity in sera was 44%, that is the maximum value of indicator compared with the other groups. The group of women with fibroids, who had five or more successful pregnancies, is characterized by the moderate level of reactivity with the 6His-PCP4hs-GFP antigen. This category accounts for 75% of sera, that is the highest value of indicator among all groups. Finally, the group of women with recurrent fibroids is characterized by equal number of sera with high and moderate activity, that shows a greater tendency for production of antibodies against PCP4/PEP19 in this group of patients compared with multiparous women.
Conclusion: For the first time, human PCP4 was in preparative amounts sufficient for analysis of its reaction with antibodies from blood serum in different groups of patients. Testing showed that most samples in all groups had significant titers of antibodies against PCP4. The activity of these antibodies varies widely both in sera of men and women. Antibodies against PCP4 are most common in women with recurrent fibroids compared with multiparous women, especially without leiomyomas. Thus, it is unlikely that PCP4 immunization сarries the risk of any pathology. The titers of antibodies to PCP4 in the general group of multiparous women were significantly lower compared with men and patients with recurrent fibroids. Therefore, using PCP4 as the basis to develop a preventive vaccine against leiomyomas is not advisable.
Authors' contributions: Kuznetsova M.V. – literature review, development of the concept and design of the study; Pozdnyzkova N.V. – protein cloning; Vypryazhkina V.E. – identification of proteins, blot analysis; Trubnikova E.V., Zelensky D.V. – statistical data processing; Kuznetsova M.V., Shevelev A.B. – text writing; Vishnyakova P.А. – final text editing.
Conflicts of interest: The authors confirm that they have no conflicts of interest to declare.
Funding: The study was conducted within the RNF project 23-15-00069 "Prophilactic vaccine development to prevent uterine leiomyoma in patients planning pregnancy".
Ethical Approval: The study was approved by the Ethics Committee of the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia
Patient Consent for Publication: The patients have signed informed consent for participation in the study and publication of the obtained data.
Authors' Data Sharing Statement: The data supporting the findings of this study are available on request from the corresponding author after approval from the principal investigator.
For citation: Kuznetsova M.V., Shevelev A.B., Pozdnyakova N.V., Samoilova D.V., Karyagina V.E.,
Tonoyan N.M., Trubnikova E.V., Zelensky D.V., Vishnyakova P.A. Exploration of protein PCP4 as
a potential tumor marker in uterine leiomyoma.
Akusherstvo i Ginekologiya/Obstetrics and Gynecology. 2025; (8): 159-171 (in Russian)
https://dx.doi.org/10.18565/aig.2025.171
Keywords
Uterine fibroids (also known as uterine leiomyomas) affect women aged 35 – 50 years and are most common benign tumors of the reproductive system [1]. According to various estimates, the incidence of this condition is up to 50% among women of reproductive age. Among gynecological pathology, uterine fibroids rank second after inflammatory diseases [2]. Among women diagnosed with uterine leiomyomas, 15–30% have different symptom severity, including pain syndrome, infertility, dysfunction of neighboring organs, abnormal uterine bleeding, anemia, and other severe complications [3]. Despite the benign nature, leiomyomas have potential to metastasize outside the uterus and penetrate into different tissues in the body, in particular, into the lungs [4]. In gynecological hospitals in Russia, up to 50-70% of surgeries are performed specifically for symptomatic uterine leiomyomas. Moreover, in 40% – 60% of cases, hysterectomy is performed specifically for this disease. In European countries, more than 300 000 surgical interventions for uterine fibroids are performed per year [5], while in the United Stated, about 600,000 hysterectomies are performed per year, of which about 200 000 are done for uterine fibroids [6].
It is well known that uterine fibroids can grow rapidly during pregnancy. This can lead to impaired placental growth and fetal malformations. In the presence of subserosal fibroids, adhesions are formed between the internal organs, that leads to difficulties with uterine contractions in labor and after birth. Due to this, the surgeons often have to perform emergency myomectomy in early pregnancy, or cesarean section before the expected delivery date [7]. Postoperative recurrence is a challenge in treatment of the disease, since according to our data, the recurrence rate after myomectomy for multiple fibroids is over 70%. At the same time, in almost every fourth case, there is a need for treatment including repeated surgical intervention [8].
Hysterectomy is the most radical way to prevent leiomyoma recurrence. However, this option is unacceptable for women planning pregnancy. Moreover, a significant problem arising after myomectomy is injury to the uterus, formation of scars, that leads to impaired uterine function during pregnancy, and the risk of uterine rupture caused by stretching and contractions before and after birth. In addition, high progesterone levels in the blood contribute to the rapid and remarkable growth of fibroids during pregnancy [9]. Thus, one of the most significant medical and social problems in management of leiomyomas is the development of treatment methods that can prevent recurrence of the disease in patients planning or experiencing pregnancy.
In our previously published paper it has been suggested that in women with five or more successful pregnancies in history, reduced risk of developing leiomyomas is due to production of antibodies to embryonic proteins, which simultaneously can be tumor markers specific for leiomyomas [10]. Given this, it was suggested that preventive vaccine can be developed based on tumor markers, that could prevent the risk of recurrence of leiomyomas due to recognition by antibodies and destruction of myometrial cells undergoing tumor transformation, and fibroid precursor cells.
According to the above mentioned review article, as well as previously published papers by other authors [11, 12], as well as the NCBI GenBank database (https://www.ncbi.nlm.nih.gov/gene), one of the candidates for tumor markers of leiomyomas is Purkinje cell protein 4 (PCP4), also known as PEP19, which has elevated expression in all types of leiomyomas. However, absence or reduced PCP4 expression is in normal tissues of adult body, including myometrium.
According to current concepts, PCP4 is a calmodulin-binding protein. Low expression of PCP4 was found in Purkinje cells in the mouse cerebellum [13]. In addition, the presence of PCP4 mRNA was detected in the hippocampus and cerebral cortex. Immunohistochemical staining experiments detected PCP4 in the structures of neocortex, other cortical structures and parts of the hippocampal formation, in the olfactory bulb and the caudate nucleus, in some areas of the amygdala, in the thalamus (especially the dorsal lateral geniculate nucleus), and hypothalamus. Another study [14] reported that PCP4 plays and important role in regulation of aldosterone secretion in adrenal cells.
Some studies describe PCP4 as a marker for cancers. PCP4 overexpression is characteristic for different tumors. Thus, the study by Yoshimura T. et al. [15] reported high PCP4 levels in carcinoma cells of salivary gland. Another study [16] proved that PCP4 is involved in regulation of the expression ASCL1 and NEUROD1 in M17 glioma cell line. In addition, it was found that PCP4 can activate expression of the aromatase gene, that affects hormonal regulation of tumor growth and its sensitivity to certain chemicals [17].
As for the myometrium and uterine fibroids, a number of studies have shown low expression of PCP4 is in normal myometrial cells, and its production increases during pregnancy [18].
Given the above, to test the hypothesis about the suitability of PCP4 as a vaccine antigen for immunization, that can reduce the risk of developing leiomyomas, the presence of antibodies against PCP4 in the blood of patients in different groups was examined: (1) in multiparous women without leiomyomatosis, (2) multiparous women with leiomyomas, (3) women with recurrent leiomyomas; (4) men in the control group. The first step was cloning of cDNA fragment of the PCP4 gene in leiomyoma, then soluble PCP4-GFP chimeric protein based on E. coli was constructed. After that, the recombinant protein was purified, serum antibody titer of patients was measured using indirect enzyme-linked immunosorbent assay (ELISA).
The objective of the study was to evaluate the possible role of PCP4 as a potential tumor marker of uterine leiomyoma by measurement of antibody titer against this protein in blood serum and the applicability of this technique for evaluation of proteins as potential vaccine antigens.
Materials and methods
Clinical material
Tissue samples from women with uterine fibroids were collected during myomectomy and hysterectomy. Two tissue fragments from each uterine fibroid were placed in physiological saline solution. Then one tissue sample from each probe was frozen at -80ºС for subsequent storage in the Biobank collection at the Academician V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia (Kulakov Center). Pathomorphological verification of another tissue sample was performed to confirm the type of tumor as fibroid. In the process of testing, tissue samples were sent upon request to the Laboratory of molecular genetics for RNA extraction.
Tissue sample from leiomyoma was collected from the patient with multiple uterine fibroids, which was stored at -80°C before RNA extraction, and was used as the starting material to derive cDNA from the PCP4 gene. The highest level of this gene expression was previously detected in this sample using reverse transcription polymerase chain reaction (RT-PCR).
Serum samples from blood donors were collected among patients undergoing treatment at the Kulakov Center, who have signed informed consent to participate in the study. Due to this, medical history of all donors was available.
Venous blood samples (5–7 ml) from each donor were collected in the vacutainer tubes, incubated at room temperature for 15 minutes and centrifuged at 2000g for 10 minutes. After that, the resulting sera was separated from the clot, placed in a clean test tube and divided into aliquots of 0.5 ml and stored at -20°C until use. The serum aliquot directly involved in testing was stored at +4°C for 7 days.
Obtaining cDNA and genetically engineered construction
To isolate RNA, the leiomyoma sample was frozen in liquid nitrogen and homogenized. Total RNA was isolated using RNeasy Mini Kit (Qiagen, USA). Total cDNA was obtained using standard laboratory method [19].
The primers PCP4-HS-F1(BamHI) (ggggatccatgagtgagcgacaaggtgc) and Nco-PCP-R (tacccatggactgagacccagccttcttc) were used for PCR reactions. PCR was performed in a total volume of 15 μl, including 1 μl cDNA solution the DreamTaq PCR Master Mix 2X kit with the following thermal cycling parameters: preheating at 94°C for 3 min, 35 cycles; at 94°C for 30 sec., at 62°C for 30 sec., at 72°C for 12 sec.
The resulting 202 bp PCR product was purified by preparative elution from 1% agarose gel using the Zymo gel recovery kit (Zymo Research, USA), and was used for cloning into the pRSET-EmGFP vector (Thermo Fisher Scientific, USA, cat. No. V35320). For this purpose, it was treated with restriction endonucleases BamHI-HF (New England Biolabs, USA, cat. No. R3136S) and NcoI-HF (New England Biolabs, USA, cat. No. R3193) followed by purification using DNA Clean & Concentrator microcolumns (Zymo Research, USA). Similarly, the pRSET-EmGFP vector was treated with the same restriction enzymes and purified in the same way.
The resulting DNA fragments were mixed and ligated. The reaction was carried out at +22°C for 30 minutes. After that 3 μl reaction mixture was used to transform XL-1 competent cells. The grown colonies were tested for the presence of the insert using colony PCR with primers PCP4-HS-F1(BamHI) and RSET-oR1 (cgtcccattcgccattcagg). Colonies were considered to be positive, when using the material of colonies as a matrix, PCR products of 1136 bp in length were formed appropriate to the PCP4hs-EmGFP fusion gene. The conformity of the obtained the pRSET-PCP4hs-EmGFP structure to the expected construct was checked by Sanger sequencing after isolation of plasmid DNA from the culture using standard method.
Protein isolation and purification
The DNA of the pRSET-PCP4hs-EmGFP construct was used to transform competent E. coli NiCo21 cells (New England Biolabs, USA) designed for the expression of bacteriophage T7 under the control of the promoter gene 10. A single colony of the ampicillin-resistant transformants was used to inoculate a flask containing LB (Luria-Bertani) medium (10 g/L Bacto peptone, 5 g/L yeast extract, 10 g/L NaCl) supplemented with 100 μg/mL ampicillin and 0.2 mM isopropyl-b-D- thiogalactopyranoside (IPTG). The inculated flask was incubated at 37°C with shaking at 250 rpm for 24 h. The cells were pelleted by centrifuging and lysed with lysozyme. The lysate was sonicated and centrifuged. After that, the clarified lysate was used for protein purification by metal chelate affinity chromatography using HisPur™ Ni-NTA Resin (Thermo Scientific, cat. No. 88222, USA) in accordance with the manufacturer’s instruction for affinity adsorbent. The sample was loaded onto the column in buffer containing 20 mM Tris-HCl, pH 8.0, 0.3 M NaCl. The column was washed with 20 mM Tris-HCl, pH 8.0, NaCl 0.5 M. Elution buffer contained 20 mM Tris-HCl, pH 8.0, imidazole 0.5 M.
After purification, the eluate containing the 6His-PCP4hs-GFP protein was cleaned from salt by gel filtration on PD-10 column equilibrated with phosphate-buffered saline (pH 7.4). Protein homogeneity was analyzed using SDS electrophoresis following the Laemmli method.
Electrophoresis and immunoblotting
Protein electrophoresis under denaturing conditions was performed accoding to the previously described method. The following 4-fold concentrated buffers were used: electrode buffer containing 25 mM Tris-base – 92 mM glycine (pH 8.6), 0.1% sodium dodecyl sulfate (SDS); 4X upper (concentrating) buffer containing 50 mM Tris-HCl (pH 6.8), 0.4% SDS; lower (separating) gel buffer containing urea – 150 mM Tris-HCl (pH 8.8), Laemmli sample buffer containing urea 25 mM Tris-base, 192 μm glycine (pH 8.6), 1% SDS, 7 M urea, 50 mM dithiothreitol, 0.001% bromophenol blue. Electrophoresis was carried out at 250 V. The gel was of 5 cm long and 0.8 mm wide.
After electrophoresis, the gel was stained with 1% Coomassie R250 in 10% acetic acid and decolorized with 10% acetic acid. The separated proteins were transferred from the polyacrylamide gel (PAAG) onto Immobilon PVDF membrane (Millipore, USA) using semi-dry transfer (or electroblotting) system with Mini-Protean 3 cell (Bio-Rad, USA) for 1.5 h at current density of 0.8 mA/cm2 at +20°C. After protein transfer, the membrane was stained with 1% Ponceau S Red in 10% acetic acid. Prestained protein markers (BioRad, USA consisted of prestained protein standards 250, 150, 100, 75, 50, 37, 25, 20, 15 and 10 kDa.
After proteins were transferred to the membrane, it was washed in deionized water. Non-specific binding was blocked by incubation for 4–20 hours in 0.1% bovine serum albumin in PBS (10 mM sodium phosphate buffer (pH 7.2) containing 100 mM NaCl). The binding of antibodies in patient sera diluted 1000-fold was performed in PBS buffer with 0.03% Tween-20. After this, the membrane was washed three times in PBS with the addition of 0.03% Tween-20. The washed membrane was incubated for 2 h with moderate stirring speed in a solution containing protein G peroxidase conjugate at the concentration of 1.1 mg/mL (Hytest, Russia) with 1:10.000 delution in PBS buffer with 0.03% Tween-20. Then the membrane was washed three times in PBS buffer with 0.03% Tween-20. Peroxidase activity was visualized in solution containing 20 μg/ml 3,3'-diaminobenzidine and 0.01% H2O2. The reaction was stopped by immersion in 10% sulfuric acid, followed by washing with deionized water and air drying of the membrane. The resulting protein was sent for mass spectrometry analysis, which confirmed the presence of PCP4 in the samples submitted for analysis.
Determination of titers of antibodies against the 6His-PCP4hs-GFP protein using indirect solid-phase immunosorbent assay (ELISA)
The purified recombinant human 6His-PCP4hs-GFP protein was added to polystyrene well plate with medium absorption capacity (Grenier, Germany) at the final concentration of 10 μg/mL in 94 mM carbonate-bicarbonate buffer pH 9.5 in a volume of 100 μl per well. The plate was incubated for 14–16 h at +4°C.
Non-specific binding of polystyrene was blocked with a solution of 10 μg/mL bovine serum albumin in PBS buffer containing 20 mM potassium phosphate, pH 7.2, 100 mM NaCl (100 μL/well), which was added to the previous buffer containing the antigen, and incubated for 1 hour at room temperature in incubation shaker.
The solution was removed from the wells of cell culture plate. Each well was washed four times with 150 µL/well of PBS-T buffer (PBS buffer with 0.05% Tween-20).
In each row of 8-well plate, the titration of one of the samples was prepared with serial serum dilutions 2: 100, 200, 400, 800, 1600, 3200, 6400 in a volume of 100 μL. The titration was carried out in a separate plate to avoid sorption, in 1% (10 mg/ml) of BSA in PBS-T. The last well of the last row remained unoccupied and was used to control non-specific binding of secondary antibodies (serum titration ended with a 6400-fold dilution). Antibodies were incubated with antigen for one hour with shaking.
The wells of the plate were washed four times to remove unbound antibodies using PBS-T buffer in the volume of 150 µL/well.
Protein G peroxidase conjugate at a concentration of 1.1 mg/mL (Hytest, Russia) was added to all wells of the plate with 1:10.000 dilution in PBS-T buffer.
The wells of the plate were washed six times to remove unbound conjugate using PBS-T buffer in the volume of 150 µL per well.
The enzymatic reaction was carried out using a single-component 3,3'5,5'-tetramethylbenzidine (TMB) with 100 µl/well for 5 minutes on the thermoshaker.
The reaction was stopped by adding 30 µL of stop reagent (10% sulfuric acid) to the wells. The A450 value in each well was measured using the plate reader.
Statistical analysis
Software package Statistica 13.3 (StatSoft Inc., USA) was used for statistical data processing. The normal distribution of quantitative data was tested using the Shapiro–Wilk test. Given that the majority of quantitative parameters demonstrated deviation from the normal distribution, they are represented as median (Me) and interquartile range [Q1; Q3]. Absolute and relative frequencies (% of the total number of observations), as well as the arithmetic mean and standard deviation (M (SD)) were calculated.
The Kruskal–Wallis test was used to compare quantitative variables between three and more independent subgroups. The Mann–Whitney U test was used to compare quantitative variables between two independent groups. Statistically significant differences in qualitative variables were assessed using Pearson’s chi-square test with Yates correction for continuity. The differences were considered to be statistically significant at р≤0.05.
Results
Evaluation of mRNA content of the PCP4 gene in tissue samples from leiomyoma and PCR cloning of the corresponding cDNA
In our study, the cDNA of leiomyoma with the driver mutation in the MED12 gene was used as a template for amplification of the coding region of the PCP4/PEP19 gene using the primers PCP4-HS-F1(BamHI) and Nco-PCP-R.
The pRSET-EmGFP vector (Thermo Fisher Scientific, USA) containing the reporter gene of emerald green fluorescent protein (EmGFP) with 6His tag at the N-terminus for metal chelate affinity chromatography was used to create the expression construct based on PCP4/PEP19 cDNA. As a result of cleavage of this vector at the BamHI and NcoI restriction enzyme sites, the full-length PCP4/PEP19 gene including the initiator ATG codon was inserted between 6His tag and the EmGFP gene (Fig.1).

The encoded fusion product at the junction between 6His tag and PCP4/PEP19 contained artificially inserted Gly-Ser residues at the site of BamHI restriction enzyme. These residues constituted a hydrophilic flexible linker that facilitated the binding of 6His tag to the Ni-containing sorbent during metal affinity chromatography. The enterokinase DLYDDDDK cleavage site was located towards the N-terminus of these residues, that made it possible to remove the sequence of 6His tag from fusion protein, when necessary.
At the junction between PCP4/PEP19 and the emerald green fluorescent protein (EmGFP), AGSQS residues conforming with the destructured C-terminal PCP4/PEP19 were found, that also provided flexibility of the linker connecting the globular parts of both proteins and, presumably, did not hinder their normal folding. The estimated mass of fusion protein encoded by the pRSET-PCP4hs-EmGFP plasmid construct was 37.8 kDa. Production strain was obtained by insertion of this plasmid construct into the E. coli NiCo21 strain (New England Biolabs, USA), and was cultured in LB medium with lactose operon inducer IPTG at the concentration of 0.2 mM. According to densitometry data of denaturing polyacrylamide gel, after staining with Coomassie R-250, the target product yield was about 18% of the total mass of the cellular protein (Fig.2).
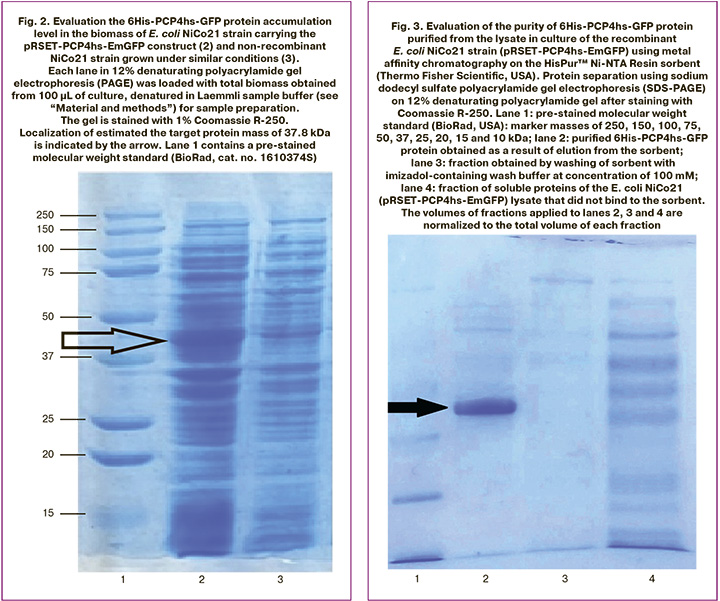
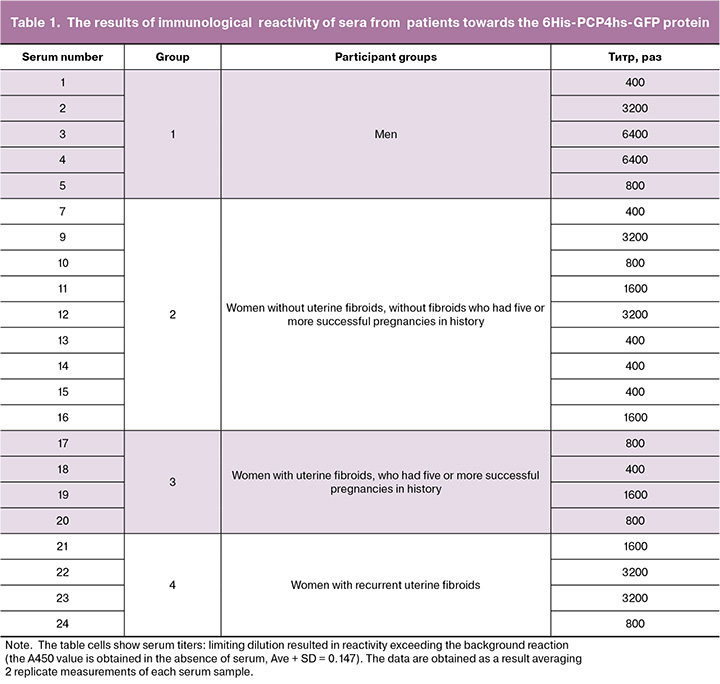
Preparative purification of the target product 6His-PCP4hs-GFP from the lysate of recombinant E. coli NiCo21 strain (pRSET-PCP4hs-EmGFP) obtained in the volume of 50 ml of the medium according to the method described in the section “Material and methods” allowed us to obtain 2.8 mg of protein with >90% purity (Fig. 3).
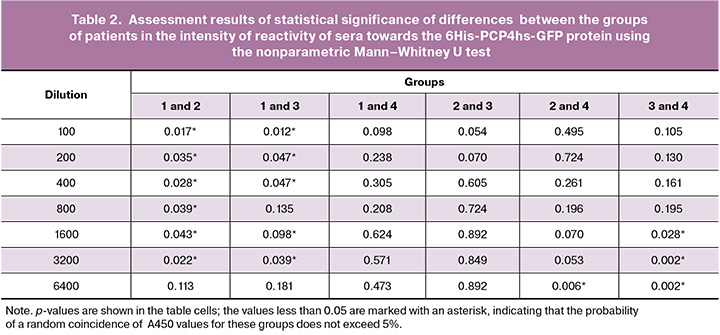
Four groups of patients were formed to assess the immunological activity of sera towards the 6His-PCP4hs-GFP protein. Group 1 was the control group composed of men (n=5) (sera samples No. 1–5). Group 2 consisted of women without uterine fibroids who had five or more successful pregnancies in history (n=9) (sera samples No. 7, 9–16). Group 3 included women with uterine fibroids, who had five or more successful pregnancies in history (n=4) (sera samples No. 17–20). Group 4 included women with recurrent uterine fibroids (n=4) (sera samples No. 21–24). The 6His-PCP4hs-GFP protein was adsorbed on the surface of polystyrene immunological plates as described in the “Materials and methods”. section. Non-specific binding was blocked, then sera samples from patients in 4 groups were applied to the wells in seven series of two-step delution from 1:100 to 1:6400. After incubation of the sera in the wells for binding to the applied antigen, unbound antibodies were removed and washed, and the amount of bound antibodies was determined using a conjugate of protein G with horseradish peroxidase and TMB peroxidase substrate according to the standard method described in the “Materials and methods” section. The experimental results are presented in Table 1. After serum incubation in the wells for binding to the applied antigen, unbound antibodies were removed and washed. The amount of bound antibodies was determined using the horseradish peroxidase (HRP) conjugate of protein G and TMB peroxidase substrate according to the standard method described in the “Materials and methods” section. The experimental results are shown in Table 1.
The data in Table 1 show high level of serum reactivity of sera towards the analyzed protein in all groups. At the same time, both high and low levels of serum reactivity were detected in all groups. Statistical significance of differences between the groups in the intensity of serum reactivity was assessed using the nonparametric Mann–Whitney U test. The results of analysis are represented in Table 2.
The data in Table 2 convincingly show that the highest immunological reactivity towards the 6His-PCP4hs-GFP protein was demonstrated by serum samples from men in the control group, whereas no significant differences were found between the groups of women. The minimal difference in sera was found in groups 2 and 4 at a dilution of 1:6400. At the same time, the reactivity of sera from women with recurrent uterine fibroids was higher compared with women without uterine fibroids, who had five or more successful pregnancies. Comparison of women in groups 3 and 4 showed statistically significant differences in the values of optical density obtained by serum delution 1:1600, 1:3200 и 1:6400. At the same time, reactivity of sera from women with recurrent uterine fibroids was higher versus women with uterine fibroids, who had five or more successful pregnancies.
Since certain serum titers spread widely in each group, it was decided to distribute serum activity into three categories: low activity – titer 1:400 and below, moderate activity – titers 1:800 and 1:1600, and high activity – titer 1:3200 and above. After that, serum percentage for each category in the groups was determined. The results are represented in Table 3.
The data in table 3 show that activity of sera from men with the 6His-PCP4hs-GFP antigen. The proportion of sera with high reactivity is 60%, and this is the maximum value for all groups. The proportion of the sera with low activity is smaller – 20%. In the group of women without uterine fibroids who had five or more successful pregnancies, the proportion of sera with low activity is 44%, and this is the maximum value compared with other groups. In the group of women with uterine fibroids who had five or more successful pregnancies, the average level of reactivity towards the 6His-PCP4hs-GFP antigen is most common. The proportion of this category of serum reactivity is 75%, and this is the maximum value among all groups. Finally, in the group of women with recurrent uterine fibroids, equal number of sera with high average activity is most common. This shows that in this group of patients, there is a greater tendency for antibody production against PCP4/PEP19 compared with multiparous women.
To validate ELISA results, a selective testing the specificity of antibody binding from the used panel of sera to the 6His-PCP4hs-GFP recombinant protein was conducted. The results of the experiment are represented in Figure 4.
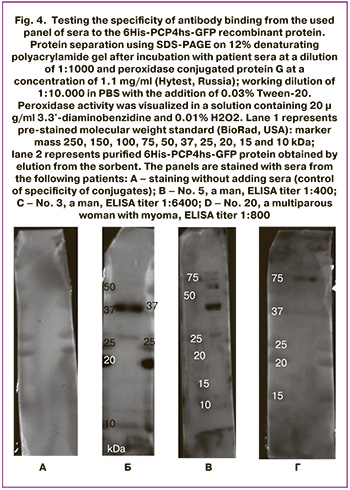
Comparison of testing results of reactivity of patient sera towards the 6His-PCP4hs-GFP protein by immunoblotting and ELISA shows that the titer value in ELISA weakly correlates with the presence or absence of band in immunoblotting. Thus, serum samples No. 3 and No. 5 from men (panels B and C, Fig. 4) stained the target antigen with a mass of 37 kDa with the same intensity in immunoblotting, although the titer values in ELISA displayed opposite poles of reaction intensity: serum sample No. 5 showed a titer of 1:400, and serum sample No. 3 – 1:6400. At the same time, serum sample No. 20, which had an intermediate titer of 1:800 in ELISA, did not show a reaction with the target antigen in immunoblotting. It can be assumed that strong discrepancies between the results obtained using ELISA and immunoblotting are explained by the fact that the native protein conformation was involved in the reaction using ELISA, while protein denaturated during sample preparation was involved in the reaction using immunoblotting. Thus in immunoblotting, conformation-dependent epitopes of the PCP4/PEP19 protein were excluded from binding to antibodies.
Discussion
Analysis of previously published data did not provide a clear answer to the question about the role of the PCP4/PEP19 protein in the functioning of normal tissues and tumors. Some studies [20, 21] reported the important role of PCP4 in the function of the myocardium, primarily in pacemaker cells, as well as its positive effect on reducing the risk of cardiomyocyte hypertrophy. Other studies [13, 22] reported PCP4 expression in cells in different parts of the brain. In one of the studies [23] there are data representing that the presence of the PCP4 protein is important for regulation of aldosterone secretion in the adrenal glands. Finally, another study [24] reported of the presence of PCP4 in myometrial cells. Taken together, these available data suggest that PCP4 is an important component in regulation of normal tissue functions. Given the presence of this protein in normal adult tissues, it can be expected that the production of PCP4 antibodies is blocked by the mechanisms of immunological tolerance.
At the same time some authors [25–27] consider PCP4 as a tumor marker characteristic for different tumor types (salivary gland carcinoma, neuroblastoma, pancreatic carcinoma). Previously published study [10] substantiates the significance of PCP4 as a tumor marker for leiomyoma. It is possible that reason for the data inconsistency is different concentrations of the protein of interest, which are found in different tissue types. It can be assumed that low expression of PCP4 is in normal tissues, while abnormal overexpression is in tumors.
In general, the obtained data show that most of the examined sera demonstrate significant, well-detectable antibody titers to PCP4. At the same time, the activity of these antibodies varies widely. In particular, in sera from men and women with recurrent myomas, antibodies against PCP4 are found more often than in the sera from multiparous women, especially in those who do not develop leiomyomas.
On the one hand, this allows to conclude that the presence of antibodies against PCP4 in human blood does not lead to significant pathologies as a result of an autoimmune reaction towards the tissues containing PCP4 (for example, the brain, myocardium, and adrenal glands). Thus, it is unlikely that PCP4 immunization is associated with the risk of any pathology. On the other hand, the fact that antibody titers of PCP4 in the group of multiparous women are significantly lower than in men and patients with recurrent uterine fibroids suggests that the presence of these antibodies does not contribute to prevention of the occurrence and growth of leiomyomas. Therefore, using PCP4 as the basis to develop a preventive vaccine against leiomyomas is not advisable.
As a hypothesis explaining the origin of the antigen in response to which antibodies to PCP4 are produced in certain individuals, it can be assumed that benign tumors are the source of this protein, which can spontaneously occur at any age both in females and males. This assumption agrees quite well with the thesis about the tendency of some tumors to overproduce PCP4 and explains extensive differences in the intensity of the immune response to PCP4 in patients of each studied group.
Conclusion
For the first time, human PCP4 was obtained in preparative amounts sufficient for analysis of its reaction with antibodies from blood serum in different groups of patients. Testing showed that most samples in all groups had significant titers of antibodies against PCP4. The activity of these antibodies varies widely both in sera from men and women. Antibodies against PCP4 are most common in women with recurrent uterine fibroids compared with multiparous women, especially who have no uterine fibroids. Thus, it is unlikely that PCP4 immunization carries the risk of disorders. The titers of antibodies against PCP4 in the general group of multiparous women were significantly lower compared with men and female patients with recurrent uterine fibroids. Therefore, using PCP4 as the basis to develop a preventive vaccine against leiomyomas is not advisable.
References
- Уварова Е.В., Адамян Л.В., Стрижакова М.А. и др. Рецидивирующая миома матки у юной пациентки (клиническое наблюдение). Репродуктивное здоровье детей и подростков. 2005; 1: 53-7. [Uvarova E.V., Adamyan L.V., Strizhakova M.A. et al. Recurrent uterine myoma in a young patient (clinical observation). Reproductive health of children and adolescents. 2005; (1): 53-7 (in Russian)].
- Краснопольский В.И., Буянова С.Н., Щукина Н.А., Попов А.А. Оперативная гинекология. М.: МЕДпресс-информ; 2010. 319 с. [Krasnopolsky V.I., Buyanova S.N., Shchukina N.A., Popov A.A. Operative gynecology. Moscow: MEDpress-inform; 2010. 319 p. (in Russian)].
- Pavone D., Clemenza S., Sorbi F., Fambrini M., Petraglia F. Epidemiology and risk factors of uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2018; 46: 3-11. https://dx.doi.org/10.1016/j.bpobgyn.2017.09.004
- Печетов А.А., Леднев А.Н., Ратникова Н.К., Волчанский Д.А. Доброкачественная метастазирующая лейомиома матки с метастазированием в легкие: проблемы диагностики и лечения. Хирургия. Журнал имени Н.И. Пирогова. 2020; (9): 85-8. [Pechetov A.A., Lednev A.N., Ratnikova N.K., Volchanskii D.A. Benign metastasizing uterine leiomyoma with lung metastasis: problems of diagnosis and treatment. Pirogov Russian Journal of Surgery. 2020; (9): 85-8 (in Russian)]. https://dx.doi.org/10.17116/hirurgia202009185
- Torres-de la Roche L.A., Becker S., Cezar C., Hermann A., Larbig A., Leicher L. et al. Pathobiology of myomatosis uteri: the underlying knowledge to support our clinical practice. Arch. Gynecol. Obstet. 2017; 296(4): 701-7. https://dx.doi.org/10.1007/s00404-017-4494-6
- Wu J.M., Wechter M.E., Geller E.J., Nguyen T.V., Visco A.G. Hysterectomy rates in the Unites States, 2003. Obstet. Gynecol. 2007; 110(5): 1091-5 https://dx.doi.org/10.1097/01.aog.0000285997.38553.4b
- González V.G, Moreta A.H., Triana A.M., Sierra L.R., García I.C., Méndez N.I. Prolapsed cervical myoma during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020; 252: 150-4. https://dx.doi.org/10.1016/j.ejogrb.2020.06.039
- Согоян Н.С., Кузнецова М. В., Донников А. Е., Мишина Н.Д., Михайловская Г.В., Шубина Е.С., Зеленский Д.В., Муллабаева С.М., Адамян Л.В. Семейная предрасположенность к развитию лейомиомы матки: поиск генетических факторов, повышающих риск развития заболевания. Проблемы репродукции. 2020; 26(5): 51-7. [Sogoyan N.S., Kuznetsova M.V., Donnikov A.E., Mishina N.D., Mikhailovskaya G.V., Shubina E.S., Zelensky D.V., Mullabaeva S.M., Adamyan L.V. Familial predisposition to uterine leiomyoma: searching for genetic factors that increase the risk of leyomyoma development. Russian Journal of Human Reproduction. 2020; 26(5): 51-7 (in Russian)]. https://dx.doi.org/10.17116/repro20202605151
- Marugo M., Centonze M., Bernasconi D., Fazzuoli L., Berta S., Giordano G. Estrogen and progesterone receptors in uterine leiomyomas. Acta Obstet. Gynecol. Scand. 1989; 68(8): 731-5. https://dx.doi.org/10.3109/00016348909006147
- Kuznetsova M.V., Tonoyan N.M., Trubnikova E.V., Zelensky D.V., Svirepova K.A., Adamyan L.V. et al. Novel approaches to possible targeted therapies and prophylaxis of uterine fibroids. Diseases. 2023; 11(4): 156. https://dx.doi.org/10.3390/diseases11040156
- Ceja-Navarro J.A., Brodie E.L., Vega F.E. A technique to dissect the alimentary canal of the coffee berry borer (Hypothenemus hampei), with isolation of internal microorganisms. Journal of Entomological and Acarological Research. 2012; 44(3): e21.117-9. https://dx.doi.org/10.4081/jear.2012.e21
- Maekawa T., Kashkar H., Coll N.S. Dying in self-defence: a comparative overview of immunogenic cell death signalling in animals and plants. Cell Death Differ. 2023; 30(2): 258-68. https://dx.doi.org/10.1038/s41418-022-01060-6
- Renelt M., von Bohlen und Halbach V., von Bohlen und Halbach O. Distribution of PCP4 protein in the forebrain of adult mice. Acta Histochem. 2014; 116(6): 1056-61. https://dx.doi.org/10.1016/j.acthis.2014.04.012
- Felizola S.J., Nakamura Y., Ono Y., Kitamura K., Kikuchi K., Onodera Y. et al. PCP4: a regulator of aldosterone synthesis in human adrenocortical tissues. J. Mol. Endocrinol. 2014; 52(2): 159-67. https://dx.doi.org/10.1530/JME-13-0248
- Yoshimura T., Higashi S., Yamada S., Noguchi H., Nomoto M., Suzuki H. et al. PCP4/PEP19 and HER2 are novel prognostic markers in mucoepidermoid carcinoma of the salivary gland. Cancers (Basel). 2022; 14(1): 54. https://dx.doi.org/10.3390/cancers14010054
- Kitazono I., Hamada T., Yoshimura T., Kirishima M., Yokoyama S., Akahane T. et al. PCP4/PEP19 downregulates neurite outgrowth via transcriptional regulation of Ascl1 and NeuroD1 expression in human neuroblastoma M17 cells. Lab. Invest. 2020; 100(12): 1551-63. https://dx.doi.org/10.1038/s41374-020-0462-z
- Honjo K., Hamada T., Yoshimura T., Yokoyama S., Yamada S., Tan Y.Q. et al. PCP4/PEP19 upregulates aromatase gene expression via CYP19A1 promoter I.1 in human breast cancer SK-BR-3 cells. Oncotarget. 2018; 9(51): 29619-33. https://dx.doi.org/10.18632/oncotarget.25651
- He L., Lee G.T., Zhou H., Buhimschi I.A., Buhimschi C.S., Weiner C.P. et al. Expression, regulation and function of the calmodulin accessory protein PCP4/PEP-19 in myometrium. Reprod. Sci. 2019; 26(12): 1650-60. https://dx.doi.org/19.1177/1933719119828072
- Бурменская О.В., Трофимов Д.Ю., Кометова В.В., Сергеев И.В., Маерле А.В., Родионов В.В., Сухих Г.Т. Разработка и опыт использования транскрипционной сигнатуры генов в диагностике молекулярных подтипов рака молочной железы. Акушерство и гинекология. 2020; 2: 132-40. [Burmenskaya O.V., Trofimov D.Yu., Kometova V.V., Sergeev I.V., Maerle A.V., Rodionov V.V., Sukhikh G.T. Development and experience of using the transcriptional gene signature in the diagnosis of molecular breast cancer subtypes. Obstetrics and Gynecology. 2020; (2): 132-40 (in Russian)]. https://dx.doi.org/10.18565/aig.2020.2.132-140
- Prodan N., Ershad F., Reyes-Alcaraz A., Li L., Mistretta B., Gonzalez L. et al. Direct reprogramming of cardiomyocytes into cardiac Purkinje-like cells. iScience. 2022; 25(11): 105402. https://dx.doi.org/10.1016/j.isci.2022.105402
- Xie Y.Y., Sun M.M., Lou X.F., Zhang C., Han F., Zhang B.Y. et al. Overexpression of PEP-19 suppresses angiotensin II-induced cardiomyocyte hypertrophy. J. Pharmacol. Sci. 2014; 125(3): 274-82. doi: 10.1254/jphs.13208fp
- Mouton-Liger F., Sahún I., Collin T., Lopes Pereira P., Masini D., Thomas S. et al. Developmental molecular and functional cerebellar alterations induced by PCP4/PEP19 overexpression: implications for Down syndrome. Neurobiol. Dis. 2014; 63: 92-106. https://dx.doi.org/10.1016/j.nbd.2013.11.016
- Aji G., Li F., Chen J., Leng F., Hu K., Cheng Z. et al. Upregulation of PCP4 in human aldosterone-producing adenomas fosters human adrenocortical tumor cell growth via AKT and AMPK pathway. Int. J. Clin. Exp. Pathol. 2018; 11(3): 1197-207.
- He J., Baxter S.L., Xu J., Xu J., Zhou X., Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat. Med. 2019; 25(1): 30-6. https://dx.doi.org/10.1038/s41591-018-0307-0
- Yoshimura A., Yamada T., Okuma Y., Fukuda A., Watanabe S., Nishioka N. et al. Impact of tumor programmed death ligand-1 expression on osimertinib efficacy in untreated EGFR-mutated advanced non-small cell lung cancer: a prospective observational study. Transl. Lung Cancer Res. 2021; 10(8): 3582-93. https://dx.doi.org/10.21037/tlcr-21-461
- Kitazono I., Hamada T., Yoshimura T., Kirishima M., Yokoyama S., Akahane T. et al. PCP4/PEP19 downregulates neurite outgrowth via transcriptional regulation of Ascl1 and NeuroD1 expression in human neuroblastoma M17 cells. Lab. Invest. 2020; 100(12): 1551-63. https://dx.doi.org/10.1038/s41374-020-0462-z
- Guo Z.S. The 2018 Nobel Prize in medicine goes to cancer immunotherapy (editorial for BMC cancer). BMC Cancer. 2018; 18(1): 1086. https://dx.doi.org/10.1186/s12885-018-5020-3
Received 26.06.2025
Accepted 11.08.2025
About the Authors
Maria V. Kuznetsova, PhD, Senior Researcher at the Institute of Reproductive Genetics, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4, +7(495)438-13-41, mkarja@mail.ru, https://orcid.org/0000-0003-3790-0427Alexey B. Shevelev, Chief Researcher, N.I. Vavilov Institute of General Genetics of the Russian Academy of Sciences, 119991, Russia, GSP-1, Moscow, Gubkin str., 3, +7(915)087-52-93, shevel_a@hotmail.com, https://orcid.org/0000-0003-3564-7405
Natalia V. Pozdnyakova, PhD, Senior Researcher at the Laboratory of Radionuclide and Radiation Technologies in Experimental Oncology, Blokhin National Research Medical Center of Oncology, Ministry of Health of Russia, 115230, Russia, Moscow, Kashirskoe Shosse, 23, +7(977)767-99-90, natpo2002@mail.ru,
https://orcid.org/0000-0002-5765-3016
Darya V. Samoilova, Researcher at the Central Pathoanatomical Laboratory of the Academician A.P. Avtsyn Research Institute of Human Morphology, Russian Scientific Center of Surgery named after Academician B.V. Petrovsky, 109548, Russia, Moscow, Ugreshskaya str., 2-7, +7(916)594-89-06, dashasam@mail.ru,
https://orcid.org/0000-0001-5639-0835
Viktoria E. Karyagina, PhD, Senior Researcher at the Laboratory of Molecular Genetic Methods, Institute of Translation Medicine, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4, +7(495)438-13-41.
Narine M. Tonoyan, PhD, Doctor, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4, SPIN: 8547-9399, Scopus AuthorID: 57213609878, https://orcid.org/0000-0002-1631-1829
Elena V. Trubnikova, Dr. Bio. Sci., Associate Professor, Chief Researcher at the Laboratory of Genetics, Kursk State University, 305000, Russia, Kursk, Radishchev str., 33; Leading Researcher, N.I. Vavilov Institute of General Genetics of the Russian Academy of Sciences, 119991, Russia, GSP-1, Moscow, Gubkin str., 3, +7(910)311-18-86,
tr_e@list.ru, https://orcid.org/0000-0001-5025-9406
Dmitry V. Zelensky, PhD student, Kursk State University, 305000, Russia, Kursk, Radishchev str., 33, +7(999)700-38-81, dmitriizelenskii@mail.ru,
https://orcid.org/0000-0006-0490-7760
Polina A. Vishnyakova, PhD, Head of the Laboratory of Regenerative Medicine, V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Ministry of Health of Russia, 117997, Russia, Moscow, Oparin str., 4; Head of the Laboratory of Molecular Pathophysiology, Research Institute of Molecular and Cellular Medicine, Patrice Lumumba Peoples’ Friendship University of Russia, Scopus AuthorID: 57190971385, https://orcid.org/0000-0001-8650-8240
Corresponding author: Maria V. Kuznetsova, mkarja@mail.ru



